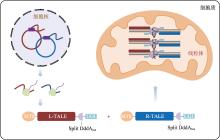
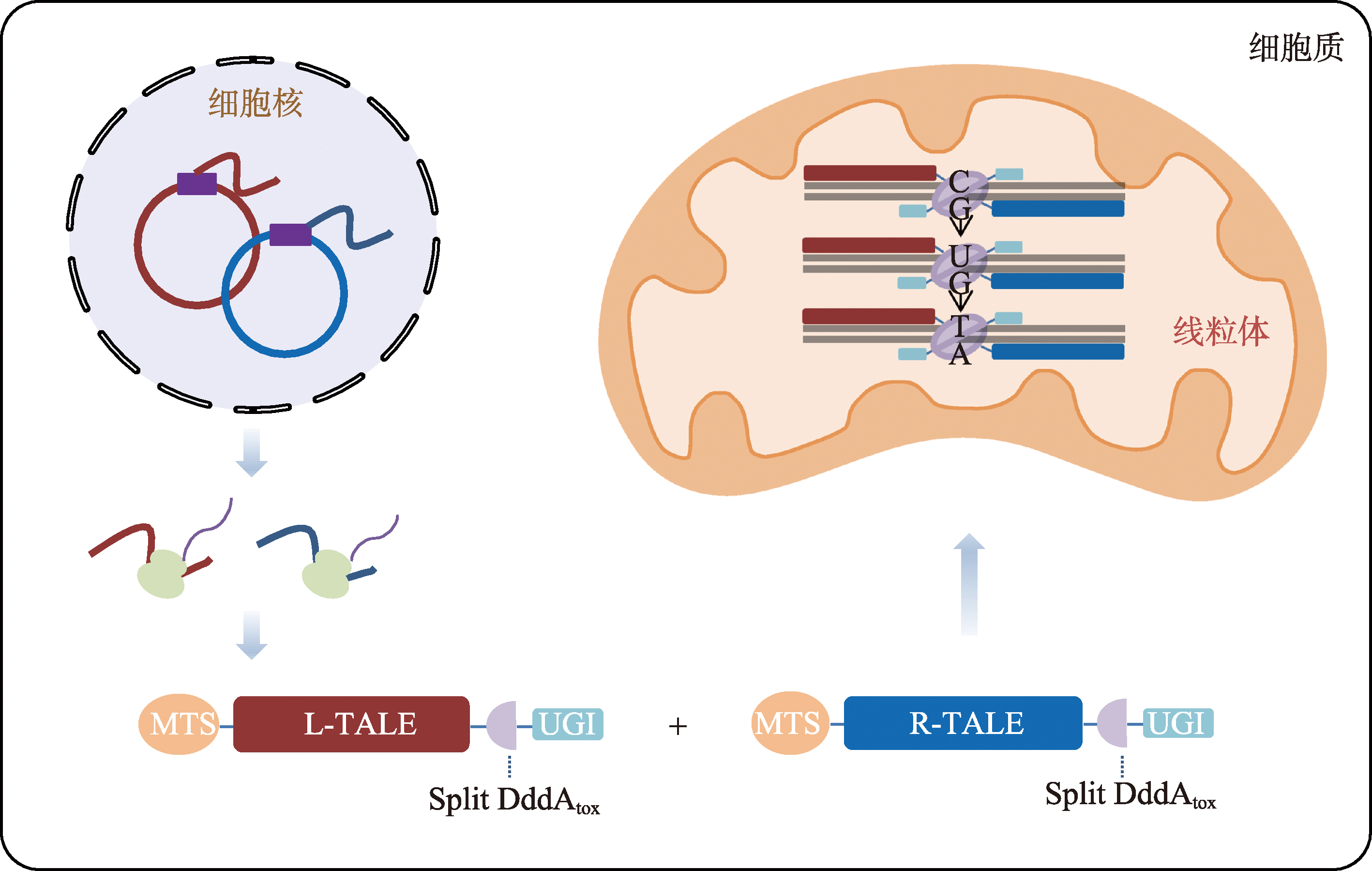
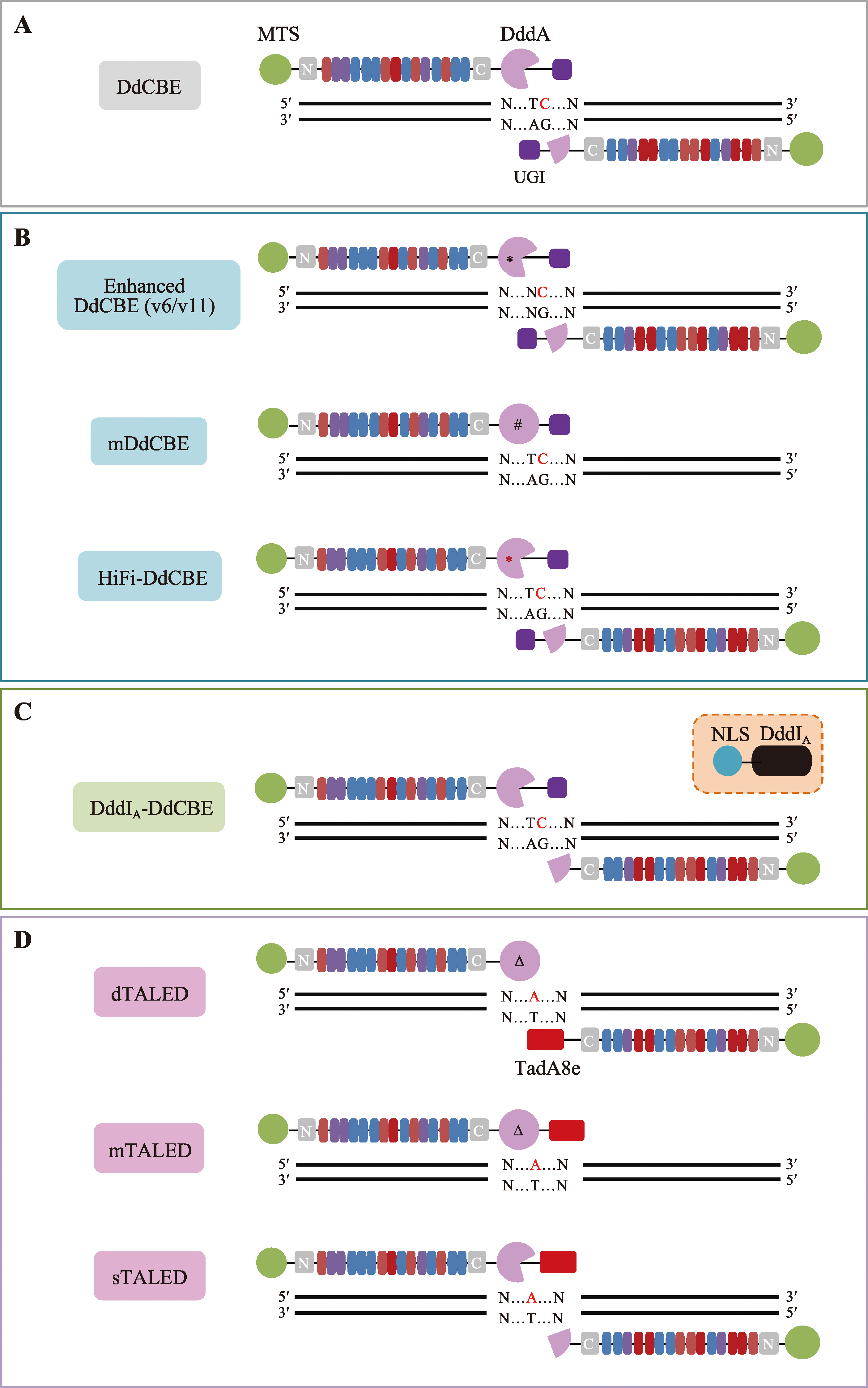
Hereditas(Beijing) ›› 2023, Vol. 45 ›› Issue (8): 632-642.doi: 10.16288/j.yczz.23-045
• Review • Previous Articles Next Articles
Advances in mitochondrial DNA base editing technology
Ruijia Song( ), Lu Han(
), Lu Han( ), Haifeng Sun, Bin Shen(
), Haifeng Sun, Bin Shen( )
)
- State Key Laboratory of Reproductive Medicine and Offspring Health, Nanjing Medical University, Nanjing 211166, China
-
Received:2023-03-01Revised:2023-06-21Online:2023-08-20Published:2023-07-04 -
Contact:Bin Shen E-mail:songruijia@stu.njmu.edu.cn;hanlu@stu.njmu.edu.cn;binshen@njmu.edu.cn. -
Supported by:National Key R&D Program of China(2021YFC2700600);National Natural Science Foundation of China(31970796)
Cite this article
Ruijia Song, Lu Han, Haifeng Sun, Bin Shen. Advances in mitochondrial DNA base editing technology[J]. Hereditas(Beijing), 2023, 45(8): 632-642.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
| [1] | Jia ZW. Mitochondria and pluripotent stem cells function. Hereditas(Beijing), 2016, 38(7): 603-611. |
| 贾振伟. 线粒体与多潜能干细胞功能. 遗传, 2016, 38(7): 603-611. | |
| [2] |
Tuppen HAL, Blakely EL, Turnbull DM, Taylor RW. Mitochondrial DNA mutations and human disease. Biochim Biophys Acta, 2010, 1797(2): 113-128.
doi: 10.1016/j.bbabio.2009.09.005 pmid: 19761752 |
| [3] |
Neupert W. SnapShot: mitochondrial architecture. Cell, 2012, 149(3): 722-722.e1.
doi: 10.1016/j.cell.2012.04.010 pmid: 22541440 |
| [4] | Schatz G. Mitochondrial DNA. In:Encyclopedia of biological chemistry. Elsevier, 2013, 132-134. |
| [5] | Gray MW. Mitochondrial DNA. In:Brenner’s encyclopedia of genetics. Elsevier, 2013, 436-438. |
| [6] |
Anderson S, Bankier AT, Barrell BG, de Bruijn MH, Coulson AR, Drouin J, Eperon IC, Nierlich DP, Roe BA, Sanger F, Schreier PH, Smith AJ, Staden R, Young IG. Sequence and organization of the human mitochondrial genome. Nature, 1981, 290(5806): 457-465.
doi: 10.1038/290457a0 |
| [7] |
Ziada AS, Smith MSR, Côté HCF. Updating the free radical theory of aging. Front Cell Dev Biol, 2020, 8: 575645.
doi: 10.3389/fcell.2020.575645 |
| [8] |
Taylor RW, Turnbull DM. Mitochondrial DNA mutations in human disease. Nat Rev Genet, 2005, 6(5): 389-402.
doi: 10.1038/nrg1606 pmid: 15861210 |
| [9] |
Biesalski HK. Free radical theory of aging. Curr Opin Clin Nutr Metab Care, 2002, 5(1): 5-10.
doi: 10.1097/00075197-200201000-00002 |
| [10] |
Filograna R, Mennuni M, Alsina D, Larsson NG. Mitochondrial DNA copy number in human disease: the more the better? FEBS Lett, 2021, 595(8): 976-1002.
doi: 10.1002/1873-3468.14021 pmid: 33314045 |
| [11] |
Silva-Pinheiro P, Minczuk M. The potential of mitochondrial genome engineering. Nat Rev Genet, 2022, 23(4): 199-214.
doi: 10.1038/s41576-021-00432-x |
| [12] |
Gammage PA, Moraes CT, Minczuk M. Mitochondrial genome engineering: the revolution may not be CRISPR-Ized. Trends Genet, 2018, 34(2): 101-110.
doi: S0168-9525(17)30191-9 pmid: 29179920 |
| [13] | Pinheiro P, Gammage PA, Minczuk M. Mitochondrially targeted zinc finger nucleases. In: The human mitochondrial genome. Elsevier, 2020, 499-514. |
| [14] |
Gammage PA, Rorbach J, Vincent AI, Rebar EJ, Minczuk M. Mitochondrially targeted ZFNs for selective degradation of pathogenic mitochondrial genomes bearing large- scale deletions or point mutations. EMBO Mol Med, 2014, 6(4): 458-466.
doi: 10.1002/emmm.201303672 pmid: 24567072 |
| [15] |
Gammage PA, Viscomi C, Simard ML, Costa ASH, Gaude E, Powell CA, Van Haute L, McCann BJ, Rebelo-Guiomar P, Cerutti R, Zhang L, Rebar EJ, Zeviani M, Frezza C, Stewart JB, Minczuk M. Genome editing in mitochondria corrects a pathogenic mtDNA mutation in vivo. Nat Med, 2018, 24(11): 1691-1695.
doi: 10.1038/s41591-018-0165-9 pmid: 30250142 |
| [16] |
Gammage PA, Gaude E, Van Haute L, Rebelo-Guiomar P, Jackson CB, Rorbach J, Pekalski ML, Robinson AJ, Charpentier M, Concordet JP, Frezza C, Minczuk M. Near-complete elimination of mutant mtDNA by iterative or dynamic dose-controlled treatment with mtZFNs. Nucleic Acids Res, 2016, 44(16): 7804-7816.
doi: 10.1093/nar/gkw676 pmid: 27466392 |
| [17] | McCann BJ, Cox A, Gammage PA, Stewart JB, Zernicka-Goetz M, Minczuk M. Delivery of mtZFNs into early mouse embryos. In: Liu J (ed). Zinc Finger Proteins, vol. 1867. New York, NY: Springer New York, 2018, 215-228. |
| [18] | Minczuk M. Engineered zinc finger proteins for manipulation of the human mitochondrial genome. In: Mackay JP, Segal DJ eds. Engineered Zinc Finger Proteins vol. 649. Totowa, NJ: Humana Press, 2010, 257-270. |
| [19] | Gammage PA, Van Haute L, Minczuk M. Engineered mtZFNs for manipulation of human mitochondrial DNA heteroplasmy. In: McKenzie M ed. MitochondrialDNA, vol. 1351. New York, NY: Springer New York, 2016, 145-162. |
| [20] |
Reddy P, Ocampo A, Suzuki K, Luo JP, Bacman SR, Williams SL, Sugawara A, Okamura D, Tsunekawa Y, Wu J, Lam D, Xiong X, Montserrat N, Esteban CR, Liu GH, Sancho-Martinez I, Manau D, Civico S, Cardellach F, del Mar O’Callaghan M, Campistol J, Zhao HM, Campistol JM, Moraes CT, Izpisua Belmonte JC. Selective elimination of mitochondrial mutations in the germline by genome editing. Cell, 2015, 161(3): 459-469.
doi: S0092-8674(15)00371-2 pmid: 25910206 |
| [21] |
Hashimoto M, Bacman SR, Peralta S, Falk MJ, Chomyn A, Chan DC, Williams SL, Moraes CT. MitoTALEN: A general approach to reduce mutant mtDNA loads and restore oxidative phosphorylation function in mitochondrial diseases. Mol Ther, 2015, 23(10): 1592-1599.
doi: 10.1038/mt.2015.126 pmid: 26159306 |
| [22] | Bacman SR, Gammage PA, Minczuk M, Moraes CT. Manipulation of mitochondrial genes and mtDNA heteroplasmy. In: Methods in cell biology vol. 155. Elsevier, 2020, 441-487. |
| [23] |
Bacman SR, Kauppila JHK, Pereira CV, Nissanka N, Miranda M, Pinto M, Williams SL, Larsson NG, Stewart JB, Moraes CT. MitoTALEN reduces mutant mtDNA load and restores TRNAAla levels in a mouse model of heteroplasmic mtDNA mutation. Nat Med, 2018, 24(11): 1696-1700.
doi: 10.1038/s41591-018-0166-8 |
| [24] |
Bacman SR, Williams SL, Pinto M, Peralta S, Moraes CT. Specific elimination of mutant mitochondrial genomes in patient-derived cells by mitoTALENs. Nat Med, 2013, 19(9): 1111-1113.
doi: 10.1038/nm.3261 pmid: 23913125 |
| [25] |
Mikhailov N, Hämäläinen RH. Modulating mitochondrial DNA heteroplasmy with mitochondrially targeted endonucleases. Ann Biomed Eng, 2022, doi: 10.1007/s10439-022-03051-7.
doi: 10.1007/s10439-022-03051-7 |
| [26] |
Rees HA, Liu DR. Base editing: precision chemistry on the genome and transcriptome of living cells. Nat Rev Genet, 2018, 19(12): 770-788.
doi: 10.1038/s41576-018-0059-1 |
| [27] |
Komor AC, Kim YB, Packer MS, Zuris JA, Liu DR. Programmable editing of a target base in genomic DNA without double-stranded DNA cleavage. Nature, 2016, 533(7603): 420-424.
doi: 10.1038/nature17946 |
| [28] |
Gaudelli NM, Komor AC, Rees HA, Packer MS, Badran AH, Bryson DI, Liu DR. Programmable base editing of A•T to G•C in genomic DNA without DNA cleavage. Nature, 2017, 551(7681): 464-471.
doi: 10.1038/nature24644 |
| [29] |
Nishida K, Arazoe T, Yachie N, Banno S, Kakimoto M, Tabata M, Mochizuki M, Miyabe A, Araki M, Hara KY, Shimatani Z, Kondo A. Targeted nucleotide editing using hybrid prokaryotic and vertebrate adaptive immune systems. Science, 2016, 353(6305): aaf8729.
doi: 10.1126/science.aaf8729 |
| [30] |
Mok BY, de Moraes MH, Zeng J, Bosch DE, Kotrys AV, Raguram A, Hsu F, Radey MC, Peterson SB, Mootha VK, Mougous JD, Liu DR. A bacterial cytidine deaminase toxin enables CRISPR-free mitochondrial base editing. Nature, 2020, 583(7817): 631-637.
doi: 10.1038/s41586-020-2477-4 |
| [31] | Krokan HE, Bjørås M. Base excision repair. Cold Spring Harb Perspect Biol, 2013, 5(4): a012583. |
| [32] |
Aushev M, Herbert M. Mitochondrial Genome Editing Gets Precise. Nature, 2020, 583(7817): 521-522.
doi: 10.1038/d41586-020-01974-6 |
| [33] |
Barrera-Paez JD, Moraes CT. Mitochondrial genome engineering coming-of-age. Trends Genet, 2022, 38(8): 869-880.
doi: 10.1016/j.tig.2022.04.011 pmid: 35599021 |
| [34] | Bi R, Li Y, Xu M, Zheng QZ, Zhang DF, Li X, Ma GL, Xiang BL, Zhu XJ, Zhao H, Huang XX, Zheng P, Yao YG. Direct evidence of CRISPR-Cas9-mediated mitochondrial genome editing. Innovation (Camb), 2022, 3(6): 100329. |
| [35] |
Riepsaame J. Editing the mitochondrial genome: no CRISPR required. Trends Genet, 2020, 36(11): 809-810.
doi: 10.1016/j.tig.2020.08.001 pmid: 32819722 |
| [36] |
Lee H, Lee S, Baek G, Kim A, Kang BC, Seo H, Kim JS. Mitochondrial DNA editing in mice with DddA-TALE fusion deaminases. Nat Commun, 2021, 12(1): 1190.
doi: 10.1038/s41467-021-21464-1 pmid: 33608520 |
| [37] |
Guo JY, Chen XX, Liu ZW, Sun HF, Zhou Y, Dai YC, Ma YE, He L, Qian XZ, Wang JY, Zhang J, Zhu YC, Zhang J, Shen B, Zhou F. DdCBE mediates efficient and inheritable modifications in mouse mitochondrial genome. Mol Ther Nucleic Acids, 2021, 27: 73-80.
doi: 10.1016/j.omtn.2021.11.016 |
| [38] |
Lee S, Lee H, Baek G, Namgung E, Park JM, Kim S, Hong S, Kim JS. Enhanced mitochondrial DNA editing in mice using nuclear-exported TALE-linked deaminases and nucleases. Genome Biol, 2022, 23(1): 211.
doi: 10.1186/s13059-022-02782-z pmid: 36224582 |
| [39] |
Guo JY, Zhang X, Chen XX, Sun HF, Dai YC, Wang JY, Qian XZ, Tan L, Lou X, Shen B. Precision modeling of mitochondrial diseases in zebrafish via DdCBE-mediated mtDNA base editing. Cell Discov, 2021, 7(1): 78.
doi: 10.1038/s41421-021-00307-9 pmid: 34480028 |
| [40] |
Qi XL, Chen XX, Guo JY, Zhang X, Sun HF, Wang JY, Qian XZ, Li B, Tan L, Yu L, Chen W, Zhang LF, Ma YW, Shen B. Precision modeling of mitochondrial disease in rats via DdCBE-mediated mtDNA editing. Cell Discov, 2021, 7(1): 95.
doi: 10.1038/s41421-021-00325-7 pmid: 34663794 |
| [41] |
Chen YP, Lüttmann FF, Schoger E, Schöler HR, Zelarayán LC, Kim KP, Haigh JJ, Kim J, Braun T. Reversible reprogramming of cardiomyocytes to a fetal state drives heart regeneration in mice. Science, 2021, 373(6562): 1537-1540.
doi: 10.1126/science.abg5159 pmid: 34554778 |
| [42] |
Bergmann O, Zdunek S, Felker A, Salehpour M, Alkass K, Bernard S, Sjostrom SL, Szewczykowska M, Jackowska T, Dos Remedios C, Malm T, Andrä M, Jashari R, Nyengaard JR, Possnert G, Jovinge S, Druid H, Frisén J. Dynamics of cell generation and turnover in the human heart. Cell, 2015, 161(7): 1566-1575.
doi: 10.1016/j.cell.2015.05.026 pmid: 26073943 |
| [43] |
Silva-Pinheiro P, Nash PA, Van Haute L, Mutti CD, Turner K, Minczuk M. In vivo mitochondrial base editing via adeno-associated viral delivery to mouse post-mitotic tissue. Nat Commun, 2022, 13(1): 750.
doi: 10.1038/s41467-022-28358-w pmid: 35136065 |
| [44] |
Mok YG, Lee JM, Chung E, Lee J, Lim K, Cho SI, Kim JS. Base editing in human cells with monomeric DddA-TALE fusion deaminases. Nat Commun, 2022, 13(1): 4038.
doi: 10.1038/s41467-022-31745-y pmid: 35821233 |
| [45] |
Chen XX, Liang D, Guo JY, Zhang JQ, Sun HF, Zhang XL, Jin JC, Dai YC, Bao QM, Qian XZ, Tan L, Hu P, Ling XF, Shen B, Xu ZF. DdCBE-Mediated mitochondrial base editing in human 3PN embryos. Cell Discov, 2022, 8(1): 8.
doi: 10.1038/s41421-021-00358-y pmid: 35102135 |
| [46] |
St John JC, Facucho-Oliveira J, Jiang Y, Kelly R, Salah R. Mitochondrial DNA transmission, replication and inheritance: a journey from the gamete through the embryo and into offspring and embryonic stem cells. Hum Reprod Update, 2010, 16(5): 488-509.
doi: 10.1093/humupd/dmq002 pmid: 20231166 |
| [47] |
Wei YH, Xu CL, Feng H, Xu K, Li ZF, Hu J, Zhou L, Wei Y, Zuo ZR, Zuo EW, Li W, Yang H, Zhang ML. Human cleaving embryos enable efficient mitochondrial base- editing with DdCBE. Cell Discov, 2022, 8(1): 7.
doi: 10.1038/s41421-021-00372-0 |
| [48] |
Wrighton KH. Cytosine base editors go off-target. Nat Rev Genet, 2019, 20(5): 254-255.
doi: 10.1038/s41576-019-0110-x pmid: 30872767 |
| [49] |
Zuo EW, Sun YD, Wei W, Yuan TL, Ying WQ, Sun H, Yuan LY, Steinmetz LM, Li YX, Yang H. GOTI, a method to identify genome-wide off-target effects of genome editing in mouse embryos. Nat Protoc, 2020, 15(9): 3009-3029.
doi: 10.1038/s41596-020-0361-1 pmid: 32796939 |
| [50] |
Zuo EW, Sun YD, Wei W, Yuan TL, Ying WQ, Sun H, Yuan LY, Steinmetz LM, Li YX, Yang H. Cytosine base editor generates substantial off-target single-nucleotide variants in mouse embryos. Science, 2019, 364(6437): 289-292.
doi: 10.1126/science.aav9973 pmid: 30819928 |
| [51] |
Wei YH, Li ZF, Xu K, Feng H, Xie L, Li D, Zuo ZR, Zhang ML, Xu CL, Yang H, Zuo EW. Mitochondrial base editor DdCBE causes substantial DNA off-target editing in nuclear genome of embryos. Cell Discov, 2022, 8(1): 27.
doi: 10.1038/s41421-022-00391-5 pmid: 35304438 |
| [52] | Lei ZX, Meng HW, Lv ZC, Liu MH, Zhao HN, Wu H, Zhang XX, Liu LL, Zhuang Y, Yin KL, Yan YC, Yi CQ. Detect-seq reveals out-of-protospacer editing and target- strand editing by cytosine base editors. Nat Methods, 2021, 18(6): 643-651. |
| [53] |
Lei ZX, Meng HW, Liu LL, Zhao HN, Rao XC, Yan YC, Wu H, Liu M, He AB, Yi CQ. Mitochondrial base editor induces substantial nuclear off-target mutations. Nature, 2022, 606(7915): 804-811.
doi: 10.1038/s41586-022-04836-5 |
| [54] |
Rowley MJ, Corces VG. Organizational principles of 3D genome architecture. Nat Rev Genet, 2018, 19(12): 789-800.
doi: 10.1038/s41576-018-0060-8 pmid: 30367165 |
| [55] |
Hansen AS, Pustova I, Cattoglio C, Tjian R, Darzacq X. CTCF and cohesin regulate chromatin loop stability with distinct dynamics. eLife, 2017, 6: e25776.
doi: 10.7554/eLife.25776 |
| [56] |
Lee S, Lee H, Baek G, Kim JS. Precision mitochondrial DNA editing with high-fidelity DddA-derived base editors. Nat Biotechnol, 2023, 41(3): 378-386.
doi: 10.1038/s41587-022-01486-w |
| [57] |
Miller JC, Holmes MC, Wang JB, Guschin DY, Lee YL, Rupniewski I, Beausejour CM, Waite AJ, Wang NS, Kim KA, Gregory PD, Pabo CO, Rebar EJ. An improved zinc-finger nuclease architecture for highly specific genome editing. Nat Biotechnol, 2007, 25(7): 778-785.
doi: 10.1038/nbt1319 pmid: 17603475 |
| [58] |
Szczepek M, Brondani V, Büchel J, Serrano L, Segal DJ, Cathomen T. Structure-based redesign of the dimerization interface reduces the toxicity of zinc-finger nucleases. Nat Biotechnol, 2007, 25(7): 786-793.
doi: 10.1038/nbt1317 pmid: 17603476 |
| [59] |
Mok BY, Kotrys AV, Raguram A, Huang TP, Mootha VK, Liu DR. CRISPR-free base editors with enhanced activity and expanded targeting scope in mitochondrial and nuclear DNA. Nat Biotechnol, 2022, 40(9): 1378-1387.
doi: 10.1038/s41587-022-01256-8 pmid: 35379961 |
| [60] |
Thuronyi BW, Koblan LW, Levy JM, Yeh WH, Zheng C, Newby GA, Wilson C, Bhaumik M, Shubina-Oleinik O, Holt JR, Liu DR. Continuous evolution of base editors with expanded target compatibility and improved activity. Nat Biotechnol, 2019, 37(9): 1070-1079.
doi: 10.1038/s41587-019-0193-0 pmid: 31332326 |
| [61] |
Roth TB, Woolston BM, Stephanopoulos G, Liu DR.Phage-assisted evolution of bacillus methanolicus methanol dehydrogenase 2. Acs Synth Biol, 2019, 8(4): 796-806.
doi: 10.1021/acssynbio.8b00481 pmid: 30856338 |
| [62] |
Dickinson BC, Packer MS, Badran AH, Liu DR. A system for the continuous directed evolution of proteases rapidly reveals drug-resistance mutations. Nat Commun, 2014, 5(1): 5352.
doi: 10.1038/ncomms6352 |
| [63] |
Cho SI, Lee S, Mok YG, Lim K, Lee J, Lee JM, Chung E, Kim JS. Targeted A-to-G base editing in human mitochondrial DNA with programmable deaminases. Cell, 2022, 185(10): 1764-1776.e12.
doi: 10.1016/j.cell.2022.03.039 |
| [64] |
Richter MF, Zhao KT, Eton E, Lapinaite A, Newby GA, Thuronyi BW, Wilson C, Koblan LW, Zeng J, Bauer DE, Doudna JA, Liu DR. Phage-assisted evolution of an adenine base editor with improved Cas domain compatibility and activity. Nat Biotechnol, 2020, 38(7): 883-891.
doi: 10.1038/s41587-020-0453-z pmid: 32433547 |
| [65] | Morgan MA, Lange L, Schambach A. Prime time for base editing in the mitochondria. Signal Transduct Target Ther, 2022, 7(1): 213. |
| [66] |
Willis JCW, Silva-Pinheiro P, Widdup L, Minczuk M, Liu DR. Compact zinc finger base editors that edit mitochondrial or nuclear DNA in vitro and in vivo. Nat Commun, 2022, 13(1): 7204.
doi: 10.1038/s41467-022-34784-7 pmid: 36418298 |
| [67] |
Lim K, Cho SI, Kim JS. Nuclear and mitochondrial DNA editing in human cells with zinc finger deaminases. Nat Commun, 2022, 13(1): 366.
doi: 10.1038/s41467-022-27962-0 pmid: 35042880 |
| [68] |
Anzalone AV, Randolph PB, Davis JR, Sousa AA, Koblan LW, Levy JM, Chen PJ, Wilson C, Newby GA, Raguram A, Liu DR. Search-and-replace genome editing without double-strand breaks or donor DNA. Nature, 2019, 576(7785): 149-157.
doi: 10.1038/s41586-019-1711-4 |
| [69] |
Chen PJ, Liu DR. Prime editing for precise and highly versatile genome manipulation. Nat Rev Genet, 2023, 24(3): 161-177.
doi: 10.1038/s41576-022-00541-1 |
| [70] | Zong Y, Gao CX. Progress on base editing systems. Hereditas(Beijing), 2019, 41(9): 777-800. |
| 宗媛, 高彩霞. 碱基编辑系统研究进展. 遗传, 2019, 41(9): 777-800. |
| [1] | Fengyue Hu, Kejian Wang. The STEME system: a novel tool for directed evolution in vivo [J]. Hereditas(Beijing), 2020, 42(3): 231-235. |
| [2] | Yuan Zong, Caixia Gao. Progress on base editing systems [J]. Hereditas(Beijing), 2019, 41(9): 777-800. |
| [3] | Jiaoyang Tian, Yuchun Li, Qingpeng Kong, Yaping Zhang. The origin and evolution history of East Asian populations from genetic perspectives [J]. Hereditas(Beijing), 2018, 40(10): 814-824. |
| [4] | Yu Wei,Xiaohui Zhang,Dali Li. The “new favorite” of gene editing technology—single base editors [J]. Hereditas(Beijing), 2017, 39(12): 1115-1121. |
| [5] | Meifen Xu, Yiqun He, Junwei Geng, Yanzi Meng, Han Yu, Zhi Lin, Suxue Shi, Ling Xue, Zhongqiu Lu, Minxin Guan. The mitochondrial tRNAMet/tRNAGlnA4401G and tRNACysG5821A mutations may be associated with hypertension in two Han Chinese families [J]. HEREDITAS, 2014, 36(2): 127-134. |
| [6] | Li Liu, Yuquan Shao, Baorong Zhang, Pingping Jiang, Ailian Du, Minxin Guan. Mitochondrial genome analysis in the probands of six Chinese families with MELAS [J]. HEREDITAS(Beijing), 2014, 36(11): 1159-1167. |
| [7] | Haysa Ayelhan, Yan Guo, Wei Meng, Tianyan Yang, Yanwu Ma. Phylogeny and divergence time estimation of Schizothoracinae fishes in Xinjiang [J]. HEREDITAS(Beijing), 2014, 36(10): 1013-1020. |
| [8] | MA Zhi-Jie ZHONG Jin-Cheng HAN Jian-Lin XU Jing-Tao LIU Zhong-Na BAI Wen-Lin. Research progress on molecular genetic diversity of the yak (Bos grunniens) [J]. HEREDITAS, 2013, 35(2): 151-160. |
| [9] | WANG Jin-Feng, ZHANG Ya-Ping, YU Li. Summary of phylogeny in family Felidae of Carnivora [J]. HEREDITAS, 2012, 34(11): 1365-1378. |
| [10] | SONG Yan-Rui, LIU Zhong, GU Chu-Lian, JIAN Li-Juan, YAN Qiang-Feng. Advances in the molecular pathogenesis of hypertrophic cardiomyopathy [J]. HEREDITAS, 2011, 33(6): 549-557. |
| [11] | JI Yan-Chun, LIU Xiao-Ling, ZHAO Fu-Xin, ZHANG Juan-Juan, ZHANG Yu, ZHOU Xiang-Tian, QU Jia, GUAN Min-Xin. The mitochondrial ND5 T12338C mutation may be associated with Leber’s hereditary optic neuropathy in two Chinese families [J]. HEREDITAS, 2011, 33(4): 322-328. |
| [12] | WEI Jin-Pu, BO Hua-Feng, LI Gong-Quan, DUAN Fei. Distribution and evolution of simple repeats in the mtDNA D-loop in mammalian [J]. HEREDITAS, 2011, 33(1): 67-74. |
| [13] | KE Yang, HUANG Yuan, LEI Fu-Min. Sequencing and analysis of the complete mitochondrial genome of Podoces hendersoni (Ave, Corvidae) [J]. HEREDITAS, 2010, 32(9): 951-960. |
| [14] | ZHANG Yong-Mei, JI Yan-Chun, LIU Xiao-Ling, ZHOU Xiang-Tian, DIAO Fu-Xin, SUN Yan-Gong, HUI Qi-Beng, ZHANG Juan-Juan, LIU Yan, JI Jia, GUAN Min-Xin. Leber’s hereditary optic neuropathy may be associated with the mi-tochondrial tRNA A14693G mutation in three Chinese families [J]. HEREDITAS, 2010, 32(4): 353-359. |
| [15] | LIU Yan, PENG Chu-Liu, TONG Shi, JI Jia, ZHOU Xiang-Tian, DIAO Fu-Xin, ZHANG Juan-Juan, ZHANG Yong-Mei, ZHANG Shu. Leber's hereditary optic neuropathy and limbs abnormity claudication may be associated with the mitochondrial ND1 T3866C mutation [J]. HEREDITAS, 2010, 32(2): 141-147. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||