Hereditas(Beijing) ›› 2021, Vol. 43 ›› Issue (1): 52-65.doi: 10.16288/j.yczz.20-357
• Review • Previous Articles Next Articles
Progress on genic male sterility gene in soybean
Xiaoyuan Sun, Yifan Wang, Yunhui Wang, Jiayu Lin, Jinhong Li, Yuantao Qiu, Xiaolong Fang, Fanjiang Kong( ), Meina Li(
), Meina Li( )
)
- School of Life Sciences, Guangzhou University, Guangzhou 510006, China
-
Received:2020-10-23Revised:2020-12-07Online:2021-01-20Published:2021-01-08 -
Contact:Kong Fanjiang,Li Meina E-mail:kongfj@gzhu.edu.cn;limeina@gzhu.edu.cn -
Supported by:Supported by the National Natural Science Foundation of China No(31871648)
Cite this article
Xiaoyuan Sun, Yifan Wang, Yunhui Wang, Jiayu Lin, Jinhong Li, Yuantao Qiu, Xiaolong Fang, Fanjiang Kong, Meina Li. Progress on genic male sterility gene in soybean[J]. Hereditas(Beijing), 2021, 43(1): 52-65.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
Table 1
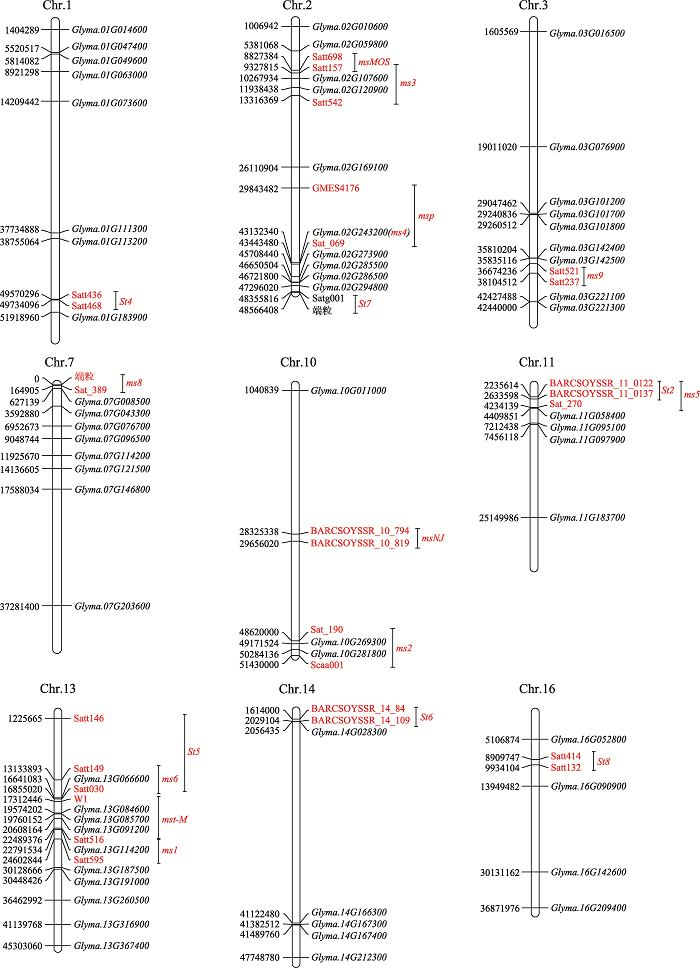
Information of genic male sterility mutants and genes in soybean"
| 大豆GMS 突变体 | 基因 | 基因位置 | 雄性不育类型 | 来源 | 文献 | ||
|---|---|---|---|---|---|---|---|
| 染色体 | 连锁群 | 标记 | |||||
| fs1fs2 | fs1fs2 | - | - | - | 结构型雄性不育 | 自然 | [ |
| msp | msp | 2 | D1b | GMES4176-Sat_069 (Satt172) | 雄性不育,雌性可育 | 自然 | [ |
| St1 | St1 | - | - | - | 雄性不育,雌性不育 | 自然 | [ |
| St2 | St2 | 11 | B1 | BARCSOYSSR_11_122- BARCSOYSSR_11_137 | 雄性不育,雌性不育 | 自然 | [ |
| St3 | St3 | - | - | - | 雄性不育,雌性不育 | 自然 | [ |
| St4 | St4 | 1 | D1a | Satt436-Satt468 | 雄性不育,雌性不育 | 自然 | [ |
| St5 | St5 | 13 | F | Satt030-Satt146 | 雄性不育,雌性不育 | 自然 | [ |
| St6 | St6 | 14 | B2 | BARCSOYSSR_14_84- BARCSOYSSR_14_109 | 雄性不育,雌性不育 | 自然 | [ |
| St7 | St7 | 2 | D1b | Satg001-端粒 | 雄性不育,雌性不育 | 自然 | [ |
| w4-mutable line | St8 | 16 | J | Satt132-Satt414 | 雄性不育,雌性不育 | 自然 | [ |
| ms1 | ms1 | 13 | F | Satt516-Satt595 | 雄性不育,雌性可育 | 自然 | [ |
| ms2 | ms2 | 10 | O | Sat_190-Scaa001 | 雄性不育,雌性可育 | 自然 | [ |
| ms3 | ms3 | 2 | D1b | Satt157-Satt542 | 雄性不育,雌性可育 | 自然 | [ |
| ms4 | ms4 | 2 | D1b | Glyma.02G243200 | 雄性不育,雌性可育 | 自然 | [ |
| ms5 | ms5 | 11 | B1 | BARCSOYSSR_11_0122-Sat_270 | 雄性不育,雌性可育 | 诱变 | [ |
| ms6 | ms6 | 13 | F | Satt149-Satt030 | 雄性不育,雌性可育 | 自然 | [ |
| ms7 | ms7 | 9 | - | - | 雄性不育,雌性可育 | 诱变 | [ |
| ms8 | ms8 | 7 | M | 端粒-Sat_389 | 雄性不育,雌性可育 | 自然 | [ |
| ms9 | ms9 | 3 | N | Satt521-Satt237 | 雄性不育,雌性可育 | 自然 | [ |
| NJ89-1 | ms0 | - | - | - | 雄性不育,雌性可育 | 自然 | [ |
| 88-428-BY | - | - | - | - | 光敏雄性不育 | 自然 | [ |
| N7241S | - | - | - | - | 雄性不育,雌性可育 | 自然 | [ |
| NJS-13H | msNJ | 10 | O | BARCSOYSSR_10_794- BARCSOYSSR_10_819 | 雄性不育,雌性可育 | 自然 | [ |
| msMOS | - | 2 | D1b | Satt157-Satt698 | 雄性不育,雌性可育 | 自然 | [ |
| D8804-7 | Introducing exogenous DNA | - | - | - | 雄性不育,雌性不育 | 导入外源DNA | [ |
| NJS-1H | - | - | - | - | 雄性不育,雌性不育 | 化学诱变 | [ |
| Wh921 | - | - | - | - | 雄性不育,雌性可育 | 自然 | [ |
| St-M | Mst-M | 13 | F | W1(Glyma.13G072100)-Satt516 | 雄性不育,雌性可育 | 自然 | [ |
Table 2
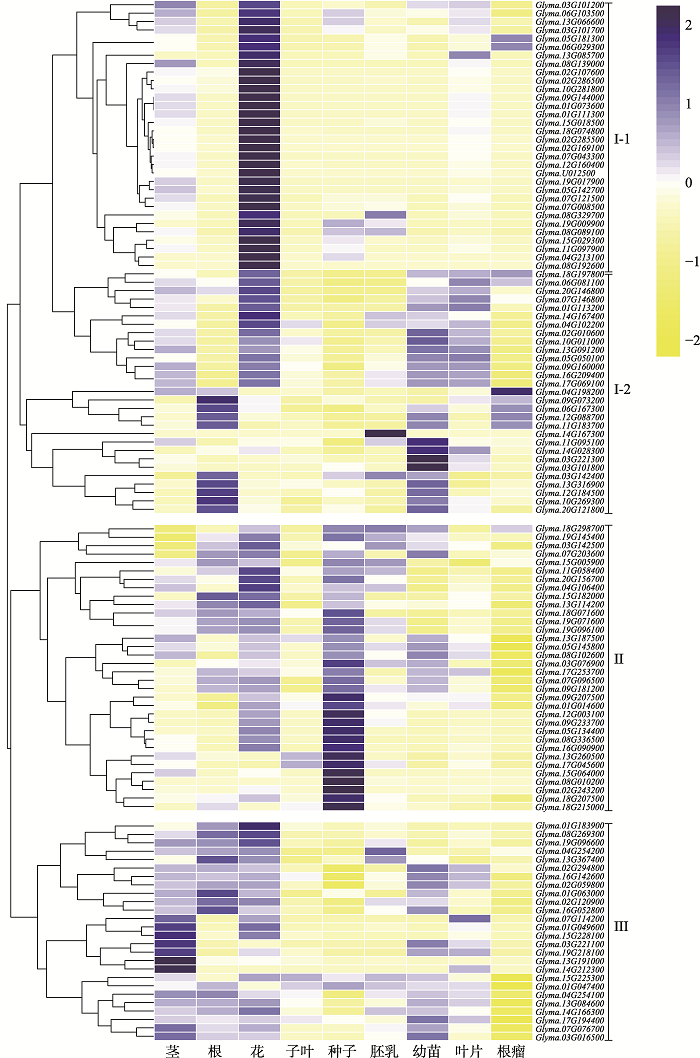
Soybean homologs of GMS genes in Arabidopsis, rice and maize"
| 拟南芥GMS基因 | 水稻GMS基因 | 玉米GMS基因 | 大豆同源基因 |
|---|---|---|---|
| Ms1 | PTC1 | ms7 | Glyma.01G047400、Glyma.02G107600 |
| Ms2 | DPW | ms6021 | Glyma.02G169100 |
| TDF1 | OsTDF1 | ms9 | Glyma.19G017900、Glyma.13G066600 |
| DYT1 | UDT1 | ms32 | Glyma.02G059800、Glyma.15G064000、Glyma.16G142600 |
| CYP704B1 | OsCYP704B2 | ms26 | Glyma.07G043300 |
| CYP703A2 | OsCYP703A3 | apv1 | Glyma.12G160400、Glyma.13G260500 |
| TPD1 | MIL2/OsTDL1a | mac1 | Glyma.04G198200、Glyma.06G167300、Glyma.08G010200 |
| TGA10 | OsTGA10 | Glyma.03G142400 | |
| EMS1 | MSP1 | Glyma.20G156700 | |
| MYB33 | OsGAMYB | Glyma.13G187500、Glyma.15G225300 | |
| MYB65 | |||
| DEX1 | OsDEX1 | Glyma.03G076900、Glyma.01G111300 | |
| AMS | TDR | Glyma.10G281800 | |
| ACOS5 | OsACOS12 | Glyma.08G329700 | |
| CalS5 | GSL5 | Glyma.04G213100 | |
| LAP5, PKSB | OsLAP5/OsPKS2 | Glyma.11G097900 | |
| LAP6, PKSA | OsLAP6/OsPKS1 | Glyma.01G073600 | |
| ABCG26 | OsABCG15/PDA1 | Glyma.18G074800 | |
| KNS4/UPEX1 | ms8 | Glyma.09G233700、Glyma.12G003100、Glyma.14G166300、 Glyma.13G084600、Glyma.04G254100 | |
| MMD1 | Glyma.02G243200(MS4)、Glyma.14G212300 | ||
| TGA9 | Glyma.12G184500、Glyma.13G316900, Glyma.12G088700、Glyma.11G183700 | ||
| MYB80 | Glyma.08G336500、Glyma.18G071600 | ||
| ATGPAT1 | Glyma.14G028300、Glyma.02G286500 | ||
| AtGPAT6 | Glyma.18G197800、Glyma.07G146800、Glyma.01G113200 | ||
| TEK | Glyma.02G285500 | ||
| ARF17 | Glyma.04G254200 | ||
| RBOHE | Glyma.15G182000、Glyma.09G073200 | ||
| CDKG1 | Glyma.05G145800、Glyma.08G102600 | ||
| AtSK32 | Glyma.07G076700、Glyma.03G016500 | ||
| LBD10 | Glyma.04G106400、Glyma.11G058400、Glyma.01G183900 | ||
| LBD27 | Glyma.08G192600 | ||
| ROXY1 | Glyma.16G052800 | ||
| ROXY2 | Glyma.19G096600 | ||
| MYB26 | Glyma.07G008500 | ||
| RPG1 | Glyma.19G009900 | ||
| DRL1/TKPR1 | Glyma.15G018500 | ||
| NEF1 | Glyma.18G298700 | ||
| CER3/FLP1/WAX2 | Glyma.17G069100、Glyma.13G091200 | ||
| CDM1 | Glyma.10G269300、Glyma.20G121800 | ||
| NPU | Glyma.18G215000 | ||
| TES | Glyma.07G096500、Glyma.09G181200、Glyma.13G114200、 Glyma.17G045600 | ||
| MIL1 | ms22 | Glyma.16G052800、Glyma.19G096600、Glyma.05G142700 | |
| TIP2/bHLH142 | ms23 | Glyma.16G090900、Glyma.08G089100、Glyma.08G269300 | |
| OsNP1 | IPE1 | Glyma.05G050100、 | |
| OsGPAT3 | ms33 | Glyma.10G011000、Glyma.03G221100、Glyma.19G218100、 Glyma.02G010600、Glyma.03G221300、Glyma.14G167300、 Glyma.14G167400、Glyma.13G085700 | |
| OsSTRL2 | ms45 | Glyma.13G367400、Glyma.15G005900、Glyma.15G029300 | |
| OsG1 | Glyma.11G095100 | ||
| OsC6 | Glyma.20G146800 | ||
| OsABCG26 | Glyma.09G160000、Glyma.16G209400 | ||
| OsDPW2 | Glyma.19G096100 | ||
| EAT1/DTD | Glyma.05G134400 | ||
| OsFIGNL1 | Glyma.19G071600 | ||
| WDA1 | Glyma.07G121500、Glyma.03G101700、 Glyma.03G101800、 Glyma.07G114200、Glyma.03G101200 | ||
| OsFTIP7 | Glyma.19G145400、Glyma.03G142500 | ||
| OsDTC1 | Glyma.18G207500 | ||
| OsDTM1 | Glyma.04G102200、Glyma.06G103500 | ||
| OsCSA | Glyma.01G049600 | ||
| MTR1 | Glyma.05G181300、Glyma.08G139000 | ||
| OsRAFTIN | Glyma.06G081100 | ||
| OsUAM3 | Glyma.02G120900、Glyma.01G063000 | ||
| OsGT1 | Glyma.06G029300、Glyma.17G253700 | ||
| CAP1 | Glyma.17G194400 | ||
| OsADF | Glyma.02G294800 | ||
| OsAPI5 | Glyma.07G203600 | ||
| Ocl4 | Glyma.01G014600、Glyma.09G207500 | ||
| IG1 | Glyma.U012500、Glyma.15G228100、Glyma.13G191000 | ||
| ms30 | Glyma.02G273900 | ||
| Ms44 | Glyma.09G144000 |
| [1] |
Kim YJ, Zhang DB . Molecular control of male fertility for crop hybrid breeding. Trends Plant Sci, 2018,23(1):53-65.
doi: 10.1016/j.tplants.2017.10.001 pmid: 29126789 |
| [2] | Zhang SQ . Advances on yield formation of hybrid wheat. Chin Agric Bull, 2019,35(6):1-5. |
| 张胜全 . 杂交小麦产量形成的研究进展. 中国农学通报, 2019,35(6):1-5. | |
| [3] |
Li JJ, Nadeem M, Sun GL, Wang XB, Qiu LJ . Male sterility in soybean: occurrence, molecular basis and utilization. Plant Breeding, 2019,138(6):659-676.
doi: 10.1111/pbr.v138.6 |
| [4] |
Chen LT, Liu YG . Male sterility and fertility restoration in crops. Annu Rev Plant Biol, 2014,65(1):579-606.
doi: 10.1146/annurev-arplant-050213-040119 |
| [5] |
Horn R, Gupta KJ, Colombo N . Mitochondrion role in molecular basis of cytoplasmic male sterility. Mitochondrion, 2014,19:198-205.
doi: 10.1016/j.mito.2014.04.004 |
| [6] |
Huang JZ, E ZG, Zhang HL, Shu QY. Workable male sterility systems for hybrid rice: genetics, biochemistry, molecular biology, and utilization. Rice, 2014,7(1):13.
doi: 10.1186/s12284-014-0013-6 pmid: 26055995 |
| [7] |
Cheng SH, Zhuang JY, Fan YY, Du JH, Cao LY . Progress in research and development on hybrid rice: a super- domesticate in China. Ann Bot, 2007,100(5):959-966.
doi: 10.1093/aob/mcm121 pmid: 17704538 |
| [8] | Xu XM, Zhang SG, Liang KJ . Progress and discussion in breeding of indica rice CMS lines in China. Chin Agric Bull, 2007,23(3):176-180. |
| 许旭明, 张受刚, 梁康迳 . 中国水稻籼型三系不育系选育的进展与讨论. 中国农学通报, 2007,23(3):176-180. | |
| [9] | Chen LY, Lei DY, Tang WB, Xiao YH . Thoughts and practice on some problems about research and application of two-line hybrid rice. Rice Sci, 2011,18(2):79-85. |
| [10] | Fu ZY, Qin YT, Tang JH . Reviews of photo-or/and thermo-sensitive genic male sterile gene in major crops. J Chin Biotechnol, 2018,38(1):115-125. |
| 付志远, 秦永田, 汤继华 . 主要作物光温敏核雄性不育基因的研究进展与应用. 中国生物工程杂志, 2018,38(1):115-125. | |
| [11] |
Chang ZY, Chen ZF, Wang N, Xie G, Lu JW, Yan W, Zhou JL, Tang XY, Deng XW . Construction of a male sterility system for hybrid rice breeding and seed production using a nuclear male sterility gene. Proc Natl Acad Sci USA, 2016,113(49):14145-14150.
doi: 10.1073/pnas.1613792113 pmid: 27864513 |
| [12] |
Thu SW, Rai KM, Sandhu D, Rajangam A, Balasubramanian VK, Palmer RG, Mendu V . Mutation in a PHD-finger protein MS4 causes male sterility in soybean. BMC Plant Biol, 2019,19(1):378.
doi: 10.1186/s12870-019-1979-4 pmid: 31455245 |
| [13] |
Johns CW, Palmer RG . Floral development of a flower- structure mutant in soybeans, Glycine max( L.) Merr.(Leguminosae). Amer J Bot, 1982,69(5):829-842.
doi: 10.1002/ajb2.1982.69.issue-5 |
| [14] |
Stelly DM, Palmer RG . A partially male-sterile mutant line of soybeans, Glycine max(L.) Merr.: characterization of the msp phenotype variation. Euphytica, 1980,29(3):539-546.
doi: 10.1007/BF00023200 |
| [15] |
Frasch RM, Weigand C, Perez PT, Palmer RG, Sandhu D . Molecular mapping of 2 environmentally sensitive male- sterile mutants in soybean. J Hered, 2011,102(1):11-16.
doi: 10.1093/jhered/esq100 |
| [16] |
Hao Y, Zhang JY, Zhang CB, Bao P, Zhang WL, Wang PN, Ding XY, Liu BH, Feng XZ, Zhao LM . Genetic effects and plant architecture influences on outcrossing rate in soybean. J Integr Agr, 2019,18(9):1971-1979.
doi: 10.1016/S2095-3119(18)62054-4 |
| [17] |
Owen FV . A sterile character in soybeans. Plant Physiol, 1928,3(2):223-226.
doi: 10.1104/pp.3.2.223 pmid: 16652565 |
| [18] |
Hadley HH, Starnes WJ . Sterility in soybeans caused by asynapsis. Crop Sci, 1964,4(4):421-424.
doi: 10.2135/cropsci1964.0011183X000400040027x |
| [19] |
Palmer RG, Kaul MLH . Genetics, cytology, and linkage studies of a desynaptic soybean mutant. J Hered, 1983,74(4):260-264.
doi: 10.1093/oxfordjournals.jhered.a109780 |
| [20] |
Palmer RG, Horner HT . Genetics and cytology of a genic male-sterile, female-sterile mutant from a transposon- containing soybean population. J Hered, 2000,91(5):378-383.
doi: 10.1093/jhered/91.5.378 pmid: 10994704 |
| [21] |
Brim CA, Young MF . Inheritance of a male-sterile character in soybeans. Crop Sci, 1971,11(4):564-566.
doi: 10.2135/cropsci1971.0011183X001100040032x |
| [22] |
Albertsen MC, Palmer RG . A comparative light-and electron-microscopic study of microsporogenesis in male sterile ( MS,) and male fertile soybeans(Glycine max (L.) Merr.). Amer J Bot 1979,66(3):253-265.
doi: 10.1002/ajb2.1979.66.issue-3 |
| [23] |
Graybosch RA, Palmer RG . Male sterility in soybean (Glycine max). I. phenotypic expression of the ms2 mutant. Amer J Bot, 1985,72(11):1738-1750.
doi: 10.1002/ajb2.1985.72.issue-11 |
| [24] |
Palmer RG, Johns CW, Muir PS . Genetics and cytology of the ms3 male-sterile soybean. J Hered, 1980,71(5):343-348.
doi: 10.1093/oxfordjournals.jhered.a109383 |
| [25] |
Delannay X, Palmer RG . Genetics and cytology of the ms4 male-sterile soybean. J Hered, 1982,73(3):219-223.
doi: 10.1093/oxfordjournals.jhered.a109621 |
| [26] |
Ott A, Yang Y, Bhattacharyya M, Horner HT, Palmer RG, Sandhu D . Molecular mapping of D1, D2 and ms5 revealed linkage between the cotyledon color locus D2 and the male-sterile locus ms5 in soybean. Plants, 2013,2(3):441-454.
doi: 10.3390/plants2030441 pmid: 27137386 |
| [27] | Skorupska H, Palmer RG . Genetics and cytology of the ms6 male-sterile soybean. J Hered, 1989,80(4):304-310. |
| [28] |
Palmer RG . Genetics of four male-sterile, female-fertile soybean mutants. Crop Sci, 2000,40(1):78-83.
doi: 10.2135/cropsci2000.40178x |
| [29] | Palmer RG, Gai JY, Sun HA, Burton JW . Production and evaluation of hybrid soybean. Plant Breed Rev, 2001,21:263-307. |
| [30] | Yang SP, Gai JY, Xu HQ . A genetical and cytomorphological study on the male sterile mutant NJ89-1 in soybeans. Soyb Sci, 1998,17(1):32-38. |
| 杨守萍, 盖钧镒, 徐汉卿 . 大豆雄性不育突变体NJ89-1的遗传学与细胞学鉴定. 大豆科学, 1998,17(1):32-38. | |
| [31] | Wang F, Wei BG, Li GQ, Li YH . A cytological observation of the pollen mother cells of the photoperiod-sensitive male sterile soybean plant of 88-428BY-827. Sci Agric Sin, 2004,37(8):1110-1113. |
| 王芳, 卫保国, 李贵全, 李艳花 . 大豆光敏雄性不育株88-428BY-827小孢子母细胞的细胞学观察. 中国农业科学, 2004,37(8):1110-1113. | |
| [32] | Zhao TJ, Yang SP, Gai JY . Discovery of a dominant nuclear male sterile mutant N7241S in soybean and analysis of its inheritance. Sci Agric Sin, 2005,38(1):22-26. |
| 赵团结, 杨守萍, 盖钧镒 . 大豆显性核雄性不育突变体N7241S的发现与遗传分析. 中国农业科学, 2005,38(1):22-26. | |
| [33] |
Wiebbecke CE, Graham MA, Cianzio SR, Palmer RG . Day temperature influences the male-sterile locus ms9 in soybean. Crop Sci, 2012,52(4):1503-1510.
doi: 10.2135/cropsci2011.08.0410 |
| [34] |
Speth B, Rogers JP, Boonyoo N, Vanmeter AJ, Baumbach J, Ott A, Moore J, Cina T, Palmer RG, Sandhu D . Molecular mapping of five soybean genes involved in male-sterility, female-sterility. Genome, 2015,58(4):143-149.
doi: 10.1139/gen-2015-0044 pmid: 26213292 |
| [35] |
Chardin C, Girin T, Roudier F, Meyer C, Krapp A . The plant RWP-RK transcription factors: key regulators of nitrogen responses and of gametophyte development. J Exp Bot, 65(19):5577-5587.
doi: 10.1093/jxb/eru261 pmid: 24987011 |
| [36] |
Kato KK, Palmer RG . Molecular mapping of the male-sterile, female-sterile mutant gene (st8) in soybean. J Hered, 2003,94(5):425-428.
doi: 10.1093/jhered/esg085 pmid: 14557397 |
| [37] |
Cervantes-Martinez I, Xu M, Zhang L, Huang Z, Kato KK, Horner HT, Palmer RG . Molecular mapping of male- sterility loci ms2 and ms9 in soybean. Crop Sci, 2007,47(1):374-379.
doi: 10.2135/cropsci2006.03.0143 |
| [38] |
Yang Y, Speth BD, Boonyoo N, Baumert E, Atkinson TR, Palmer RG, Sandhu D . Molecular mapping of three male-sterile, female-fertile mutants and generation of a comprehensive map of all known male sterility genes in soybean. Genome, 2014,57(3):155-160.
doi: 10.1139/gen-2014-0018 |
| [39] |
Nie ZX, Zhao TJ, Liu MF, Dai JY, He TT, Lyu D, Zhao JM, Yang SP, Gai JY . Molecular mapping of a novel male- sterile gene msNJ in soybean [Glycine max(L.) Merr.]. Plant Reprod, 2019,32(4):371-380.
doi: 10.1007/s00497-019-00377-6 pmid: 31620875 |
| [40] |
Cervantes-Martinez I, Sandhu D, Xu M, Ortiz-Pérez E, Kato KK, Horner HT, Palmer RG . The male sterility locus ms3 is present in a fertility controlling gene cluster in soybean. J Hered, 2009,100(5):565-570.
doi: 10.1093/jhered/esp054 pmid: 19617521 |
| [41] |
Graybosch RA, Palmer RG . Analysis of a male-sterile character in soybeans. J Hered, 1987,78(2):66-70.
doi: 10.1093/oxfordjournals.jhered.a110338 |
| [42] | Buss GR . Research Notes: Inheritance of a male-sterile mutant from irradiated Essex soybeans. Soyb Genet Newslett, 1983,10(33):104-108. |
| [43] | Zhao LM, Liu DP, Sun H, Yuan Y, Huang M . A sterile material of soybean gainned by introducing exogenous DNA. Soyb Sci, 1995,14(1):83-87. |
| 赵丽梅, 刘德璞, 孙寰, 袁英, 黄梅 . 外源DNA导入大豆获得一不育材料. 大豆科学, 1995,14(1):83-87. | |
| [44] | Li SG, Zhao TJ, Gai JY . Cytological and genetical characterization of a nuclear male-sterille soybean mutant NJS-1H. Soyb Sci, 2010,29(2):181-185. |
| 李曙光, 赵团结, 盖钧镒 . 大豆突变体NJS-1H核雄性不育性的细胞学与遗传学分析. 大豆科学, 2010,29(2):181-185. | |
| [45] | Zhang L, Huang ZP, Li JK, Dai OH . Preliminary study on soybean male sterile mutant Wh921 and its heterosis. Chin J Oil Crop Sci, 1999,21(1):20-23. |
| 张磊, 黄志平, 李杰坤, 戴瓯和 . 大豆雄性不育突变体Wh921及其杂种优势初步研究. 中国油料作物学报, 1999,21(1):20-23. | |
| [46] |
Zhao QS, Tong Y, Yang CY, Yang YQ, Zhang MC . Identification and mapping of a new soybean male-sterile gene, mst-M. Front Plant Sci, 2019,10(94):1-9.
doi: 10.3389/fpls.2019.00001 |
| [47] |
Wan XY, Wu SW, Li ZW, Dong ZY, An XL, Ma B, Tian YH, Li JP . Maize genic male-sterility genes and their applications in hybrid breeding: progress and perspectives. Mol Plant, 2019,12(3):321-342.
doi: 10.1016/j.molp.2019.01.014 pmid: 30690174 |
| [48] |
Zheng ZF, Xia Q, Dauk M, Shen WY, Selvaraj G, Zou JT . Arabidopsis AtGPAT1, a member of the membrane-bound glycerol-3-phosphate acyltransferase gene family, is essential for tapetum differentiation and male fertility. Plant Cell, 2003,15(8):1872-1887.
doi: 10.1105/tpc.012427 pmid: 12897259 |
| [49] |
Grienenberger E, Kim SS, Lallemand B, Geoffroy P, Heintz D, de Azevedo Souza C, Heitz T, Douglas CJ, Legrand M. Analysis of TETRAKETIDE α-PYRONE REDUCTASE function in Arabidopsis thaliana reveals a previously unknown, but conserved, biochemical pathway in sporopollenin monomer biosynthesis. Plant Cell, 2010,22(12):4067-4083.
doi: 10.1105/tpc.110.080036 pmid: 21193572 |
| [50] |
Yang CY, Xu ZY, Song J, Conner K, Vizcay Barrena G, Wilson ZA . Arabidopsis MYB26/MALE STERILE35 regulates secondary thickening in the endothecium and is essential for anther dehiscence. Plant Cell, 2007,19(2):534-548.
doi: 10.1105/tpc.106.046391 pmid: 17329564 |
| [51] |
Lou Y, Xu XF, Zhu J, Gu JN, Blackmore S, Yang ZN . The tapetal AHL family protein TEK determines nexine formation in the pollen wall. Nat Commun, 2014,5(1):3855.
doi: 10.1038/ncomms4855 |
| [52] |
Dobritsa AA, Shrestha J, Morant M, Pinot F, Matsuno M, Swanson R, Møller BL, Preuss D . CYP704B1 is a long-chain fatty acid omega-hydroxylase essential for sporopollenin synthesis in pollen of Arabidopsis. Plant Physiol, 2009,151(2):574-589.
doi: 10.1104/pp.109.144469 pmid: 19700560 |
| [53] |
Morant M, Jørgensen K, Schaller H, Pinot F, Møller BL, Werck-Reichhart D, Bak S . CYP703 is an ancient cytochrome P450 in land plants catalyzing in-chain hydroxylation of lauric acid to provide building blocks for sporopollenin synthesis in pollen. Plant Cell, 2007,19(5):1473-1487.
doi: 10.1105/tpc.106.045948 pmid: 17496121 |
| [54] |
Kim SS, Grienenberger E, Lallemand B, Colpitts CC, Kim SY, de Azevedo Souza C, Geoffroy P, Heintz D, Krahn D, Kaiser M, Kombrink E, Heitz T, Suh DY, Legrand M, Douglas CJ. LAP6/POLYKETIDE SYNTHASE A and LAP5/POLYKETIDE SYNTHASE B encode hydroxyalkyl α-pyrone synthases required for pollen development and sporopollenin biosynjournal in Arabidopsis thaliana. Plant Cell, 2010,22(12):4045-4066.
doi: 10.1105/tpc.110.080028 pmid: 21193570 |
| [55] |
Paxson-Sowders DM, Dodrill CH, Owen HA, Makaroff CA . DEX1, a novel plant protein, is required for exine pattern formation during pollen development in Arabidopsis. Plant Physiol, 2001,127(4):1739-1749.
pmid: 11743117 |
| [56] |
Guan YF, Huang XY, Zhu J, Gao JF, Zhang HX, Yang ZN . RUPTURED POLLEN GRAIN1, a member of the MtN3/saliva gene family, is crucial for exine pattern formation and cell integrity of microspores in Arabidopsis. Plant Physiol, 2008,147(2):852-863.
doi: 10.1104/pp.108.118026 pmid: 18434608 |
| [57] |
Kim MJ, Kim M, Lee MR, Park SK, Kim J . LATERAL ORGAN BOUNDARIES DOMAIN(LBD) 10 interacts with SIDECAR POLLEN/LBD27 to control pollen development in Arabidopsis. Plant J, 2015,81(5):794-809.
doi: 10.1111/tpj.12767 pmid: 25611322 |
| [58] |
Xu J, Yang CY, Yuan Z, Zhang DS, Gondwe MY, Ding ZW, Liang WQ, Zhang DB, Wilson ZA . The ABORTED MICROSPORES regulatory network Is required for postmeiotic male reproductive development in Arabidopsis thaliana. Plant Cell, 2010,22(1):91-107.
doi: 10.1105/tpc.109.071803 pmid: 20118226 |
| [59] |
Cai CF, Zhu J, Lou Y, Guo ZL, Xiong SX, Wang K, Yang ZN . The functional analysis of OsTDF1 reveals a conserved genetic pathway for tapetal development between rice and Arabidopsis. Sci Bull, 2015,60(12):1073-1082.
doi: 10.1007/s11434-015-0810-3 |
| [60] |
Zhao GC, Shi JX, Liang WQ, Xue FY, Luo Q, Zhu L, Qu GR, Chen MJ, Schreiber L, Zhang DB . Two ATP Binding Cassette G transporters, Rice ATP binding cassette G26 and ATP binding cassette G15, collaboratively regulate rice male reproduction. Plant Physiol, 2015,169(3):2064-2079.
doi: 10.1104/pp.15.00262 pmid: 26392263 |
| [61] |
Li H, Yuan Z, Vizcay-Barrena G, Yang CY, Liang WQ, Zong J, Wilson ZA, Zhang DB . PERSISTENT TAPETAL CELL1 encodes a PHD-finger protein that is required for tapetal cell death and pollen development in rice. Plant Physiol, 2011,156(2):615-630.
doi: 10.1104/pp.111.175760 |
| [62] |
Zhu XL, Yu J, Shi JX, Tohge T, Fernie AR, Meir S, Aharoni A, Xu DW, Zhang DB, Liang WQ . The polyketide synthase OsPKS2 is essential for pollen exine and ubisch body patterning in rice. J Integr Plant Biol, 2017,59(9):612-628.
doi: 10.1111/jipb.12574 |
| [63] |
Li YL, Li DD, Guo ZL, Shi QS, Xiong SX, Zhang C, Zhu J, Yang ZN . OsACOS12, an orthologue of Arabidopsis acyl-CoA synthetase5, plays an important role in pollen exine formation and anther development in rice. BMC Plant Biol, 2016,16(1):256.
doi: 10.1186/s12870-016-0943-9 pmid: 27871243 |
| [64] |
Tan HX, Liang WQ, Hu JP, Zhang DB . MTR1 encodes a secretory fasciclin glycoprotein required for male reproductive development in rice. Dev Cell, 2012,22(6):1127-1137.
doi: 10.1016/j.devcel.2012.04.011 pmid: 22698279 |
| [65] |
Fu ZZ, Yu J, Cheng XW, Zong X, Xu J, Chen MJ, Li ZY, Zhang DB, Liang WQ . The rice basic helix-loop-helix transcription factor TDR INTERACTING PROTEIN2 is a central switch in early anther development. Plant cell, 2014,26(4):1512-1524.
doi: 10.1105/tpc.114.123745 |
| [66] |
Zou T, Li SC, Liu MX, Wang T, Xiao Q, Chen D, Li Q, Liang YL, Zhu J, Liang YY, Deng QM, Wang SQ, Zheng AP, Wang LX, Li P . An atypical strictosidine synthase, OsSTRL2, plays key roles in anther development and pollen wall formation in rice. Sci Rep, 2017,7(1):6863.
doi: 10.1038/s41598-017-07064-4 pmid: 28761138 |
| [67] |
Moon S, Kim SR, Zhao GC, Yi J, Yoo Y, Jin P, Lee SW, Jung KH, Zhang DB, An G . Rice GLYCOSYLTRANSFERASE1 encodes a glycosyltransferase essential for pollen wall formation. Plant Physiol, 2013,161(2):663-675.
doi: 10.1104/pp.112.210948 |
| [68] |
Shi X, Sun XH, Zhang ZG, Feng D, Zhang Q, Han LD, Wu JX, Lu TG . GLUCAN SYNTHASE-LIKE 5 (GSL5) plays an essential role in male fertility by regulating callose metabolism during microsporogenesis in rice. Plant Cell Physiol, 2015,56(3):497-509.
doi: 10.1093/pcp/pcu193 pmid: 25520407 |
| [69] |
Wan XY, Wu SW, Li ZW, An XL, Tian YH . Lipid Metabolism: critical roles in male fertility and other aspects of reproductive development in plants. Mol Plant, 2020,13(7):955-983.
doi: 10.1016/j.molp.2020.05.009 pmid: 32434071 |
| [70] |
Ito T, Nagata N, Yoshiba Y, Ohme-Takagi M, Ma H, Shinozaki K . Arabidopsis MALE STERILITY1 encodes a PHD-type transcription factor and regulates pollen and tapetum development. Plant Cell, 2007,19(11):3549-3562.
doi: 10.1105/tpc.107.054536 pmid: 18032630 |
| [71] |
Zhang DF, Wu SW, An XL, Xie K, Dong ZY, Zhou Y, Xu LW, Fang W, Liu SS, Liu SS, Zhu TT, Li JP, Rao LQ, Zhao JR, Wan XY . Construction of a multicontrol sterility system for a maize male-sterile line and hybrid seed production based on the ZmMs7 gene encoding a PHD-finger transcription factor. Plant Biotechnol J, 2018,16(2):459-471.
doi: 10.1111/pbi.12786 pmid: 28678349 |
| [72] |
Lou Y, Zhou HS, Han Y, Zeng QY, Zhu J, Yang ZN . Positive regulation of AMS by TDF1 and the formation of a TDF1-AMS complex are required for anther development in Arabidopsis thaliana. New Phytol, 2018,217(1):378-391.
doi: 10.1111/nph.14790 pmid: 28940573 |
| [73] |
Ko SS, Li MJ, Ku MSB, Ho YC, Lin YJ, Chuang MH, Hsing HX, Lien YC, Yang HT, Chang HC, Chan MT . The bHLH142 transcription factor coordinates with TDR1 to modulate the expression of EAT1 and regulate pollen development in rice. Plant Cell, 2014,26(6):2486-2504.
doi: 10.1105/tpc.114.126292 |
| [74] | Lu PL, Chai MF, Yang JG, Ning G, Wang GL, Ma H . The Arabidopsis CALLOSE DEFECTIVE MICROSPORE1 gene is required for male fertility through regulating callose metabolism during microsporogenesis. Plant Physiol, 2014,164(4):1893-1904. |
| [75] |
Suzuki T, Narciso JO, Zeng W, Van De Meene A, Yasutomi M, Takemura S, Lampugnani ER, Doblin MS, Bacic A, Ishiguro S. KNS4/UPEX1: A type II Arabinogalactan β-(1,3)-Galactosyltransferase required for pollen exine development. Plant Physiol, 2017,173(1):183-205.
doi: 10.1104/pp.16.01385 pmid: 27837085 |
| [76] |
Men X, Shi JX, Liang WQ, Zhang QF, Lian GB, Quan S, Zhu L, Luo ZJ, Chen MJ, Zhang DB . Glycerol-3- Phosphate Acyltransferase 3 (OsGPAT3) is required for anther development and male fertility in rice. J Exp Bot, 2017,68(3):513-526.
doi: 10.1093/jxb/erw445 pmid: 28082511 |
| [77] |
Chen XB, Goodwin SM, Boroff VL, Liu XL, Jenks MA . Cloning and characterization of the WAX2 gene of Arabidopsis involved in cuticle membrane and wax production. Plant Cell, 2003,15(5):1170-1185.
doi: 10.1105/tpc.010926 pmid: 12724542 |
| [78] |
Zhu J, Chen H, Li H, Gao JF, Jiang H, Wang C, Guan YF, Yang ZN . Defective in Tapetal Development and Function 1 is essential for anther development and tapetal function for microspore maturation in Arabidopsis. Plant J, 2008,55(2):266-277.
doi: 10.1111/j.1365-313X.2008.03500.x pmid: 18397379 |
| [79] |
Yang CY, Spielman M, Coles JP, Li Y, Ghelani S, Bourdon V, Brown RC, Lemmon BE, Scott RJ, Dickinson HG . TETRASPORE encodes a kinesin required for male meiotic cytokinesis in Arabidopsis. Plant J, 2003,34(2):229-240.
doi: 10.1046/j.1365-313x.2003.01713.x pmid: 12694597 |
| [80] |
Nishihama R, Soyano T, Ishikawa M, Araki S, Tanaka H, Asada T, Irie K, Ito M, Terada M, Banno H, Yamazaki Y, Machida Y . Expansion of the cell plate in plant cytokinesis requires a kinesin-like protein/MAPKKK complex. Cell, 2002,109(1):87-99.
doi: 10.1016/s0092-8674(02)00691-8 pmid: 11955449 |
| [81] | Wang SM, Sun H, Zhao LM, Wang YQ, Peng B, Fan XH, Zhang BS. Progress and problem analysis on soybean male sterility and heterosis exploitation in China. Soyb Sci, 2009, 28(6): 1089-1096+1102. |
| 王曙明, 孙寰, 赵丽梅, 王跃强, 彭宝, 范旭红, 张宝石 . 中国大豆雄性不育和杂种优势利用研究进展与问题分析. 大豆科学, 2009, 28(6): 1089-1096+1102. | |
| [82] | Wu SW, Wang XY . Using biotechnology to establish the technical system of male sterile cross breeding and seed production of main crops. J Chin Biotechnol, 2018,38(1):78-87. |
| 吴锁伟, 万向元 . 利用生物技术创建主要作物雄性不育杂交育种和制种的技术体系. 中国生物工程杂志, 2018,38(1):78-87. | |
| [83] | Hou BK, Yu HM, Xia GM . Expression vector used in chloroplast genetic transformation. Hereditas(Beijing), 2002,24(1):100-103. |
| 侯丙凯, 于惠敏, 夏光敏 . 用于叶绿体遗传转化的表达载体. 遗传, 2002,24(1):100-103. | |
| [84] | Li XQ, Yuan LP, Deng QY, Xiao JH . Potential ways to use spontaneous genic male sterility in the molecular breeding of hybrid crops. Bull Bot, 2003,20(5):625-631. |
| 李新奇, 袁隆平, 邓启云, 肖金华 . 在杂交作物分子育种中利用普通核雄性不育的几个可能途径. 植物学报, 2003,20(5):625-631. | |
| [85] | Li XQ, Zhao CP, Xiao JH, Xie FM . Technical analysis of utilization of spontaneous and artificial genic male sterility in molecular breeding of hybrid crops. Sci Technol Rev, 2006,24(11):39-44. |
| 李新奇, 赵昌平, 肖金华, 谢放鸣 . 基因转化创造植物杂种优势利用新方式的途径分析. 科技导报, 2006,24(11):39-44. |
| [1] | Biwei Lai, Lei Chen, Sijia Lu. The current status of photoperiod adaptability in soybean [J]. Hereditas(Beijing), 2023, 45(9): 793-800. |
| [2] | Zhongling Wen, Minkai Yang, Xingyu Chen, Chenyu Hao, Ran Ren, Shujuan Chu, Hongwei Han, Hongyan Lin, Guihua Lu, Jinliang Qi, Yonghua Yang. Bacterial composition, function and the enrichment of plant growth promoting rhizobacteria (PGPR) in differential rhizosphere compartments of Al-tolerant soybean in acidic soil [J]. Hereditas(Beijing), 2021, 43(5): 487-500. |
| [3] | Zhuozhuo Mao, Yu Gong, Guixia Shi, Yali Li, Deyue Yu, Fang Huang. Cloning of the soybean E2 ubiquitin-conjugating enzyme GmUBC1 and its expression in Arabidopsis thaliana [J]. Hereditas(Beijing), 2020, 42(8): 788-798. |
| [4] | Yongyao Xie,Jintao Tang,Bowen Yang,Jun Hu,Yao-Guang Liu,Letian Chen. Current advance on molecular genetic regulation of rice fertility [J]. Hereditas(Beijing), 2019, 41(8): 703-715. |
| [5] | Huiqing Li, Chao Chen, Ranran Chen, Xuewei Song, Jina Li, Yanming Zhu, Xiaodong Ding. Preliminary analysis of the role of GmSnRK1.1 and GmSnRK1.2 in the ABA and alkaline stress response of the soybean using the CRISPR/Cas9-based gene double-knockout system [J]. Hereditas(Beijing), 2018, 40(6): 496-507. |
| [6] | Nan Wang, Shizhen Zhao, Menghua Lv, Fengning Xiang, Shuo Li. Research progress on identification of QTLs and functional genes involved in salt tolerance in soybean [J]. Hereditas(Beijing), 2016, 38(11): 992-1003. |
| [7] | Dan Zhang,Haina Song,Hao Cheng,Deyue Yu. Mapping and cloning of low phosphorus tolerance genes in soybeans [J]. HEREDITAS(Beijing), 2015, 37(4): 336-343. |
| [8] | Hongmei Qiu, Wenyuan Hao, Shuqin Gao, Xiaoping Ma, Yuhong Zheng, Fanfan Meng, Xuhong Fan, Yang Wang, Yueqiang Wang, Shuming Wang. Gene mining of sulfur-containing amino acid metabolic enzymes in soybean [J]. HEREDITAS(Beijing), 2014, 36(9): 934-942. |
| [9] | Nan Wu, Piwu Wang, Dan Li, Liqiang Dai, Chengzhong Zheng, Shi Lu, Yuan Cai, Zhuo Zhang, Jing Qu, Haifeng Xia. Function of chalcone reductase gene CHR1 in soybean [J]. HEREDITAS(Beijing), 2014, 36(7): 707-712. |
| [10] | TAN Bing GUO Yong QIU Li-Juan. Whole genome discovery of genes related to branching and co-localization with QTLs in soybean [J]. HEREDITAS, 2013, 35(6): 793-804. |
| [11] | SHEN Yan, HUANG Peng, ZHANG Bo. A protocol for TALEN construction and gene targeting in zebrafish [J]. HEREDITAS, 2013, 35(4): 533-544. |
| [12] | GAO Li-Fang GUO Yong HAO Zai-Bin QIU Li-Juan. Integration and “Overview” analysis of QTLs related to plant height in soybean [J]. HEREDITAS, 2013, 35(2): 215-224. |
| [13] | SONG Jian, GUO Yong, YU Li-Jie, QIU Li-Juan. Progress in genes related to seed-coat color in soybean [J]. HEREDITAS, 2012, 34(6): 687-694. |
| [14] | SONG Bing, WANG Pi-Wu, FU Yong-Ping, FAN Xu-Hong, XIA Hai-Feng, GAO-Wei, HONG Yang, WANG He, ZHANG Zhuo, MA Jian. Cloning and functional analysis of SCTF-1 encoding a C2H2-type zinc finger protein from soybean [J]. HEREDITAS(Beijing), 2012, 34(6): 749-756. |
| [15] | YANG Ze-Mao, XIE Xiao-Fang, HUANG Xian-Bo, WANG Feng-Qing, TONG Zhi-Jun, DUAN Yuan-Ling, LAN Tao, WU Wei-Ren. Mapping of Sanming dominant genic male sterility gene in rice [J]. HEREDITAS, 2012, 34(5): 615-620. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||