Hereditas(Beijing) ›› 2025, Vol. 47 ›› Issue (12): 1300-1325.doi: 10.16288/j.yczz.25-032
• Review • Previous Articles Next Articles
Current understanding of mitochondrial DNA genetic diseases and gene therapy
Cheng Tang1,2( ), Shunqing Xu1, Hanzeng Li1(
), Shunqing Xu1, Hanzeng Li1( )
)
1. School of Environmental Science and Engineering ,Hainan University Haikou 570228, China 2. School of Life and Health Sciences ,Hainan University Haikou 570228, China
-
Received:2025-02-07Revised:2025-05-23Online:2025-05-27Published:2025-05-27 -
Contact:Hanzeng Li E-mail:23220860010019@hainanu.edu.cn;hanzeng.li@hainanu.edu.cn -
Supported by:Hainan Provincial Natural Science Foundation(823QN230);National Natural Science Foundation of China(32400724);National Natural Science Foundation of China(22466015)
Cite this article
Cheng Tang, Shunqing Xu, Hanzeng Li. Current understanding of mitochondrial DNA genetic diseases and gene therapy[J]. Hereditas(Beijing), 2025, 47(12): 1300-1325.
share this article
Table 1
Statistics of some typical mitochondrial diseases"
| 分类 | 中文名称 | 英文名称 | 部分常见突变基因及位点 | 参考文献 |
|---|---|---|---|---|
| 异质点突变 相关疾病 | 线粒体脑肌病伴乳酸酸中毒和中风样发作 | Mitochondrial encephalomyopathy with lactic acidosis and stroke-like episodes (MELAS) | MT-TL1基因m.3243A>G等 | [ |
| 利氏综合征 | Leigh syndrome (LS) | MT-ATP6基因m.8993T>C以及MT-ND3基因m.10191T>C等 | [ | |
| 共济失调和视网膜色素变性 | Neuropathy, ataxia, and retinitis pigmentosa (NARP) | MT-ATP6基因m.8993T>G/C等 | [ | |
| 肌阵挛性癫痫伴红色粗纤维 | Myoclonic epilepsy with ragged red fibers (MERRF) | MT-TK基因m.8344A>G等 | [ | |
| 同质点突变 相关疾病 | Leber遗传性视神经病变 | Leber hereditary optic neuropathy (LHON) | MT-ND1基因m.3460G>A、MT-ND4基因m.11778G>A、MT-ND6基因m.14484T>C等 | [ |
Table 2
Statistics of existing mitochondrial editing methods"
| 分类 | 中文名称 | 英文名称 | 编辑位点 | 治疗策略 | 参考文献 |
|---|---|---|---|---|---|
| 线粒体靶向核酸酶对线粒体进行编辑 | 线粒体靶向定位的锌指核酸酶 | Mitochondrial zinc-finger nucleases (mtZFNs) | 无 | 降低异质性 | [ |
| 线粒体靶向转录激活样效应因子核酸酶 | Mitochondria-targeted transcription activator- like effector nucleases (mitoTALENs) | 无 | [ | ||
| 线粒体靶向定位的巨核核酸酶 | Mitochondrial-targeted ARCUS (mitoARCUS) | 无 | [ | ||
| 碱基编辑器对线粒体进行编辑 | 线粒体靶向的DddA衍生胞嘧啶碱基编辑器 | Mitochondrial-targeted DddA-derived cytosine base editor (mitoDdCBE) | C>T | 纠正突变位点 | [ |
| TALE连接的腺嘌呤脱氨酶 | TALE-linked deoxyadenosine deaminase (TALED) | A>G | [ | ||
| 线粒体DNA碱基编辑器 | Mitochondrial base editors (mitoBEs) | A>G或C>T | [ |
Table 3
Statistics on the spplication of existing mitochondrial editing methods"
| 名称 | 编辑序列偏好性 | 部分体内外应用研究 | 编辑 位点 | 编辑效率 | 脱靶概率 | 参考文献 |
|---|---|---|---|---|---|---|
| mtZFNs | / | 人骨肉瘤143B细胞 | / | 四个周期后m.8993T>G异质性从80%降到7% | 较为显著 | [ |
| 小鼠胚胎成纤维细胞(MEF)、小鼠 | 在MEF细胞中,异质性比例从65%下降到了45%;小鼠心脏细胞内的突变mtDNA比例从73%下降到37% | 未说明 | [ | |||
| mitoTALENs | / | 小鼠胚胎成纤维细胞(MEF)、小鼠 | / | 在MEF细胞中,异质性降低的比例大概在20%~60%;在小鼠中,各个部位的异质性比例也有所降低 | 未检测到mtDNA大片段缺失 | [ |
| 水稻、油菜 | 无定量数据/(主要用于开发新品种作物) | 未说明 | [ | |||
| mitoARCUS | / | 小鼠胚胎成纤维细胞(MEF)、小鼠 | / | 在MEF细胞中m.5024C>T突变降低从50%降低至约25%~30%;在幼鼠和成年鼠中突变mtDNA均能降低约90% | 未说明 | [ |
| 人骨肉瘤143B细胞、异种移植小鼠模型 | 在人骨肉瘤143B细胞中3天内突变mtDNA从95%到0.3%;在种移植小鼠模型中,最高剂量组肿瘤组织中野生型mtDNA比例从6.5%提升至13.9% | 未说明 | [ | |||
| mitoDdCBE | 第一版有严格的5′-TC序列依赖性,优化过的第二版除5′-TC环境外,可进一步编辑5′-AC和5′-CC。 | HEK293T | / | 在MT-ND6基因位点编辑效率在16%~27%;MT-ND1、MT-ND2、MT-ND4、MT-ND5和MT-ATP8基因位点编辑效率在4.6%~49% | 未检测到显著性突变 | [ |
| HEK293T | DddA6的碱基编辑器将TC编辑效率平均提高了3.3倍;DddA11变体将AC和CC位点的平均编辑效率从<10%提升至15%~30% | 未检测到显著性突变 | [ | |||
| 人类3PN胚胎 | 60% (8细胞阶段)、25% (3PN胚胎) | 未说明 | [ | |||
| 双细胞胚胎 | 46%~72% | 出现大量核基因脱靶突变 | [ | |||
| HEK293T | 未给出明确数据 | 0.46%~17.51% (核基因脱靶) | [ | |||
| TALED | / | HEK293T | A>G | sTALEDs平均A>G编辑频率为27%±3%,平均C>T编辑频率为32%±4%;mTALEDs和dTALEDs的平均编辑频率为19%±4% | sTALEDs平均脱靶频率为0.019%± 0.002%;mTALEDs和dTALEDs分别为0.009%±0.001%和0.008%±0.001% | [ |
| 植物叶绿体 | 46% (rrn16);25% (psbA);51% (psaA) | 未检测到显著性突变 | [ | |||
| mitoBEs | / | 小鼠 | A>G或G>C | 在F0小鼠MT-ATP6基因的T8591C和MT-ND5基因的A12784G,mitoABEv2的平均编辑效率分别是46%和44%,最大效率分别为68%和82%;mitoABE的平均效率为30%和38%,最大效率为50%和55% | 未检测到显著性突变 | [ |
| [1] |
Paradies G, Paradies V, Ruggiero FM, Petrosillo G. Role of cardiolipin in mitochondrial function and dynamics in health and disease: molecular and pharmacological aspects. Cells, 2019, 8(7): 728.
pmid: 31315173 |
| [2] |
Kuznetsov AV, Margreiter R. Heterogeneity of mitochondria and mitochondrial function within cells as another level of mitochondrial complexity. Int J Mol Sci, 2009, 10(4): 1911-1929.
pmid: 19468346 |
| [3] |
Godoy JA, Rios JA, Picón-Pagès P, Herrera-Fernández V, Swaby B, Crepin G, Vicente R, Fernández-Fernández JM, Muñoz FJ. Mitostasis, calcium and free radicals in health, aging and neurodegeneration. Biomolecules, 2021, 11(7): 1012.
pmid: 34356637 |
| [4] |
Buschlen S, Amillet JM, Guiard B, Fournier A, Marcireau C, Bolotin-Fukuhara M. The S. Cerevisiae HAP complex, a key regulator of mitochondrial function, coordinates nuclear and mitochondrial gene expression. Comp Func Genomics, 2003, 4(1): 37-46.
pmid: 18629096 |
| [5] |
Calvo S, Jain M, Xie XH, Sheth SA, Chang B, Goldberger OA, Spinazzola A, Zeviani M, Carr SA, Mootha VK. Systematic identification of human mitochondrial disease genes through integrative genomics. Nat Genet, 2006, 38(5): 576-582.
pmid: 16582907 |
| [6] |
Ryan MT, Hoogenraad NJ. Mitochondrial-nuclear communications. Annu Rev Biochem, 2007, 76: 701-722.
pmid: 17227225 |
| [7] |
Bar-Yaacov D, Blumberg A, Mishmar D. Mitochondrial- nuclear co-evolution and its effects on OXPHOS activity and regulation. Biochim Biophys Acta, 2012, 1819(9-10): 1107-1111.
pmid: 22044624 |
| [8] |
Hill GE. Mitonuclear ecology. Mol Biol Evol, 2015, 32(8): 1917-1927.
pmid: 25931514 |
| [9] |
Zhou Q, Xu H, Yan L, Ye L, Zhang XY, Tan B, Yi Q, Tian J, Zhu J. PGC-1α promotes mitochondrial respiration and biogenesis during the differentiation of hiPSCs into cardiomyocytes. Genes Dis, 2021, 8(6): 891-906.
pmid: 34522716 |
| [10] |
Ryu KW, Fung TS, Baker DC, Saoi M, Park J, Febres-Aldana CA, Aly RG, Cui RB, Sharma A, Fu Y, Jones OL, Cai X, Pasolli HA, Cross JR, Rudin CM, Thompson CB. Cellular ATP demand creates metabolically distinct subpopulations of mitochondria. Nature, 2024, 635(8039):746-754.
pmid: 39506109 |
| [11] |
Taylor RW, Turnbull DM. Mitochondrial DNA mutations in human disease. Nat Rev Genet, 2005, 6(5): 389-402.
pmid: 15861210 |
| [12] |
Mattson MP, Gleichmann M, Cheng AW. Mitochondria in neuroplasticity and neurological disorders. Neuron, 2008, 60(5): 748-766.
pmid: 19081372 |
| [13] |
Sekar D, Johnson J, Biruntha M, Lakhmanan G, Gurunathan D, Ross K. Biological and clinical relevance of microRNAs in mitochondrial diseases/dysfunctions. DNA Cell Biol, 2020, 39(8): 1379-1384.
pmid: 31855060 |
| [14] |
Ding XW, Robinson M, Li RZ, Aldhowayan H, Geetha T, Babu JR. Mitochondrial dysfunction and beneficial effects of mitochondria-targeted small peptide SS-31 in diabetes mellitus and alzheimer’s disease. Pharmacol Res, 2021, 171: 105783.
pmid: 34302976 |
| [15] |
Ke D, Zhang Z, Liu JT, Chen PJ, Li JL, Sun XH, Chu YH, Li LX. Ferroptosis, necroptosis and cuproptosis: Novel forms of regulated cell death in diabetic cardiomyopathy. Front Cardiovasc Med, 2023, 10: 1135723.
pmid: 36970345 |
| [16] |
Oltra SS, Colomo S, Sin L, Pérez-López M, Lázaro S, Molina-Crespo A, Choi KH, Ros-Pardo D, Martínez L, Morales S, González-Paramos C, Orantes A, Soriano M, Hernández A, Lluch A, Rojo F, Albanell J, Gómez- Puertas P, Ko JK, Sarrió D, Moreno-Bueno G. Distinct GSDMB protein isoforms and protease cleavage processes differentially control pyroptotic cell death and mitochondrial damage in cancer cells. Cell Death Differ, 2023, 30(5): 1366-1381.
pmid: 36899106 |
| [17] |
Rahman J, Rahman S. Mitochondrial medicine in the omics era. Lancet, 2018, 391(10139): 2560-2574.
pmid: 29903433 |
| [18] | Sobkiv S, Kambalyal A, Perkins G, Lysakowski A. Functional and morphological characteristics of efferent bouton mitochondria of the inner ear. The FASEB Journal, 2017, 31(1): 740.13-740.13. |
| [19] |
Rensvold JW, Shishkova E, Sverchkov Y, Miller IJ, Cetinkaya A, Pyle A, Manicki M, Brademan DR, Alanay Y, Raiman J, Jochem A, Hutchins PD, Peters SR, Linke V, Overmyer KA, Salome AZ, Hebert AS, Vincent CE, Kwiecien NW, Rush MJP, Westphall MS, Craven M, Akarsu NA, Taylor RW, Coon JJ, Pagliarini DJ. Defining mitochondrial protein functions through deep multiomic profiling. Nature, 2022, 606(7913): 382-388.
pmid: 35614220 |
| [20] |
Calvo SE, Mootha VK. The mitochondrial proteome and human disease. Annu Rev Genomics Hum Genet, 2010, 11: 25-44.
pmid: 20690818 |
| [21] |
Long Q, Zhou YS, Wu H, Du SW, Hu ML, Qi JT, Li W, Guo JY, Wu Y, Yang L, Xiang G, Wang L, Ye SH, Wen JY, Mao H, Wang JW, Zhao H, Chan WY, Liu JS, Chen YL, Li PL, Liu XG. Phase separation drives the self-assembly of mitochondrial nucleoids for transcriptional modulation. Nat Struct Mol Biol, 2021, 28(11): 900-908.
pmid: 34711968 |
| [22] |
Xia MF, Zhang YZ, Jin K, Lu ZT, Zeng ZY, Xiong W. Communication between mitochondria and other organelles: a brand-new perspective on mitochondria in cancer. Cell Biosci, 2019, 9: 27.
pmid: 30931098 |
| [23] |
Victorelli S, Salmonowicz H, Chapman J, Martini H, Vizioli MG, Riley JS, Cloix C, Hall-Younger E, Machado Espindola-Netto J, Jurk D, Lagnado AB, Sales Gomez L, Farr JN, Saul D, Reed R, Kelly G, Eppard M, Greaves LC, Dou ZX, Pirius N, Szczepanowska K, Porritt RA, Huang HJ, Huang TY, Mann DA, Masuda CA, Khosla S, Dai HM, Kaufmann SH, Zacharioudakis E, Gavathiotis E, LeBrasseur NK, Lei X, Sainz AG, Korolchuk VI, Adams PD, Shadel GS, Tait SWG, Passos JF. Apoptotic stress causes mtDNA release during senescence and drives the SASP. Nature, 2023, 622(7983): 627-636.
pmid: 37821702 |
| [24] |
Moschandrea C, Kondylis V, Evangelakos I, Herholz M, Schneider F, Schmidt C, Yang M, Ehret S, Heine M, Jaeckstein MY, Szczepanowska K, Schwarzer R, Baumann L, Bock T, Nikitopoulou E, Brodesser S, Krüger M, Frezza C, Heeren J, Trifunovic A, Pasparakis M. Mitochondrial dysfunction abrogates dietary lipid processing in enterocytes. Nature, 2024, 625(7994): 385-392.
pmid: 38123683 |
| [25] |
Petersen MC, Vatner DF, Shulman GI. Regulation of hepatic glucose metabolism in health and disease. Nat Rev Endocrinol, 2017, 13(10): 572-587.
pmid: 28731034 |
| [26] |
Manford AG, Rodríguez-Pérez F, Shih KY, Shi Z, Berdan CA, Choe M, Titov DV, Nomura DK, Rape M. A cellular mechanism to detect and alleviate reductive stress. Cell, 2020, 183(1): 46-61.e21.
pmid: 32941802 |
| [27] |
Magi S, Piccirillo S, Preziuso A, Amoroso S, Lariccia V. Mitochondrial localization of NCXs: balancing calcium and energy homeostasis. Cell Calcium, 2020, 86: 102162.
pmid: 31981913 |
| [28] |
Ma J, Hao ZH, Zhang YD, Li LJ, Huang XS, Wang Y, Chen LY, Yang G, Li W. Physical contacts between mitochondria and WPBs participate in WPB maturation. Arteriosclerosis Thromb Vasc Biol, 2024, 44(1): 108-123.
pmid: 37942609 |
| [29] |
Clay Montier LL, Deng JJ, Bai Y. Number matters: control of mammalian mitochondrial DNA copy number. J Genet Genomics, 2009, 36(3): 125-31.
pmid: 19302968 |
| [30] |
Fontana GA, Gahlon HL. Mechanisms of replication and repair in mitochondrial DNA deletion formation. Nucleic Acids Res, 2020, 48(20): 11244-11258.
pmid: 33021629 |
| [31] |
Kotrys AV, Durham TJ, Guo XYA, Vantaku VR, Parangi S, Mootha VK. Single-cell analysis reveals context- dependent, cell-level selection of mtDNA. Nature, 2024, 629(8011): 458-466.
pmid: 38658765 |
| [32] |
Stewart JB, Chinnery PF. Extreme heterogeneity of human mitochondrial DNA from organelles to populations. Nat Rev Genet, 2021, 22(2): 106-118.
pmid: 32989265 |
| [33] |
Nissanka N, Moraes CT. Mitochondrial DNA heteroplasmy in disease and targeted nuclease-based therapeutic approaches. EMBO Rep, 2020, 21(3): e49612.
pmid: 32073748 |
| [34] |
Payne BAI, Wilson IJ, Yu-Wai-Man P, Coxhead J, Deehan D, Horvath R, Taylor RW, Samuels DC, Santibanez-Koref M, Chinnery PF. Universal heteroplasmy of human mitochondrial DNA. Hum Mol Genet, 2013, 22(2): 384-390.
pmid: 23077218 |
| [35] |
Khan NA, Govindaraj P, Meena AK, Thangaraj K. Mitochondrial disorders: challenges in diagnosis & treatment. Indian J Med Res, 2015, 141(1): 13-26.
pmid: 25857492 |
| [36] |
Stewart JB, Chinnery PF. The dynamics of mitochondrial DNA heteroplasmy: implications for human health and disease. Nat Rev Genet, 2015, 16(9): 530-542.
pmid: 26281784 |
| [37] | Menacho C, Okawa S, Álvarez-Merz I, Wittich A, Muñoz-Oreja M, Lisowski P, Pentimalli TM, Rybak- Wolf A, Inak G, Zakin S, Thevandavakkam M, Petersilie L, Zaliani A, Mlody B, Seibt A, Donnelly J, Woleben K, Fernandez-Checa J, Herebian D, Mayatepek E, Rajewsky N, Spinazzola A, Schuelke M, Perlstein E, Rossi A, Distelmaier F, Holt IJ, Pless O, Rose CR, Del Sol A, Prigione A. Deep learning-driven neuromorphogenesis screenings identify repurposable drugs for mitochondrial disease. bioRxiv, 2024: 2024.07.08.602501. |
| [38] |
Chen L, Zhou MN, Li H, Liu DL, Liao P, Zong Y, Zhang CQ, Zou WG, Gao JJ. Mitochondrial heterogeneity in diseases. Signal Transduct Target Ther, 2023, 8(1): 311.
pmid: 37607925 |
| [39] |
Gorman GS, Chinnery PF, DiMauro S, Hirano M, Koga Y, McFarland R, Suomalainen A, Thorburn DR, Zeviani M, Turnbull DM. Mitochondrial diseases. Nat Rev Dis Primers, 2016, 2: 16080.
pmid: 27775730 |
| [40] |
Copeland WC. Inherited mitochondrial diseases of DNA replication. Annu Rev Med, 2008, 59: 131-146.
pmid: 17892433 |
| [41] |
Granat L, Knorr DY, Ranson DC, Chakrabarty RP, Chandel NS, Bateman JM. A drosophila model of mitochondrial disease phenotypic heterogeneity. Biol Open, 2024, 13(2): bio060278.
pmid: 38304969 |
| [42] |
Breton S. Mitochondrial russian doll genes may explain some discrepancies in links between mtDNA mutations and mitochondrial diseases. BioEssays, 2021, 43(6): e2100104.
pmid: 34010468 |
| [43] |
Ng YS, Turnbull DM. Mitochondrial disease: genetics and management. J Neurol, 2016, 263(1): 179-191.
pmid: 26315846 |
| [44] |
Russell O, Turnbull D. Mitochondrial DNA disease- molecular insights and potential routes to a cure. Exp Cell Res, 2014, 325(1): 38-43.
pmid: 24675282 |
| [45] |
Gorman GS, Schaefer AM, Ng Y, Gomez N, Blakely EL, Alston CL, Feeney C, Horvath R, Yu-Wai-Man P, Chinnery PF, Taylor RW, Turnbull DM, McFarland R. Prevalence of nuclear and mitochondrial DNA mutations related to adult mitochondrial disease. Ann Neurol, 2015, 77(5): 753-759.
pmid: 25652200 |
| [46] |
Yonova-Doing E, Calabrese C, Gomez-Duran A, Schon K, Wei W, Karthikeyan S, Chinnery PF, Howson JMM. An atlas of mitochondrial DNA genotype-phenotype associations in the UK Biobank. Nat Genet, 2021, 53(7): 982-993.
pmid: 34002094 |
| [47] |
Tomoda E, Nagao A, Shirai Y, Asano K, Suzuki T, Battersby BJ, Suzuki T. Restoration of mitochondrial function through activation of hypomodified tRNAs with pathogenic mutations associated with mitochondrial diseases. Nucleic Acids Res, 2023, 51(14): 7563-7579.
pmid: 36928678 |
| [48] |
Rahman S, Blok RB, Dahl HH, Danks DM, Kirby DM, Chow CW, Christodoulou J, Thorburn DR. Leigh syndrome: clinical features and biochemical and DNA abnormalities. Ann Neurol, 1996, 39(3): 343-351.
pmid: 8602753 |
| [49] |
Holt IJ, Harding AE, Petty RK, Morgan-Hughes JA. A new mitochondrial disease associated with mitochondrial DNA heteroplasmy. Am J Hum Genet, 1990, 46(3): 428-433.
pmid: 2137962 |
| [50] |
Shoffner JM, Lott MT, Lezza AM, Seibel P, Ballinger SW, Wallace DC. Myoclonic epilepsy and ragged-red fiber disease (MERRF) is associated with a mitochondrial DNA tRNALys mutation. Cell, 1990, 61(6): 931-937.
pmid: 2112427 |
| [51] |
Santorelli FM, Siciliano G, Casali C, Basirico MG, Carrozzo R, Calvosa F, Sartucci F, Bonfiglio L, Murri L, DiMauro S. Mitochondrial tRNACys gene mutation (A5814G): a second family with mitochondrial encephalopathy. Neuromuscul Disord, 1997, 7(3): 156-159.
pmid: 9185178 |
| [52] |
Goto Y, Nonaka I, Horai S. A mutation in the tRNALeu(UUR) gene associated with the MELAS subgroup of mitochondrial encephalomyopathies. Nature, 1990, 348(6302): 651-653.
pmid: 2102678 |
| [53] |
Yu XL, Yan CZ, Ji KQ, Lin PF, Xu XB, Dai TJ, Li W, Zhao YY. Clinical, neuroimaging, and pathological analyses of 13 chinese leigh syndrome patients with mitochondrial DNA mutations. Chin Med J(Engl), 2018, 131(22): 2705-2712.
pmid: 30425197 |
| [54] |
Finsterer J. Neuropathy, ataxia, and retinitis pigmentosa syndrome. J Clin Neuromuscul Dis, 2023, 24(3): 140-146.
pmid: 36809201 |
| [55] |
Claeys KG, Abicht A, Häusler M, Kleinle S, Wiesmann M, Schulz JB, Horvath R, Weis J. Novel genetic and neuropathological insights in neurogenic muscle weakness, ataxia, and retinitis pigmentosa (NARP). Muscle Nerve, 2016, 54(2): 328-333.
pmid: 27015314 |
| [56] |
Kawazoe T, Tobisawa S, Sugaya K, Uruha A, Miyamoto K, Komori T, Goto YI, Nishino I, Yoshihashi H, Mizuguchi T, Matsumoto N, Egawa N, Kawata A, Isozaki E. Myoclonic epilepsy with ragged-red fibers with intranuclear inclusions. Intern Med, 2022, 61(4): 547-552.
pmid: 34433719 |
| [57] |
Peverelli L, Catania A, Marchet S, Ciasca P, Cammarata G, Melzi L, Bellino A, Fancellu R, Lamantea E, Capristo M, Caporali L, La Morgia C, Carelli V, Ghezzi D, Bianchi Marzoli S, Lamperti C. Leber’s hereditary optic neuropathy: a report on novel mtDNA pathogenic variants. Front Neurol, 2021, 12: 657317.
pmid: 34177762 |
| [58] |
Ueda K, Morizane Y, Shiraga F, Shikishima K, Ishikawa H, Wakakura M, Nakamura M. Nationwide epidemiological survey of Leber hereditary optic neuropathy in Japan. J Epidemiol, 2017, 27(9): 447-450.
pmid: 28392196 |
| [59] |
Keilland E, Rupar CA, Prasad AN, Tay KY, Downie A, Prasad C. The expanding phenotype of MELAS caused by the m. 3291T>C mutation in the MT-TL1 gene. Mol Genet Metab Rep. 2016, 6:64-9.
pmid: 27014580 |
| [60] |
Hirano M, Ricci E, Koenigsberger MR, Defendini R, Pavlakis SG, DeVivo DC, DiMauro S, Rowland LP. Melas: an original case and clinical criteria for diagnosis. Neuromuscul Disord, 1992, 2(2): 125-135.
pmid: 1422200 |
| [61] |
Alves CAPF, Zandifar A, Peterson JT, Tara SZ, Ganetzky R, Viaene AN, Andronikou S, Falk MJ, Vossough A, Goldstein AC. MELAS: phenotype classification into classic-versus-atypical presentations. AJNR Am J Neuroradiol, 2023, 44(5): 602-610.
pmid: 37024306 |
| [62] |
Bakare AB, Lesnefsky EJ, Iyer S. Leigh syndrome: a tale of two genomes. Front Physiol, 2021, 12: 693734.
pmid: 34456746 |
| [63] |
Rahman S. Leigh syndrome. Handb Clin Neurol, 2023, 194: 43-63.
pmid: 36813320 |
| [64] |
Haroon S, Yoon H, Seiler C, Osei-Frimpong B, He J, Nair RM, Mathew ND, Burg L, Kose M, Venkata CRM, Anderson VE, Nakamaru-Ogiso E, Falk MJ. N-acetylcysteine and cysteamine bitartrate prevent azide-induced neuromuscular decompensation by restoring glutathione balance in two novel surf1-/- zebrafish deletion models of Leigh syndrome. Hum Mol Genet, 2023, 32(12): 1988-2004.
pmid: 36795052 |
| [65] |
Scuderi C, Borgione E, Musumeci S, Elia M, Castello F, Fichera M, Davidzon G, DiMauro S. Severe encephalomyopathy in a patient with homoplasmic A5814G point mutation in mitochondrial tRNACys gene. Neuromuscul Disord, 2007, 17(3): 258-261.
pmid: 17241783 |
| [66] |
Manfredi G, Schon EA, Bonilla E, Moraes CT, Shanske S, DiMauro S. Identification of a mutation in the mitochondrial tRNA(Cys) gene associated with mitochondrial encephalopathy. Hum Mutat, 1996, 7(2): 158-163.
pmid: 8829635 |
| [67] |
Karadimas C, Tanji K, Geremek M, Chronopoulou P, Vu T, Krishna S, Sue CM, Shanske S, Bonilla E, DiMauro S, Lipson M, Bachman R. A5814G mutation in mitochondrial DNA can cause mitochondrial myopathy and cardiomyopathy. J Child Neurol, 2001, 16(7): 531-533.
pmid: 11453453 |
| [68] |
Villanueva-Paz M, Povea-Cabello S, Villalón-García I, Suárez-Rivero JM, Álvarez-Córdoba M, de la Mata M, Talaverón-Rey M, Jackson S, Sánchez-Alcázar JA. Pathophysiological characterization of MERRF patient- specific induced neurons generated by direct reprogramming. Biochim Biophys Acta Mol Cell Res, 2019, 1866(5): 861-881.
pmid: 30797798 |
| [69] |
Chen BS, Yu-Wai-Man P, Newman NJ. Developments in the treatment of Leber hereditary optic neuropathy. Curr Neurol Neurosci Rep, 2022, 22(12): 881-892.
pmid: 36414808 |
| [70] |
Takano F, Ueda K, Godefrooij DA, Yamagami A, Ishikawa H, Chuman H, Ishikawa H, Ikeda Y, Sakamoto T, Nakamura M. Incidence of Leber hereditary optic neuropathy in 2019 in Japan: a second nationwide questionnaire survey. Orphanet J Rare Dis, 2022, 17(1): 319.
pmid: 35987635 |
| [71] |
Wang R, Bao FX, Lu MJ, Jia XY, Xiao JH, Wu Y, Zhang QJ, Liu XG. MSC-mediated mitochondrial transfer restores mitochondrial DNA and function in neural progenitor cells of Leber’s hereditary optic neuropathy. Sci China Life Sci, 2024, 67(11): 2511-2519.
pmid: 39134891 |
| [72] |
Altshuler-Keylin S, Kajimura S. Mitochondrial homeostasis in adipose tissue remodeling. Sci Signal, 2017, 10(468): eaai9248.
pmid: 28246203 |
| [73] |
Luongo TS, Lambert JP, Gross P, Nwokedi M, Lombardi AA, Shanmughapriya S, Carpenter AC, Kolmetzky D, Gao E, van Berlo JH, Tsai EJ, Molkentin JD, Chen XW, Madesh M, Houser SR, Elrod JW. The mitochondrial Na+/Ca2+ exchanger is essential for Ca2+ homeostasis and viability. Nature, 2017, 545(7652): 93-97.
pmid: 28445457 |
| [74] |
Moudy AM, Handran SD, Goldberg MP, Ruffin N, Karl I, Kranz-Eble P, DeVivo DC, Rothman SM. Abnormal calcium homeostasis and mitochondrial polarization in a human encephalomyopathy. Proc Natl Acad Sci USA, 1995, 92(3): 729-733.
pmid: 7846043 |
| [75] |
Zhang PC, Konja D, Zhang YW, Wang Y. Communications between mitochondria and endoplasmic reticulum in the regulation of metabolic homeostasis. Cells, 2021, 10(9): 2195.
pmid: 34571844 |
| [76] |
Reinisch KM, Prinz WA. Mechanisms of nonvesicular lipid transport. J Cell Biol, 2021, 220(3): e202012058.
pmid: 33605998 |
| [77] |
Kornmann B, Currie E, Collins SR, Schuldiner M, Nunnari J, Weissman JS, Walter P. An ER-mitochondria tethering complex revealed by a synthetic biology screen. Science, 2009, 325(5939): 477-481.
pmid: 19556461 |
| [78] |
Kopec KO, Alva V, Lupas AN. Homology of SMP domains to the TULIP superfamily of lipid-binding proteins provides a structural basis for lipid exchange between ER and mitochondria. Bioinformatics, 2010, 26(16): 1927-1931.
pmid: 20554689 |
| [79] |
Schauder CM, Wu XD, Saheki Y, Narayanaswamy P, Torta F, Wenk MR, De Camilli P, Reinisch KM. Structure of a lipid-bound extended synaptotagmin indicates a role in lipid transfer. Nature, 2014, 510(7506): 552-555.
pmid: 24847877 |
| [80] |
Wozny MR, Di Luca A, Morado DR, Picco A, Khaddaj R, Campomanes P, Ivanović L, Hoffmann PC, Miller EA, Vanni S, Kukulski W. In situ architecture of the ER-mitochondria encounter structure. Nature, 2023, 618(7963): 188-192.
pmid: 37165187 |
| [81] |
Backes S, Herrmann JM. Protein translocation into the intermembrane space and matrix of mitochondria: mechanisms and driving forces. Front Mol Biosci, 2017, 4: 83.
pmid: 29270408 |
| [82] |
Bhatti JS, Bhatti GK, Reddy PH. Mitochondrial dysfunction and oxidative stress in metabolic disorders - A step towards mitochondria based therapeutic strategies. Biochim Biophys Acta Mol Basis Dis, 2017, 1863(5): 1066-1077.
pmid: 27836629 |
| [83] |
Mouchiroud L, Houtkooper RH, Moullan N, Katsyuba E, Ryu D, Cantó C, Mottis A, Jo YS, Viswanathan M, Schoonjans K, Guarente L, Auwerx J. The NAD+/ sirtuin pathway modulates longevity through activation of mitochondrial UPR and FOXO signaling. Cell, 2013, 154(2): 430-441.
pmid: 23870130 |
| [84] |
Liu JF, He XY, Zheng SC, Zhu AS, Wang JY. The mitochondrial unfolded protein response: a novel protective pathway targeting cardiomyocytes. Oxid Med Cell Longev, 2022, 2022: 6430342.
pmid: 23870130 |
| [85] |
Guo MM, Qiao XH, Wang YY, Li ZH, Shi C, Chen Y, Kang L, Chen C, Zhou XL. Mitochondrial translational defect extends lifespan in C. elegans by activating UPRmt. Redox Biol, 2023, 63: 102722.
pmid: 37167879 |
| [86] |
Aras S, Purandare N, Gladyck S, Somayajulu-Nitu M, Zhang KZ, Wallace DC, Grossman LI. Mitochondrial Nuclear Retrograde Regulator 1 (MNRR1) rescues the cellular phenotype of MELAS by inducing homeostatic mechanisms. Proc Natl Acad Sci USA, 2020, 117(50): 32056-32065.
pmid: 33257573 |
| [87] |
McGuire PJ. Mitochondrial dysfunction and the aging immune system. Biology (Basel), 2019, 8(2): 26.
pmid: 31083529 |
| [88] |
Liu H, Fan HL, He PC, Zhuang HX, Liu X, Chen MT, Zhong WW, Zhang Y, Zhen C, Li YL, Jiang HL, Meng T, Xu YM, Zhao GJ, Feng D. Prohibitin 1 regulates mtDNA release and downstream inflammatory responses. EMBO J, 2022, 41(24): e111173.
pmid: 36245295 |
| [89] |
Jin J, Mu YM, Zhang HM, Sturmlechner I, Wang CY, Jadhav RR, Xia Q, Weyand CM, Goronzy JJ. CISH impairs lysosomal function in activated T cells resulting in mitochondrial DNA release and inflammaging. Nat Aging, 2023, 3(5): 600-616.
pmid: 37118554 |
| [90] |
Pohjoismäki JLO, Wanrooij S, Hyvärinen AK, Goffart S, Holt IJ, Spelbrink JN, Jacobs HT. Alterations to the expression level of mitochondrial transcription factor A, TFAM, modify the mode of mitochondrial DNA replication in cultured human cells. Nucleic Acids Res, 2006, 34(20): 5815-5828.
pmid: 17062618 |
| [91] |
Qi Y, Ye YD, Wang RX, Yu SL, Zhang Y, Lv J, Jin WW, Xia ST, Jiang W, Li YF, Zhang DH. Mitochondrial dysfunction by TFAM depletion disrupts self-renewal and lineage differentiation of human PSCs by affectting cell proliferation and YAP response. Redox Biol, 2022, 50: 102248.
pmid: 35091324 |
| [92] |
Gulen MF, Samson N, Keller A, Schwabenland M, Liu C, Glück S, Thacker VV, Favre L, Mangeat B, Kroese LJ, Krimpenfort P, Prinz M, Ablasser A. cGAS-STING drives ageing-related inflammation and neurodegeneration. Nature, 2023, 620(7973): 374-380.
pmid: 37532932 |
| [93] |
Maresca A, Del Dotto V, Romagnoli M, La Morgia C, Di Vito L, Capristo M, Valentino ML, Carelli V, ER-MITO Study Group. Expanding and validating the biomarkers for mitochondrial diseases. J Mol Med, 2020, 98(10): 1467-1478.
pmid: 32851462 |
| [94] |
Soustiel JF, Larisch S. Mitochondrial damage: a target for new therapeutic horizons. Neurotherapeutics, 2010, 7(1): 13-21.
pmid: 20129493 |
| [95] |
Garrido-Maraver J, Paz MV, Cordero MD, Bautista- Lorite J, Oropesa-Ávila M, de la Mata M, Pavón AD, de Lavera I, Alcocer-Gómez E, Galán F, Ybot González P, Cotán D, Jackson S, Sánchez-Alcázar JA. Critical role of AMP-activated protein kinase in the balance between mitophagy and mitochondrial biogenesis in MELAS disease. Biochim Biophys Acta, 2015, 1852(11): 2535-2553.
pmid: 26341273 |
| [96] |
Klionsky DJ, Emr SD. Autophagy as a regulated pathway of cellular degradation. Science, 2000, 290(5497): 1717-1721.
pmid: 11099404 |
| [97] |
Kim I, Rodriguez-Enriquez S, Lemasters JJ. Selective degradation of mitochondria by mitophagy. Arch Biochem Biophys, 2007, 462(2): 245-253.
pmid: 17475204 |
| [98] |
Kim I, Lemasters JJ. Mitophagy selectively degrades individual damaged mitochondria after photoirradiation. Antioxid Redox Signal, 2011, 14(10): 1919-1928.
pmid: 21126216 |
| [99] |
Narendra D, Tanaka A, Suen DF, Youle RJ. Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J Cell Biol, 2008, 183(5): 795-803.
pmid: 19029340 |
| [100] |
Lazarou M, Narendra DP, Jin SM, Tekle E, Banerjee S, Youle RJ. PINK1 drives Parkin self-association and HECT-like E3 activity upstream of mitochondrial binding. J Cell Biol, 2013, 200(2): 163-172.
pmid: 23319602 |
| [101] |
Chan NC, Salazar AM, Pham AH, Sweredoski MJ, Kolawa NJ, Graham RLJ, Hess S, Chan DC. Broad activation of the ubiquitin-proteasome system by Parkin is critical for mitophagy. Hum Mol Genet, 2011, 20(9): 1726-1737.
pmid: 21296869 |
| [102] |
Deas E, Plun-Favreau H, Gandhi S, Desmond H, Kjaer S, Loh SHY, Renton AEM, Harvey RJ, Whitworth AJ, Martins LM, Abramov AY, Wood NW. PINK1 cleavage at position A103 by the mitochondrial protease PARL. Hum Mol Genet, 2011, 20(5): 867-879.
pmid: 21138942 |
| [103] |
Greene AW, Grenier K, Aguileta MA, Muise S, Farazifard R, Haque ME, McBride HM, Park DS, Fon EA. Mitochondrial processing peptidase regulates PINK1 processing, import and Parkin recruitment. EMBO Rep, 2012, 13(4): 378-385.
pmid: 22354088 |
| [104] |
Lazarou M, Jin SM, Kane LA, Youle RJ. Role of PINK1 binding to the TOM complex and alternate intracellular membranes in recruitment and activation of the E3 ligase Parkin. Dev Cell, 2012, 22(2): 320-333.
pmid: 22280891 |
| [105] |
Matsuda N, Sato S, Shiba K, Okatsu K, Saisho K, Gautier CA, Sou YS, Saiki S, Kawajiri S, Sato F, Kimura M, Komatsu M, Hattori N, Tanaka K. PINK1 stabilized by mitochondrial depolarization recruits Parkin to damaged mitochondria and activates latent Parkin for mitophagy. J Cell Biol, 2010, 189(2): 211-221.
pmid: 20404107 |
| [106] |
Gegg ME, Cooper JM, Chau KY, Rojo M, Schapira AHV, Taanman JW. Mitofusin 1 and mitofusin 2 are ubiquitinated in a PINK1/parkin-dependent manner upon induction of mitophagy. Hum Mol Genet, 2010, 19(24): 4861-4870.
pmid: 20871098 |
| [107] |
Poole AC, Thomas RE, Yu S, Vincow ES, Pallanck L. The mitochondrial fusion-promoting factor mitofusin is a substrate of the PINK1/parkin pathway. PLoS One, 2010, 5(4): e10054.
pmid: 20383334 |
| [108] |
Liu H, Zhen CE, Xie JM, Luo ZH, Zeng L, Zhao GJ, Lu SH, Zhuang HX, Fan HL, Li X, Liu ZJ, Lin SY, Jiang HL, Chen YQ, Cheng JH, Cao ZY, Dai KY, Shi JH, Wang ZH, Hu YQ, Meng T, Zhou CC, Han ZY, Huang HS, Zhou QH, He PC, Feng D. TFAM is an autophagy receptor that limits inflammation by binding to cytoplasmic mitochondrial DNA. Nat Cell Biol, 2024, 26(6): 878-891.
pmid: 38783142 |
| [109] |
Ghaoui R, Sue CM. Movement disorders in mitochondrial disease. J Neurol, 2018, 265(5): 1230-1240.
pmid: 29307008 |
| [110] |
Schon EA. Mitochondrial genetics and disease. Trends Biochem Sci, 2000, 25(11): 555-560.
pmid: 11084368 |
| [111] |
Yang L, Slone J, Li Z, Lou XT, Hu YC, Queme LF, Jankowski MP, Huang T. Systemic administration of AAV-Slc25a46 mitigates mitochondrial neuropathy in Slc25a46-/- mice. Hum Mol Genet, 2020, 29(4): 649-661.
pmid: 31943007 |
| [112] |
Yang L, Slone J, Zou WW, Queme LF, Jankowski MP, Yin F, Huang TS. Systemic delivery of AAV-Fdxr mitigates the phenotypes of mitochondrial disorders in Fdxr mutant mice. Mol Ther Methods Clin Dev, 2020, 18: 84-97.
pmid: 32995353 |
| [113] |
Yu-Wai-Man P, Newman NJ, Carelli V, Moster ML, Biousse V, Sadun AA, Klopstock T, Vignal-Clermont C, Sergott RC, Rudolph G, La Morgia C, Karanjia R, Taiel M, Blouin L, Burguière P, Smits G, Chevalier C, Masonson H, Salermo Y, Katz B, Picaud S, Calkins DJ, Sahel JA. Bilateral visual improvement with unilateral gene therapy injection for Leber hereditary optic neuropathy. Sci Transl Med, 2020, 12(573): eaaz7423.
pmid: 33298565 |
| [114] |
Srivastava S, Moraes CT. Manipulating mitochondrial DNA heteroplasmy by a mitochondrially targeted restriction endonuclease. Hum Mol Genet, 2001, 10(26): 3093-3099.
pmid: 11751691 |
| [115] |
Tanaka M, Borgeld HJ, Zhang J, Muramatsu S, Gong JS, Yoneda M, Maruyama W, Naoi M, Ibi T, Sahashi K, Shamoto M, Fuku N, Kurata M, Yamada Y, Nishizawa K, Akao Y, Ohishi N, Miyabayashi S, Umemoto H, Muramatsu T, Furukawa K, Kikuchi A, Nakano I, Ozawa K, Yagi K. Gene therapy for mitochondrial disease by delivering restriction endonuclease SmaI into mitochondria. J Biomed Sci, 2002, 9(6 Pt1): 534-541.
pmid: 12372991 |
| [116] |
Bayona-Bafaluy MP, Blits B, Battersby BJ, Shoubridge EA, Moraes CT. Rapid directional shift of mitochondrial DNA heteroplasmy in animal tissues by a mitochondrially targeted restriction endonuclease. Proc Natl Acad Sci USA, 2005, 102(40): 14392-14397.
pmid: 16179392 |
| [117] |
Alexeyev M, Shokolenko I, Wilson G, LeDoux S. The maintenance of mitochondrial DNA integrity--critical analysis and update. Cold Spring Harbor Perspect Biol, 2013, 5(5): a012641.
pmid: 23637283 |
| [118] |
Peeva V, Blei D, Trombly G, Corsi S, Szukszto MJ, Rebelo-Guiomar P, Gammage PA, Kudin AP, Becker C, Altmüller J, Minczuk M, Zsurka G, Kunz WS. Linear mitochondrial DNA is rapidly degraded by components of the replication machinery. Nat Commun, 2018, 9(1): 1727.
pmid: 29712893 |
| [119] |
Moretton A, Morel F, Macao B, Lachaume P, Ishak L, Lefebvre M, Garreau-Balandier I, Vernet P, Falkenberg M, Farge G. Selective mitochondrial DNA degradation following double-strand breaks. PLoS One, 2017, 12(4): e0176795.
pmid: 28453550 |
| [120] |
Minczuk M, Papworth MA, Kolasinska P, Murphy MP, Klug A. Sequence-specific modification of mitochondrial DNA using a chimeric zinc finger methylase. Proc Natl Acad Sci USA, 2006, 103(52): 19689-19694.
pmid: 17170133 |
| [121] |
Minczuk M, Papworth MA, Miller JC, Murphy MP, Klug A. Development of a single-chain, quasi-dimeric zinc-finger nuclease for the selective degradation of mutated human mitochondrial DNA. Nucleic Acids Res, 2008, 36(12): 3926-3938.
pmid: 18511461 |
| [122] |
Bacman SR, Williams SL, Pinto M, Peralta S, Moraes CT. Specific elimination of mutant mitochondrial genomes in patient-derived cells by mitoTALENs. Nat Med, 2013, 19(9): 1111-1113.
pmid: 23913125 |
| [123] |
Zekonyte U, Bacman SR, Smith J, Shoop W, Pereira CV, Tomberlin G, Stewart J, Jantz D, Moraes CT. Mitochondrial targeted meganuclease as a platform to eliminate mutant mtDNA in vivo. Nat Commun, 2021, 12(1): 3210.
pmid: 34050192 |
| [124] |
Mok BY, de Moraes MH, Zeng J, Bosch DE, Kotrys AV, Raguram A, Hsu F, Radey MC, Peterson SB, Mootha VK, Mougous JD, Liu DR. A bacterial cytidine deaminase toxin enables CRISPR-free mitochondrial base editing. Nature, 2020, 583(7817): 631-637.
pmid: 32641830 |
| [125] |
Cho SI, Lee S, Mok YG, Lim K, Lee J, Lee JM, Chung E, Kim JS. Targeted A-to-G base editing in human mitochondrial DNA with programmable deaminases. Cell, 2022, 185(10): 1764-1776.e12.
pmid: 35472302 |
| [126] |
Yi ZY, Zhang XX, Tang W, Yu Y, Wei XX, Zhang X, Wei WS. Strand-selective base editing of human mitochondrial DNA using mitoBEs. Nat Biotechnol, 2024, 42(3): 498-509.
pmid: 37217751 |
| [127] |
Kyriakouli DS, Boesch P, Taylor RW, Lightowlers RN. Progress and prospects: gene therapy for mitochondrial DNA disease. Gene Ther, 2008, 15(14): 1017-1023.
pmid: 18496570 |
| [128] |
Gammage PA, Moraes CT, Minczuk M. Mitochondrial genome engineering: the revolution may not be CRISPR- Ized. Trends Genet, 2018, 34(2): 101-110.
pmid: 29179920 |
| [129] |
Mok BY, Kotrys AV, Raguram A, Huang TP, Mootha VK, Liu DR. CRISPR-free base editors with enhanced activity and expanded targeting scope in mitochondrial and nuclear DNA. Nat Biotechnol, 2022, 40(9): 1378-1387.
pmid: 35379961 |
| [130] |
Lim K, Cho SI, Kim JS. Nuclear and mitochondrial DNA editing in human cells with zinc finger deaminases. Nat Commun, 2022, 13(1): 366.
pmid: 35042880 |
| [131] |
Willis JCW, Silva-Pinheiro P, Widdup L, Minczuk M, Liu DR. Compact zinc finger base editors that edit mitochondrial or nuclear DNA in vitro and in vivo. Nature Commun, 2022, 13(1): 7204.
pmid: 36418298 |
| [132] |
Reddy P, Ocampo A, Suzuki K, Luo JP, Bacman SR, Williams SL, Sugawara A, Okamura D, Tsunekawa Y, Wu J, Lam D, Xiong X, Montserrat N, Esteban CR, Liu GH, Sancho-Martinez I, Manau D, Civico S, Cardellach F, Del Mar O’Callaghan M, Campistol J, Zhao HM, Campistol JM, Moraes CT, Izpisua Belmonte JC. Selective elimination of mitochondrial mutations in the germline by genome editing. Cell, 2015, 161(3): 459-469.
pmid: 25910206 |
| [133] |
Pereira CV, Bacman SR, Arguello T, Zekonyte U, Williams SL, Edgell DR, Moraes CT. mitoTev-TALE: a monomeric DNA editing enzyme to reduce mutant mitochondrial DNA levels. EMBO Mol Med, 2018, 10(9): e8084.
pmid: 30012581 |
| [134] |
Yang Y, Wu H, Kang XJ, Liang YH, Lan T, Li TJ, Tan T, Peng JY, Zhang QJ, An G, Liu YL, Yu Q, Ma ZL, Lian Y, Soh BS, Chen QF, Liu P, Chen YY, Sun XF, Li R, Zhen XM, Liu P, Yu Y, Li XP, Fan Y. Targeted elimination of mutant mitochondrial DNA in MELAS-iPSCs by mitoTALENs. Protein Cell, 2018, 9(3): 283-297.
pmid: 29318513 |
| [135] |
Yahata N, Matsumoto Y, Omi M, Yamamoto N, Hata R. Author correction: TALEN-mediated shift of mitochondrial DNA heteroplasmy in MELAS-iPSCs with m. 13513 G>A mutation. Sci Rep, 2018, 8(1): 4683.
pmid: 29535331 |
| [136] |
Suzuki Y, Holmes JB, Cerritelli SM, Sakhuja K, Minczuk M, Holt IJ, Crouch RJ. An upstream open reading frame and the context of the two AUG codons affect the abundance of mitochondrial and nuclear RNase H1. Mol Cell Biol, 2010, 30(21): 5123-5134.
pmid: 20823270 |
| [137] |
Gammage PA, Rorbach J, Vincent AI, Rebar EJ, Minczuk M. Mitochondrially targeted ZFNs for selective degradation of pathogenic mitochondrial genomes bearing large-scale deletions or point mutations. EMBO Mol Med, 2014, 6(4): 458-466.
pmid: 24567072 |
| [138] |
Gammage PA, Gaude E, Van Haute L, Rebelo-Guiomar P, Jackson CB, Rorbach J, Pekalski ML, Robinson AJ, Charpentier M, Concordet JP, Frezza C, Minczuk M. Near-complete elimination of mutant mtDNA by iterative or dynamic dose-controlled treatment with mtZFNs. Nucleic Acids Res, 2016, 44(16): 7804-7816.
pmid: 27466392 |
| [139] |
Gammage PA, Viscomi C, Simard ML, Costa ASH, Gaude E, Powell CA, Van Haute L, McCann BJ, Rebelo-Guiomar P, Cerutti R, Zhang L, Rebar EJ, Zeviani M, Frezza C, Stewart JB, Minczuk M. Genome editing in mitochondria corrects a pathogenic mtDNA mutation in vivo. Nat Med, 2018, 24(11): 1691-1695.
pmid: 30250142 |
| [140] |
Comte C, Tonin Y, Heckel-Mager AM, Boucheham A, Smirnov A, Auré K, Lombès A, Martin RP, Entelis N, Tarassov I. Mitochondrial targeting of recombinant RNAs modulates the level of a heteroplasmic mutation in human mitochondrial DNA associated with kearns sayre syndrome. Nucleic Acids Res, 2013, 41(1): 418-433.
pmid: 23087375 |
| [141] |
Taylor RW, Chinnery PF, Turnbull DM, Lightowlers RN. Selective inhibition of mutant human mitochondrial DNA replication in vitro by peptide nucleic acids. Nat Genet, 1997, 15(2): 212-215.
pmid: 9020853 |
| [142] |
Bacman SR, Kauppila JHK, Pereira CV, Nissanka N, Miranda M, Pinto M, Williams SL, Larsson NG, Stewart JB, Moraes CT. MitoTALEN reduces mutant mtDNA load and restores tRNAAla levels in a mouse model of heteroplasmic mtDNA mutation. Nat Med, 2018, 24(11): 1696-1700.
pmid: 30250143 |
| [143] |
Kazama T, Okuno M, Watari Y, Yanase S, Koizuka C, Tsuruta Y, Sugaya H, Toyoda A, Itoh T, Tsutsumi N, Toriyama K, Koizuka N, Arimura SI. Curing cytoplasmic male sterility via TALEN-mediated mitochondrial genome editing. Nat Plants, 2019, 5(7): 722-730.
pmid: 31285556 |
| [144] |
Shoop WK, Lape J, Trum M, Powell A, Sevigny E, Mischler A, Bacman SR, Fontanesi F, Smith J, Jantz D, Gorsuch CL, Moraes CT. Efficient elimination of MELAS-associated m. 3243G mutant mitochondrial DNA by an engineered mitoARCUS nuclease. Nat Metab, 2023, 5(12): 2169-2183.
pmid: 38036771 |
| [145] |
Grieger JC, Samulski RJ. Packaging capacity of adeno- associated virus serotypes: impact of larger genomes on infectivity and postentry steps. J Virol, 2005, 79(15): 9933-9944.
pmid: 16014954 |
| [146] |
Chen XX, Liang D, Guo JY, Zhang JQ, Sun HF, Zhang XL, Jin JC, Dai YC, Bao QM, Qian XZ, Tan L, Hu P, Ling XF, Shen B, Xu ZF. DdCBE-mediated mitochondrial base editing in human 3PN embryos. Cell Discov, 2022, 8(1): 8.
pmid: 35102135 |
| [147] |
Wei YH, Xu CL, Feng H, Xu K, Li ZF, Hu J, Zhou L, Wei Y, Zuo ZR, Zuo EW, Li W, Yang H, Zhang ML. Human cleaving embryos enable efficient mitochondrial base-editing with DdCBE. Cell Discovery, 2022, 8(1): 7.
pmid: 35102133 |
| [148] |
Wei YH, Li ZF, Xu K, Feng H, Xie L, Li D, Zuo ZR, Zhang ML, Xu CL, Yang H, Zuo EW. Mitochondrial base editor DdCBE causes substantial DNA off-target editing in nuclear genome of embryos. Cell Discov, 2022, 8(1): 27.
pmid: 35304438 |
| [149] |
Lei ZX, Meng HW, Liu LL, Zhao HN, Rao XC, Yan YC, Wu H, Liu M, He AB, Yi CQ. Mitochondrial base editor induces substantial nuclear off-target mutations. Nature, 2022, 606(7915): 804-811.
pmid: 35551512 |
| [150] |
Guo JY, Zhang X, Chen XX, Sun HF, Dai YC, Wang JY, Qian XZ, Tan L, Lou X, Shen B. Precision modeling of mitochondrial diseases in zebrafish via DdCBE-mediated mtDNA base editing. Cell Discov, 2021, 7(1): 78.
pmid: 34480028 |
| [151] |
Qi XL, Chen XX, Guo JY, Zhang X, Sun HF, Wang JY, Qian XZ, Li B, Tan L, Yu L, Chen W, Zhang LF, Ma YW, Shen B. Precision modeling of mitochondrial disease in rats via DdCBE-mediated mtDNA editing. Cell Discov, 2021, 7(1): 95.
pmid: 34663794 |
| [152] |
Mok YG, Hong S, Bae SJ, Cho SI, Kim JS. Targeted A-to-G base editing of chloroplast DNA in plants. Nat Plants, 2022, 8(12): 1378-1384.
pmid: 36456803 |
| [153] |
Zhang XX, Zhang X, Ren JW, Li JY, Wei XX, Yu Y, Yi ZY, Wei WS. Precise modelling of mitochondrial diseases using optimized mitoBEs. Nature, 2025, 639(8055): 735-745.
pmid: 39843744 |
| [154] |
Tornabene P, Trapani I, Minopoli R, Centrulo M, Lupo M, de Simone S, Tiberi P, Dell'Aquila F, Marrocco E, Iodice C, Iuliano A, Gesualdo C, Rossi S, Giaquinto L, Albert S, Hoyng CB, Polishchuk E, Cremers FPM, Surace EM, Simonelli F, De Matteis MA, Polishchuk R, Auricchio A. Intein-mediated protein trans-splicing expands adeno-associated virus transfer capacity in the retina. Sci Transl Med, 2019, 11(492): eaav4523.
pmid: 31092694 |
| [155] |
Jo A, Ham S, Lee GH, Lee YI, Kim S, Lee YS, Shin JH, Lee Y. Efficient mitochondrial genome editing by CRISPR/Cas9. Biomed Res Int, 2015, 2015: 305716.
pmid: 26448933 |
| [156] |
Yoo BC, Yadav NS, Orozco EM Jr, Sakai H. Cas9/gRNA- mediated genome editing of yeast mitochondria and Chlamydomonas chloroplasts. PeerJ, 2020, 8: e8362.
pmid: 31934513 |
| [157] |
Loutre R, Heckel AM, Jeandard D, Tarassov I, Entelis N. Anti-replicative recombinant 5S rRNA molecules can modulate the mtDNA heteroplasmy in a glucose- dependent manner. PLoS One, 2018, 13(6): e0199258.
pmid: 29912984 |
| [158] |
Antón Z, Mullally G, Ford HC, van der Kamp MW, Szczelkun MD, Lane JD. Mitochondrial import, health and mtDNA copy number variability seen when using type II and type V CRISPR effectors. J Cell Sci, 2020, 133(18): jcs248468.
pmid: 32843580 |
| [159] |
Hussain SA, Yalvac ME, Khoo B, Eckardt S, McLaughlin KJ. Adapting CRISPR/Cas9 system for targeting mitochondrial genome. Front Genet, 2021, 12: 627050.
pmid: 33889176 |
| [160] |
Liu PP, Long LJ, Xiong K, Yu B, Chang NN, Xiong JW, Zhu ZY, Liu D. Heritable/conditional genome editing in C. elegans using a CRISPR-Cas9 feeding system. Cell Res, 2014, 24(7): 886-889.
pmid: 24874953 |
| [161] |
Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature, 1998, 391(6669): 806-811.
pmid: 9486653 |
| [162] |
Hunter CP, Winston WM, Molodowitch C, Feinberg EH, Shih J, Sutherlin M, Wright AJ, Fitzgerald MC. Systemic RNAi in Caenorhabditis elegans. Cold Spring Harb Symp Quant Biol, 2006, 71: 95-100.
pmid: 17381285 |
| [163] |
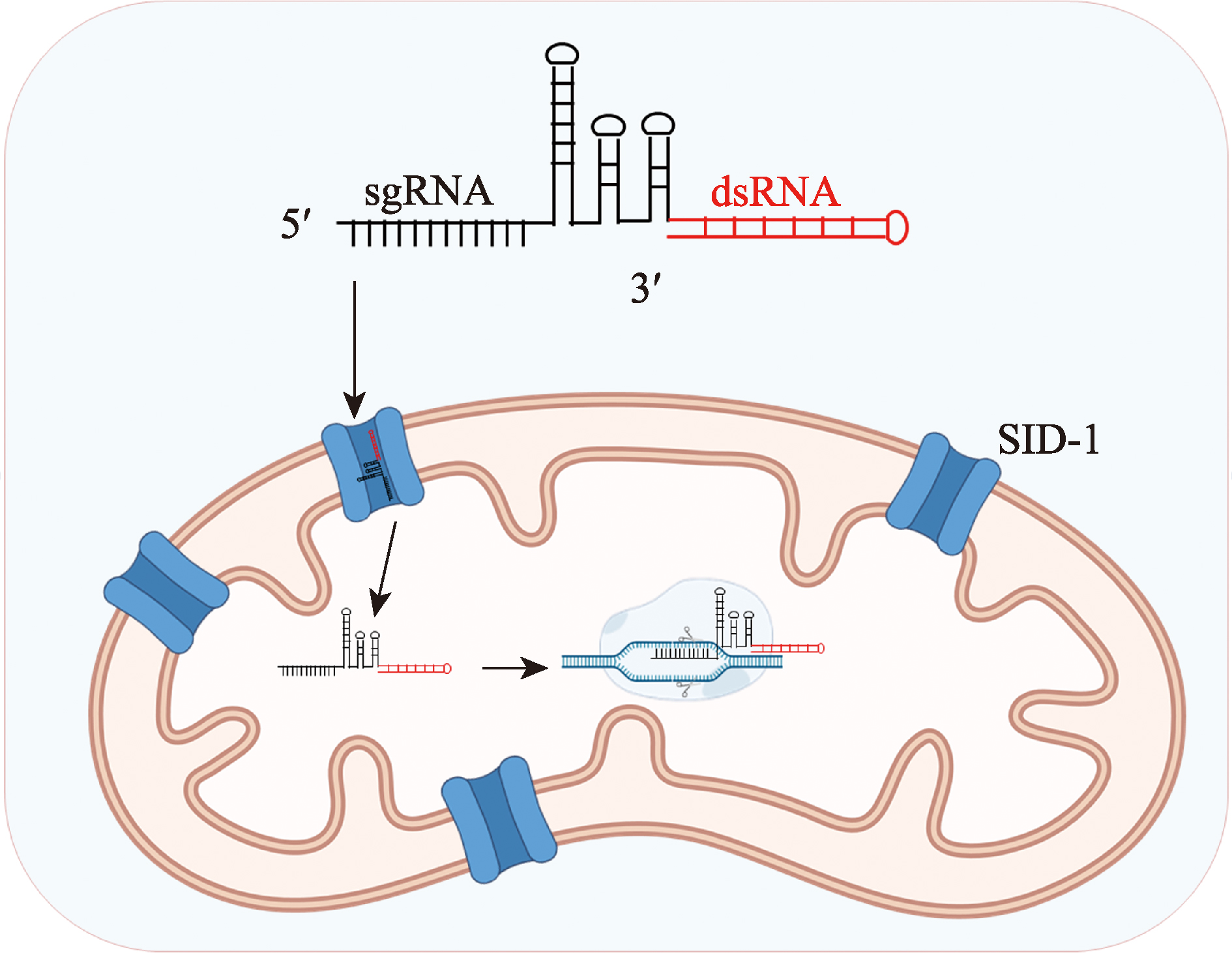
Zhang JT, Zhan CH, Fan JP, Wu D, Zhang RX, Wu D, Chen XY, Lu Y, Li M, Lin M, Gong JK, Jiang DH. Structural insights into double-stranded RNA recognition and transport by SID-1. Nat Struct Mol Biol, 2024, 31(7): 1095-1104.
pmid: 38664565 |
| [1] | Xun Zhou, Shijie Zhou, Jie Liu, Yuxiang Wang. CRISPR/Cas system targeting RNA and its derivative technology [J]. Hereditas(Beijing), 2025, 47(8): 842-860. |
| [2] | Dongxia Pan, Hui Wang, Benhai Xiong, Xiangfang Tang. Progress on CRISPR-Cas gene editing technology in sheep production [J]. Hereditas(Beijing), 2024, 46(9): 690-700. |
| [3] | Ma Baoxia, Yang Sen, Lyu Ming, Wang Yuren, Chang Liye, Han Yifan, Wang Jiangang, Guo Yang, Xu Kun. Comparison and optimization of different CRISPR/Cas9 donor-adapting systems for gene editing [J]. Hereditas(Beijing), 2024, 46(6): 466-477. |
| [4] | Yanchun Bao, Lingli Dai, Zaixia Liu, Fengying Ma, Yu Wang, Yongbin Liu, Mingjuan Gu, Risu Na, Wenguang Zhang. Progress on CRISPR/Cas9 system in the genetic improvement of livestock and poultry [J]. Hereditas(Beijing), 2024, 46(3): 219-231. |
| [5] | Bingqian Zhou, Shangpu Li, Xu Wang, Xiangyu Meng, Jingrong Deng, Jinliang Xing, Jiangang Wang, Kun Xu. Dual-localization signals enhance mitochondrial targeted presentation of engineered proteins [J]. Hereditas(Beijing), 2024, 46(11): 937-946. |
| [6] | Yi Shi, Yao Yu, Yilin Lü, Hong Lü. Design and practice of educational experiments on genetic epistasis [J]. Hereditas(Beijing), 2024, 46(11): 958-970. |
| [7] | Ruijia Song, Lu Han, Haifeng Sun, Bin Shen. Advances in mitochondrial DNA base editing technology [J]. Hereditas(Beijing), 2023, 45(8): 632-642. |
| [8] | Bingzheng Wang, Chao Zhang, Jiali Zhang, Jin Sun. Conditional editing of the Drosophila melanogaster genome using single transcripts expressing Cas9 and sgRNA [J]. Hereditas(Beijing), 2023, 45(7): 593-601. |
| [9] | Danni Liu, Haiping Wu, Guohua Zhou. Research progress of visual detection in rapid on-site detection of pathogen nucleic acid [J]. Hereditas(Beijing), 2023, 45(4): 306-323. |
| [10] | Qian Zhang, Zihao Wang, Ye Tian. Inter-tissue communication of mitochondrial stress in aging [J]. Hereditas(Beijing), 2023, 45(3): 187-197. |
| [11] | Zhongsheng Wu, Yu Gao, Yongtao Du, Song Dang, Kangmin He. The protocol of tagging endogenous proteins with fluorescent tags using CRISPR-Cas9 genome editing [J]. Hereditas(Beijing), 2023, 45(2): 165-175. |
| [12] | Zhenrong Yang, Gangqiao Zhou. The application of CRISPR genome editing technologies in the pathogenesis studies, diagnosis, prevention and treatment of infectious diseases [J]. Hereditas(Beijing), 2023, 45(11): 950-962. |
| [13] | Meizhen Liu, Liren Wang, Yongmei Li, Xueyun Ma, Honghui Han, Dali Li. Generation of genetically modified rat models via the CRISPR/Cas9 technology [J]. Hereditas(Beijing), 2023, 45(1): 78-87. |
| [14] | Xiaojun Zhang, Kun Xu, Juncen Shen, Lu Mu, Hongrun Qian, Jieyu Cui, Baoxia Ma, Zhilong Chen, Zhiying Zhang, Zehui Wei. A CRISPR/Cas9-Gal4BD donor adapting system for enhancing homology-directed repair [J]. Hereditas(Beijing), 2022, 44(8): 708-719. |
| [15] | Shuang Zhang, Shanshan Guo, Ruwen Wang, Renyan Ma, Xianmin Wu, Peijie Chen, Ru Wang. The roles of PARK gene family in myopathy [J]. Hereditas(Beijing), 2022, 44(7): 545-555. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||