Hereditas(Beijing) ›› 2024, Vol. 46 ›› Issue (2): 140-148.doi: 10.16288/j.yczz.23-262
• Review • Previous Articles Next Articles
Progress on the quality control technology of next generation sequencing library
Mengnan Cui( ), Yan Guo, Yarong Wu, Guangqian Pei, Yujun Cui(
), Yan Guo, Yarong Wu, Guangqian Pei, Yujun Cui( )
)
- State Key Laboratory of Pathogen and Biosecurity, Beijing Institute of Microbiology and Epidemiology, Beijing 100071, China
-
Received:2023-10-23Revised:2023-12-22Online:2024-02-20Published:2024-01-05 -
Contact:Yujun Cui E-mail:mengncui@163.com;cuiyujun.new@gmail.com
Cite this article
Mengnan Cui, Yan Guo, Yarong Wu, Guangqian Pei, Yujun Cui. Progress on the quality control technology of next generation sequencing library[J]. Hereditas(Beijing), 2024, 46(2): 140-148.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
Table 1
The comparison of different library concentration evaluation techniques"
| 类别 | 紫外吸收技术 | 荧光染料技术 | qPCR(SYBR-Green) | qPCR(TaqMan) | ddPCR |
|---|---|---|---|---|---|
| 典型仪器 | NanoDrop | Qubit | ABI7500 | ABI7500 | qx200 |
| 投入量(μL) | 1 | 1~20 | 2 | 2 | 2 |
| 定量范围 | 2~3700 ng/μLa | 10 pg/μL~100 ng/μLb | 0.00083~8.3pmol/L[ | 0.068~6.8 pmol/L[ | 1~105个拷贝c |
| 单次检测量 | 1 | 1 | ≤96 | ≤96 | ≤96 |
| 单次检测时间 | 30 s | 1 min | 2.5 h | 2.5 h | 微滴生成<2 min/ 8 个,PCR反应时间2 h,微滴检测< 10 min/个 |
| 试剂成本/单样品 (美元) | <0.5 | 0.7 | 3.0 | 5.9 | 7~9.8 |
| 优点 | 操作简单、检测速度快、成本低 | 操作简单、成本较低,可以特异性区分DNA、RNA,检测灵敏度高、重复性好 | 不需要设计荧光探针,灵活,成本较低,能区分文库两端是否连接接头 | 需要设计荧光探针,成本较低,能区分文库两端是否连接接头 | 绝对定量,不依赖于具有特定片段大小的标准品,不依赖于校准物的扩增效率,准确度更高、置信度和可重复性更好 |
| 缺点 | 对DNA、RNA、蛋白质没有选择性,受杂质影响大,精度差 | 无法区分文库两端是否连接接头 | 不能区分接头二聚体,依赖于生物分析仪对文库片段大小的检测 | 不能区分接头二聚体,依赖于生物分析仪对文库片段大小的检测 | 成本较高 |
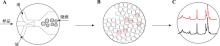
Table 2
The comparison of different library size distribution evaluation techniques"
| 类别 | 琼脂糖凝胶电泳技术 | 微流控芯片电泳技术 |
|---|---|---|
| 典型仪器 | Agilent 2100 Bioanalyzer | |
| 投入量 | 1 μL | |
| 分离片段范围 | 与琼脂糖凝胶配制浓度相关 | 25 bp~12 kb |
| 灵敏度 | 与作为DNA分子量标准有关 | 0.1 ng DNA |
| 单次检测量 | 与制胶时的孔容量有关 | 12 |
| 单次检测时间 | 3 min | |
| 优点 | 原理、操作方法等较简单,成本较低 | 所需进样量、试剂量等少、人工操作时间短、操作安全、数据可视化、分析自动化、准确度高、重复性好、灵敏度高 |
| 缺点 | 分离精度较低、操作繁琐、自动化程度低 | 试剂、芯片价格较昂贵 |
| [1] |
Chen LJ, Liu WY, Zhang Q, Xu K, Ye GM, Wu WC, Sun ZY, Liu F, Wu KL, Zhong B, Mei Y, Zhang WX, Chen Y, Li YR, Shi M, Lan K, Liu YL. RNA based mNGS approach identifies a novel human coronavirus from two individual pneumonia cases in 2019 Wuhan outbreak. Emerg Microbes Infect, 2020, 9(1): 313-319.
doi: 10.1080/22221751.2020.1725399 |
| [2] |
Zheng HY, Yan L, Yang C, Wu YR, Qin JL, Hao TY, Yang DJ, Guo YC, Pei XY, Zhao TY, Cui YJ. Population genomics study of Vibrio alginolyticus. Hereditas(Beijing), 2021, 43(4): 350-361.
doi: 10.16288/j.yczz.21-061 pmid: 33972209 |
|
郑宏源, 闫琳, 杨超, 武雅蓉, 秦婧靓, 郝彤宇, 杨大进, 郭云昌, 裴晓燕, 赵彤言, 崔玉军. 溶藻弧菌群体基因组学研究. 遗传, 2021, 43(4): 350-361.
doi: 10.16288/j.yczz.21-061 pmid: 33972209 |
|
| [3] |
Wang GZ, Long J, Zhuang Y, Leng X, Zhang YQ, Liu LBX, Fu JW, Chen Y, Li CQ, Zhou Y, Huang B, Feng CC. Application of metagenomic next-generation sequencing in the detection of pathogens in spinal infections. Spine J, 2023, 23(6): 859-867.
doi: 10.1016/j.spinee.2023.02.001 pmid: 36773890 |
| [4] |
Børsting C, Morling N. Next generation sequencing and its applications in forensic genetics. Forensic Sci Int Genet, 2015, 18: 78-89.
doi: 10.1016/j.fsigen.2015.02.002 |
| [5] |
Liu YL, Xu C, Sun YZ, Chen X, Dong WP, Yang XY, Zhou SL. Method for quick DNA barcode reference library construction. Ecol Evol, 2021, 11(17): 11627-11638.
doi: 10.1002/ece3.7788 pmid: 34522329 |
| [6] |
Laurie MT, Bertout JA, Taylor SD, Burton JN, Shendure JA, Bielas JH. Simultaneous digital quantification and fluorescence-based size characterization of massively parallel sequencing libraries. Biotechniques, 2013, 55(2): 61-67.
doi: 10.2144/000114063 pmid: 23931593 |
| [7] |
Modi A, Vai S, Caramelli D, Lari M. The Illumina sequencing protocol and the NovaSeq 6000 system. Methods Mol Biol, 2021, 2242: 15-42.
doi: 10.1007/978-1-0716-1099-2_2 pmid: 33961215 |
| [8] |
Glasel JA. Validity of nucleic acid purities monitored by 260nm/280nm absorbance ratios. Biotechniques, 1995, 18(1): 62-63.
pmid: 7702855 |
| [9] |
Huberman JA. Importance of measuring nucleic acid absorbance at 240 nm as well as at 260 and 280 nm. BioTechniques, 1995, 18(4): 636.
pmid: 7598897 |
| [10] |
Manchester KL. Value of A260/A280 ratios for measurement of purity of nucleic acids. Biotechniques, 1995, 19(2): 208-210.
pmid: 8527139 |
| [11] |
Singer VL, Jones LJ, Yue ST, Haugland RP. Characterization of picoGreen reagent and development of a fluorescence-based solution assay for double-stranded dna quantitation. Anal Biochem, 1997, 249(2): 228-238.
doi: 10.1006/abio.1997.2177 pmid: 9212875 |
| [12] |
Le Pecq JB, Paoletti C. A new fluorometric method for RNA and DNA determination. Anal Biochem, 1966, 17(1): 100-107.
pmid: 6008008 |
| [13] |
Kapuscinski J. DAPI: a DMA-specific fluorescent probe. Biotech Histochem, 1995, 70(5): 220-233.
doi: 10.3109/10520299509108199 pmid: 8580206 |
| [14] |
Heydt C, Fassunke J, Künstlinger H, Ihle MA, König K, Heukamp LC, Schildhaus HU, Odenthal M, Büttner R, Merkelbach-Bruse S. Comparison of pre-analytical FFPE sample preparation methods and their impact on massively parallel sequencing in routine diagnostics. PLoS One, 2014, 9(8): e104566.
doi: 10.1371/journal.pone.0104566 |
| [15] |
Heredia NJ. Droplet Digital™ PCR next-generation sequencing library qc assay. Methods Mol Biol, 2018, 1768: 477-488.
doi: 10.1007/978-1-4939-7778-9_27 pmid: 29717460 |
| [16] |
Chang LL, Wang D, Peng CZ, Wang Q, Xu BQ, Tong Z. A method for high-concentration agarose gel preparation and its application in high-resolution separation of low- molecular-weight nucleic acids and proteins. Int J Biol Macromol, 2023, 231: 123358.
doi: 10.1016/j.ijbiomac.2023.123358 |
| [17] | Sun YL, Lu ZX, Miller M, Perroud T, Tong YH. Application of microfluidic chip electrophoresis for high-throughput nucleic acid fluorescence fragment analysis assays. NAR Genom Bioinform, 2023, 5(1): lqad011. |
| [18] |
Hussing C, Kampmann ML, Mogensen HS, Børsting C, Morling N. Quantification of massively parallel sequencing libraries -a comparative study of eight methods. Sci Rep, 2018, 8(1): 1110.
doi: 10.1038/s41598-018-19574-w |
| [19] |
Harris JK, Sahl JW, Castoe TA, Wagner BD, Pollock DD, Spear JR. Comparison of normalization methods for construction of large, multiplex amplicon pools for next-generation sequencing. Appl Environ Microbiol, 2010, 76(12): 3863-3868.
doi: 10.1128/AEM.02585-09 |
| [20] |
Hosomichi K, Mitsunaga S, Nagasaki H, Inoue I. A bead-based normalization for uniform sequencing depth (BeNUS) protocol for multi-samples sequencing exemplified by HLA-B. BMC Genomics, 2014, 15(1): 645.
doi: 10.1186/1471-2164-15-645 |
| [21] | Masago K, Fujita S, Oya Y, Takahashi Y, Matsushita H, Sasaki E, Kuroda H. Comparison between fluorimetry (qubit) and spectrophotometry (nanodrop) in the quantification of DNA and RNA extracted from frozen and FFPE tissues from lung cancer patients: a real-world use of genomic tests. Medicina (Kaunas), 2021, 57(12): 1375. |
| [22] |
Tuononen K, Mäki-Nevala S, Sarhadi VK, Wirtanen A, Rönty M, Salmenkivi K, Andrews JM, Telaranta-Keerie AI, Hannula S, Lagström S, Ellonen P, Knuuttila A, Knuutila S. Comparison of targeted next-generation sequencing (NGS) and real-time PCR in the detection of EGFR, KRAS, and BRAF mutations on formalin-fixed, paraffin-embedded tumor material of non-small cell lung carcinoma-superiority of NGS. Genes Chromosomes Cancer, 2013, 52(5): 503-511.
doi: 10.1002/gcc.v52.5 |
| [23] |
Sah S, Chen LJ, Houghton J, Kemppainen J, Marko AC, Zeigler R, Latham GJ. Functional DNA quantification guides accurate next-generation sequencing mutation detection in formalin-fixed, paraffin-embedded tumor biopsies. Genome Med, 2013, 5(8): 77.
doi: 10.1186/gm481 pmid: 24001039 |
| [24] |
Simbolo M, Gottardi M, Corbo V, Fassan M, Mafficini A, Malpeli G, Lawlor RT, Scarpa A. DNA qualification workflow for next generation sequencing of histopathological samples. PLoS One, 2013, 8(6): e62692.
doi: 10.1371/journal.pone.0062692 |
| [25] |
Holden MJ, Haynes RJ, Rabb SA, Satija N, Yang K, Blasic JR. Factors affecting quantification of total DNA by UV spectroscopy and PicoGreen fluorescence. J Agric Food Chem, 2009, 57(16): 7221-7226.
doi: 10.1021/jf901165h |
| [26] |
Georgiou CD, Papapostolou I. Assay for the quantification of intact/fragmented genomic DNA. Anal Biochem, 2006, 358(2): 247-256.
doi: 10.1016/j.ab.2006.07.035 pmid: 16942746 |
| [27] |
Navarro E, Serrano-Heras G, Castaño MJ, Solera J. Real-time PCR detection chemistry. Clin Chim Acta, 2015, 439: 231-250.
doi: 10.1016/j.cca.2014.10.017 pmid: 25451956 |
| [28] |
Robin JD, Ludlow AT, LaRanger R, Wright WE, Shay JW. Comparison of DNA quantification methods for next generation sequencing. Sci Rep, 2016, 6: 24067.
doi: 10.1038/srep24067 pmid: 27048884 |
| [29] |
Arya M, Shergill IS, Williamson M, Gommersall L, Arya N, Patel HRH. Basic principles of real-time quantitative PCR. Expert Rev Mol Diagn, 2005, 5(2): 209-219.
doi: 10.1586/14737159.5.2.209 pmid: 15833050 |
| [30] |
Dang J, Mendez P, Lee S, Kim JW, Yoon JH, Kim TW, Sailey CJ, Jablons DM, Kim IJ. Development of a robust DNA quality and quantity assessment qPCR assay for targeted next-generation sequencing library preparation. Int J Oncol, 2016, 49(4): 1755-1765.
doi: 10.3892/ijo.2016.3654 pmid: 27511764 |
| [31] |
Dressman D, Yan H, Traverso G, Kinzler KW, Vogelstein B. Transforming single DNA molecules into fluorescent magnetic particles for detection and enumeration of genetic variations. Proc Natl Acad Sci USA, 2003, 100(15): 8817-8822.
doi: 10.1073/pnas.1133470100 pmid: 12857956 |
| [32] |
Fedurco M, Romieu A, Williams S, Lawrence I, Turcatti G. BTA, a novel reagent for DNA attachment on glass and efficient generation of solid-phase amplified DNA colonies. Nucleic Acids Res, 2006, 34(3): e22.
doi: 10.1093/nar/gnj023 pmid: 16473845 |
| [33] |
Parkinson NJ, Maslau S, Ferneyhough B, Zhang G, Gregory L, Buck D, Ragoussis J, Ponting CP, Fischer MD. Preparation of high-quality next-generation sequencing libraries from picogram quantities of target DNA. Genome Res, 2012, 22(1): 125-133.
doi: 10.1101/gr.124016.111 pmid: 22090378 |
| [34] |
Li MK, Stoneking M. A new approach for detecting low-level mutations in next-generation sequence data. Genome Biol, 2012, 13(5): R34.
doi: 10.1186/gb-2012-13-5-r34 |
| [35] |
Mamedov TG, Pienaar E, Whitney SE, TerMaat JR, Carvill G, Goliath R, Subramanian A, Viljoen HJ. A fundamental study of the PCR amplification of GC-rich DNA templates. Comput Biol Chem, 2008, 32(6): 452-457.
doi: 10.1016/j.compbiolchem.2008.07.021 pmid: 18760969 |
| [36] |
Vogelstein B, Kinzler KW. Digital PCR. Proc Natl Acad Sci USA, 1999, 96(16): 9236-9241.
doi: 10.1073/pnas.96.16.9236 pmid: 10430926 |
| [37] |
Aigrain L, Gu Y, Quail MA. Quantitation of next generation sequencing library preparation protocol efficiencies using droplet digital PCR assays-a systematic comparison of DNA library preparation kits for Illumina sequencing. BMC Genomics, 2016, 17: 458.
doi: 10.1186/s12864-016-2757-4 pmid: 27297323 |
| [38] |
Seguin-Orlando A, Schubert M, Clary J, Stagegaard J, Alberdi MT, Prado JL, Prieto A, Willerslev E, Orlando L. Ligation bias in Illumina next-generation DNA libraries: implications for sequencing ancient genomes. PLoS One, 2013, 8(10): e78575.
doi: 10.1371/journal.pone.0078575 |
| [39] |
Ziraldo R, Shoura MJ, Fire AZ, Levene SD. Deconvolution of nucleic-acid length distributions: a gel electrophoresis analysis tool and applications. Nucleic Acids Res, 2019, 47(16): e92.
doi: 10.1093/nar/gkz534 |
| [40] |
Manz A, Graber N, Widmer HM. Miniaturized total chemical analysis systems: a novel concept for chemical sensing. Sens Actuators B, 1990, 1(1-6): 244-248.
doi: 10.1016/0925-4005(90)80209-I |
| [41] |
Loughran M, Cretich M, Chiari M, Suzuki H. Separation of DNA in a versatile microchip. Sens Actuators B, 2005, 107(2): 975-979.
doi: 10.1016/j.snb.2004.12.014 |
| [42] | Liu KH, Liang N, Yao B, Luo GA, Wang YM. Development of Laser-induced fluorescence detector for deoxyribonucleic acid fragments seperation by microfluidic chip. Chin J Anal Chem, 2005, 33(9): 1350-1353. |
| 刘科辉, 梁宁, 姚波, 罗国安, 王义明. 微流控芯片-激光诱导荧光检测器的研制及核酸片段分离检测中应用. 分析化学, 2005, 33(9): 1350-1353. | |
| [43] |
Chiappetta C, Anile M, Leopizzi M, Venuta F, Della Rocca C. Use of a new generation of capillary electrophoresis to quantify circulating free DNA in non-small cell lung cancer. Clin Chim Acta, 2013, 425: 93-96.
doi: 10.1016/j.cca.2013.07.014 pmid: 23892142 |
| [44] | Wang HX, Cui ZG, Xiong LF, Zhang LJ, Kan B. Study on multiple locus VNTRs analysis of Salmonella Typhi by nucleic acid separation technology based on microfluidics. Dis Surveill, 2009, 24(3): 209-212. |
| 王洪霞, 崔志刚, 熊礼凤, 章丽娟, 阚飙. 基于微流控的核酸片段分离技术用于伤寒沙门菌MLVA分型的研究. 疾病监测, 2009, 24(3): 209-212. | |
| [45] |
Hussing C, Kampmann ML, Mogensen HS, Børsting C, Morling N. Comparison of techniques for quantification of next-generation sequencing libraries. Forensic Sci Int: Genet Suppl Ser, 2015, 5: e276-e278.
doi: 10.1016/j.fsigss.2015.09.110 |
| [1] | SUN De-Ming, WANG Tian-Qi, ZHU Xiao-Hong, YIN Kun-Lun, YUE Bing-Fei, CUI Zong-Bin, SUN Rong-Ze, ZHANG Bo. Hereditary quality standards for laboratory fish [J]. HEREDITAS, 2012, 34(9): 1202-1207. |
| [2] | TIAN Jing, CHEN Na, DIAO Zhi-Hu, CHEN Hui-Feng. The quality control of 4C-clone screening assay [J]. HEREDITAS, 2011, 33(4): 404-410. |
| [3] | CAO Zong-Fu, CAO Yan-Rong, MA Li-Guang, PENG Zuo-Qi, HU Xu-Huai, WANG Yuan-Yuan, XU Jiu-Jin, MA Xu. Standardization for sharing and utilization of Chinese genetic re-sources [J]. HEREDITAS, 2008, 30(1): 51-58. |
| [4] | LI Zhi-Feng, LI Yu-Jian, ZHAO Dong-Sheng, HANG Xing-Yi, WANG Zheng-Zhi, LUO Zhi-Gang, ZHANG Cheng-Gang. Construction of Standard Human Transcript Dataset Based on RefSeq and Human Genome Sequence Database [J]. HEREDITAS, 2006, 28(3): 329-333. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||