Hereditas(Beijing) ›› 2022, Vol. 44 ›› Issue (2): 117-133.doi: 10.16288/j.yczz.21-253
• Review • Previous Articles Next Articles
Research progress on neural crest cells and neurocristopathies and its pathogenesis
Zhuoyuan Jiang1( ), Yan Zha1, Xiaofeng Shi3,4, Yongbiao Zhang2,3,4(
), Yan Zha1, Xiaofeng Shi3,4, Yongbiao Zhang2,3,4( )
)
- 1. School of Biological Science and Medical Engineering, Beihang University, Beijing 100000, China
2. School of Medicine and Engineering, Beihang University, Beijing 100000, China
3. Beijing Advanced Innovation Center for Big Data-Based Precision Medicine, Beihang University, Beijing 100000, China
4. Key Laboratory of Big Data-Based Precision Medicine (Beihang University), Ministry of Industry and Information Technology, Beijing 100000, China;
-
Received:2021-09-29Revised:2021-12-11Online:2022-02-20Published:2022-01-04 -
Contact:Zhang Yongbiao E-mail:SY1910307@buaa.edu.cn;zhangyongbiao@buaa.edu.cn -
Supported by:Supported by the National Natural Science Foundation of China Nos(82171844);Supported by the National Natural Science Foundation of China Nos(81970898);Beijing Natural Science Foundation Project No(7204273)
Cite this article
Zhuoyuan Jiang, Yan Zha, Xiaofeng Shi, Yongbiao Zhang. Research progress on neural crest cells and neurocristopathies and its pathogenesis[J]. Hereditas(Beijing), 2022, 44(2): 117-133.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
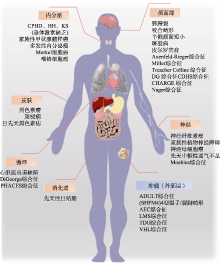
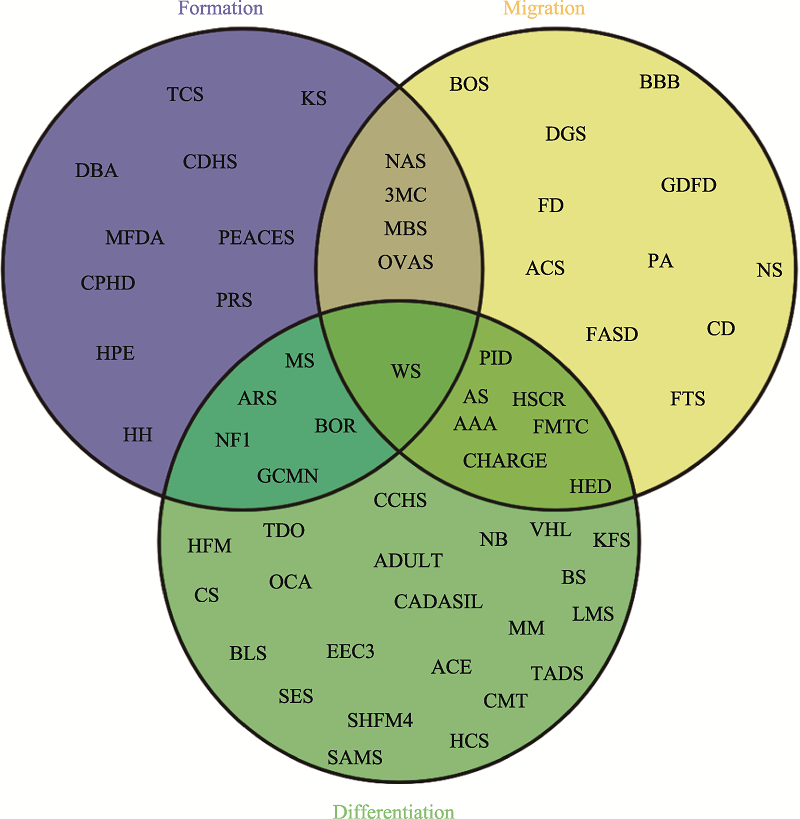
Table 1
The affected organs and phenotypes of neurocristopathies"
| 受累部位 | 临床表型 | 神经嵴病 | 参考文献 |
|---|---|---|---|
| 颅面部 | 眼:眼歪斜,睫毛稀疏,眼色素异常 耳:耳道和耳廓发育不全(小耳畸形) 颌:上颌骨发育不良(腭裂) | TCS、CS、HFM Cleft lip and palate、 CHARGE | [ |
| 内脏器官 | 贫血、血管畸形、心脏缺损、心脏流出道缺损、肝损伤、肠梗阻、肾功能不全、 呼吸障碍 | DBA、CADSIL、 PHACES、Alagille HSCR、AAA、CCHS | [ |
| 四肢 | 四肢骨骼发育异常、手指/脚趾畸形、运动能力发育迟缓 | NAS、CHARGE | [ |
| 皮肤 | 色斑皮肤、白化病皮肤、黑色素瘤、胸部骨骼异常、头发稀疏、生殖器畸形 | NS、OCA、Piebaldism HED、CHARGE | [ |
| 整体 | 生长发育迟缓 | CPHD、GDFD | [ |
Table 3
The abnormal GRN causes NCPs"
| 神经嵴细胞发育阶段 | 调控基因 | 神经嵴病 | 相关信息 | 参考文献 |
|---|---|---|---|---|
| 神经嵴细胞的形成 | SOX9 | PRS | 转录因子、骨骼发育、颅面部畸形 | [ |
| PAX3 | WS、CDHS | 转录因子、骨骼、肌肉、黑色素发育异常 | [ | |
| TFAP2A | BOFS | 调节AP-2α转录因子、PA1/2发育、鳃-眼-面畸形 | [ | |
| TCOF1 POLR1C/D | TCS | 核糖体合成异常、NCCs异常凋亡、颅面部畸形 | [ | |
| RPs | DBA | 核糖体合成异常、造血细胞凋亡、骨髓异常 | [ | |
| DHODH | MS | 核糖体合成异常、颅面部、四肢骨骼异常 | [ | |
| SF3B4 | NS | mRNA合成异常、面部、四肢骨骼异常、BMP通路 | [ | |
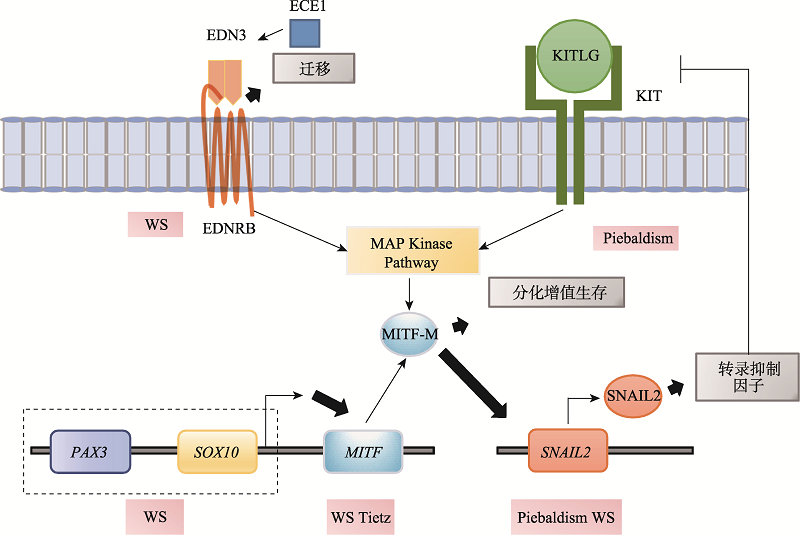
| 神经嵴细胞的迁移 | Snail2 | Piebaldism、WS | KIT和E-cadherin转录抑制因子、黑色素细胞迁移 | [ |
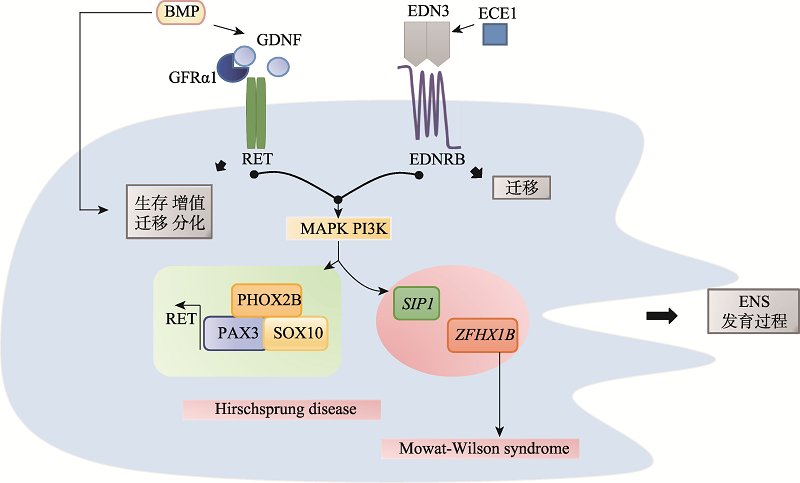
| EDNRB END3 | HSCR、WS | Eph/Ephrin信号、NCCs迁移、肠神经和黑色素细胞 | [ | |
| MID1 | BBB | 微管形成、蛋白代谢异常、NCCs迁移受阻 | [ | |
| ELP1 | FD | 细胞骨架的形成、自主神经元的迁移 | [ | |
| 神经嵴细胞的分化 | PHOX2B | NB、CDHS、HSCR | 神经元的形成、调节神经元成熟和分化、自主神经 | [ |
| MITF | WS、Tietz | 转录因子、黑色素细胞发育、影响破骨细胞发育 | [ | |
| SOX10 | WS、HSCR 黑色素瘤 | 转录因子、肠道神经元形成和黑色素细胞发育 | [ | |
| KIT | Piebaldism | 激活RET和Nanog通路、黑色素细胞分化异常 | [ | |
| NOTCH3 | CADSIL | 血管平滑肌细胞的形成、影响脑部血管发育 | [ | |
| FGF8 FDFR1 | HH、KS、CPHD | 促性腺激素释放激素神经元发育、嗅觉神经元发育 | [ | |
| TP63 | AEC、LMS、ADULT | 肿瘤蛋白p63、外胚层、口腔和肢体发育 | [ |
Table 4
Epigenetic regulation of neural crest development"
| 修饰类型 | 影响基团 | 基因 | 靶基因 | 神经嵴发育过程 | 神经嵴病 | 参考文献 | |
|---|---|---|---|---|---|---|---|
| DNA甲基化 | - | DNMT3A | Sox2、Sox3 | NCCs发育 | - | [ | |
| - | DNMT3B | - | NCCs分化 | ICF、HSCR | [ | ||
| 组蛋白 修饰 | 甲基化 | H3K27me3 | Aebp2-PRC2 | Ret、Gdnf、Ednrb、Sox10、Bmp4、 Pax3、Snail2、Phox2b、Zfhx1b | NCCs特化, 迁移 | - | [ |
| H3K9me3、H3K36me3 | KDM4A | Sox10、Snail2 | NCCs发育、 黑色素细胞发育 | - | |||
| H3K20me1、H3K9me1/2 | PHF8 | MSX1、MSXB | 颅NCCs发育 | 唇腭裂 | |||
| H3K36me3 | WHSC1、WHSC2、 LETM1 | Pdgfra | 迷走NCCs发育、 心脏发育异常 | Wolf-Hirschhorn 综合征 | |||
| H3K9 | EHMT1 | 迷走NCCs发育 | Kleefstra综合征 | [ | |||
| H3K4me1/2、H3K27 | KMT2D、UTX、KDM6A | - | 迷走NCCs分化 | 歌舞伎综合征 | [ | ||
| 乙酰化 | HDAC1 | FoxD3 | 黑色素细胞发育 | [ | |||
| - | HDAC3 | - | 迷走NCCs发育 | - | [ | ||
| - | HDAC4 | - | 颅NCCs发育 | - | [ | ||
| - | HDAC8 | Otx2、Lhx1 | 颅NCCs发育 | BDMR | [ | ||
| H3KAc | PHD12、 Sin3A、HDAC | Cad6b | NCCs迁移 | - | [ | ||
| 变异 | H3.3 | - | - | 颅NCCs间充质 | - | [ | |
| 染色质 修饰 | - | CHD7 | Sox9、Twist、Snail2 | NCCs发育 | CHARGE | [ | |
| - | WSTF | - | NCCs迁移、数量维持 | WS | [ | ||
| SWI/SNF complex | Brg1 | - | NCCs诱导、分化 | Coffin-Siris 综合征 | [ | ||
| miRNA 调控 | miR-1、miR-23a | Sec63 | CNCCs迁移、分化 | - | [ | ||
| - | - | TFAP2A | 第一二鳃弓发育 | BOFS | [ | ||
| miR-34B、miR-146a、miR-196a2、miR-200A、miR-141、miR-192、miR-195、miR-206、miR-218-1 | - | - | NCCs迁移、增值 | HSCR | [ | ||
| miR-140、miR-96、miR-141-200a、miR-429 | - | Pdgfra、Tbx1、 Dlx5、FoxD3 | 分化软骨细胞、 分化色素细胞 | - | [ | ||
| [1] | Bae CJ, Saint-Jeannet JP. Induction and specification of neural crest cells: extracellular signals and transcriptional switches. Neural Crest Cells, 2014, 27-49. |
| [2] |
Martik ML, Gandhi S, Uy BR, Gillis JA, Green SA, Simoes-Costa M, Bronner ME. Evolution of the new head by gradual acquisition of neural crest regulatory circuits. Nature, 2019, 574(7780):675-678.
doi: 10.1038/s41586-019-1691-4 |
| [3] |
Martik ML, Bronner ME. Riding the crest to get a head: neural crest evolution in vertebrates. Nat Rev Neurosci, 2021, 22(10):616-626.
doi: 10.1038/s41583-021-00503-2 pmid: 34471282 |
| [4] |
Hoppler S, Wheeler GN. DEVELOPMENTAL BIOLOGY. It's about time for neural crest. Science, 2015, 348(6241):1316-1317.
doi: 10.1126/science.aab2719 pmid: 25931447 |
| [5] |
Yuan Y, Loh YHE, Han X, Feng JF, Ho TV, He JZ, Jing JJ, Groff K, Wu AL, Chai Y. Spatiotemporal cellular movement and fate decisions during first pharyngeal arch morphogenesis. Sci Adv, 2020, 6(51): eabb0119.
doi: 10.1126/sciadv.abb0119 |
| [6] |
Soldatov R, Kaucka M, Kastriti ME, Petersen J, Chontorotzea T, Englmaier L, Akkuratova N, Yang YS, Häring M, Dyachuk V, Bock C, Farlik M, Piacentino ML, Boismoreau F, Hilscher MM, Yokota C, Qian XY, Nilsson M, Bronner ME, Croci L, Hsiao WY, Guertin DA, Brunet JF, Consalez GG, Ernfors P, Fried K, Kharchenko PV, Adameyko I. Spatiotemporal structure of cell fate decisions in murine neural crest. Science, 2019, 364(6444): eaas9536.
doi: 10.1126/science.aas9536 |
| [7] |
Zhu YL, Crowley SC, Latimer AJ, Lewis GM, Nash R, Kucenas S. Migratory neural crest cells phagocytose dead cells in the developing nervous system. Cell, 2019, 179(1): 74-89.e10.
doi: 10.1016/j.cell.2019.08.001 |
| [8] | Le Douarin NM, Dupin E. The neural crest, a fourth germ layer of the vertebrate embryo: significance in chordate evolution. Neural Crest Cells, 2014, 3-26. |
| [9] |
Bolande RP. The neurocristopathies: A unifying concept of disease arising in neural crest maldevelopment. Hum Pathol, 1974, 5(4):409-429.
doi: 10.1016/S0046-8177(74)80021-3 |
| [10] | Trainor P, Krumlauf R, Bronner-Fraser M. 19 - Neural Crest Cells. In: Handbook of Stem Cells. Edited by Lanza R, Gearhart J, Hogan B, Melton D, Pedersen R, Thomson J, West M. Burlington: Academic Press, 2004, 219-232. |
| [11] |
Vega-Lopez GA, Cerrizuela S, Tribulo C, Aybar MJ. Neurocristopathies: New insights 150 years after the neural crest discovery. Dev Biol, 2018, 444(Suppl 1):S110-S143.
doi: 10.1016/j.ydbio.2018.05.013 |
| [12] |
Alkobtawi M, Pla P, Monsoro-Burq AH. BMP signaling is enhanced intracellularly by FHL3 controlling WNT- dependent spatiotemporal emergence of the neural crest. Cell Rep, 2021, 35(12):109289.
doi: 10.1016/j.celrep.2021.109289 pmid: 34161771 |
| [13] |
Copeland J, Simoes-Costa M. Post-transcriptional tuning of FGF signaling mediates neural crest induction. Proc Natl Acad Sci USA, 2020, 117(52):33305-33316.
doi: 10.1073/pnas.2009997117 |
| [14] |
Minoux M, Rijli FM. Molecular mechanisms of cranial neural crest cell migration and patterning in craniofacial development. Development, 2010, 137(16):2605-2621.
doi: 10.1242/dev.040048 |
| [15] |
Dyson Y, Holmes A, Li A, Kulesa PM. A chemotactic model of trunk neural crest cell migration. Genesis, 2018, 56(9):e23239.
doi: 10.1002/dvg.v56.9 |
| [16] |
Burns AJ, Le Douarin NM. Enteric nervous system development: analysis of the selective developmental potentialities of vagal and sacral neural crest cells using quail-chick chimeras. Anat Rec, 2010, 262(1):16-28.
doi: 10.1002/(ISSN)1097-0185 |
| [17] |
Szabó A, Mayor R. Mechanisms of neural crest migration. Annu Rev Genet, 2018, 52:43-63.
doi: 10.1146/genet.2018.52.issue-1 |
| [18] |
Simões-Costa M, Bronner ME. Insights into neural crest development and evolution from genomic analysis. Genome Res, 2013, 23(7):1069-1080.
doi: 10.1101/gr.157586.113 pmid: 23817048 |
| [19] |
Reed RJ. Cutaneous manifestations of neural crest disorders (neurocristopathies). Int J Dermatol, 1977, 16(10):807-826.
pmid: 340393 |
| [20] | A Vega-Lopez G, J Aybar M. Neurocristopathies: how new discoveries in neural crest research changed our understanding. Cell Dev Biol, 2018, 7(2). |
| [21] |
Cordero DR, Brugmann S, Chu Y, Bajpai R, Jame M, Helms JA. Cranial neural crest cells on the move: their roles in craniofacial development. Am J Med Genet A, 2011, 155A(2):270-279.
doi: 10.1002/ajmg.a.33702 pmid: 21271641 |
| [22] |
Xu XP, Wang BQ, Jiang ZY, Chen Q, Mao K, Shi XF, Yan C, Hu JT, Zha Y, Ma C, Zhang J, Guo R, Wang LG, Zhao SQ, Liu HS, Zhang QG, Zhang YB. Novel risk factors for craniofacial microsomia and assessment of their utility in clinic diagnosis. Hum Mol Genet, 2021, 30(11):1045-1056.
doi: 10.1093/hmg/ddab055 |
| [23] |
Zhang DC, Ighaniyan S, Stathopoulos L, Rollo B, Landman K, Hutson J, Newgreen D. The neural crest: a versatile organ system. Birth Defects Res C Embryo Today, 2014, 102(3):275-298.
doi: 10.1002/bdrc.v102.3 |
| [24] |
Schulz Y, Wehner P, Opitz L, Salinas-Riester G, Bongers EMHF, van Ravenswaaij-Arts CMA, Wincent J, Schoumans J, Kohlhase J, Borchers A, Pauli S. CHD7, the gene mutated in CHARGE syndrome, regulates genes involved in neural crest cell guidance. Hum Genet, 2014, 133(8):997-1009.
doi: 10.1007/s00439-014-1444-2 |
| [25] |
Mort RL, Jackson IJ, Patton EE. The melanocyte lineage in development and disease. Development, 2015, 142(7):1387.
doi: 10.1242/dev.123729 |
| [26] |
Kelberman D, Rizzoti K, Lovell-Badge R, Robinson ICAF, Dattani MT. Genetic regulation of pituitary gland development in human and mouse. Endocr Rev, 2009, 30(7):790-829.
doi: 10.1210/er.2009-0008 pmid: 19837867 |
| [27] |
Tortora C, Meazzini MC, Garattini G, Brusati R. Prevalence of abnormalities in dental structure, position, and eruption pattern in a population of unilateral and bilateral cleft lip and palate patients. Cleft Palate Craniofac J, 2008, 45(2):154-162.
doi: 10.1597/06-218.1 |
| [28] |
Li X, Hu JT, Zhang J, Jin Q, Wang DM, Yu J, Zhang QG, Zhang YB. Genome-wide linkage study suggests a susceptibility locus for isolated bilateral microtia on 4p15. 32-4p16. 2. PLoS One, 2014, 9(7):e101152.
doi: 10.1371/journal.pone.0101152 |
| [29] |
Onwochei BC, Simon JW, Bateman JB, Couture KC, Mir E. Ocular colobomata. Surv Ophthalmol, 2000, 45(3):175-194.
pmid: 11094243 |
| [30] |
SooHoo JR, Davies BW, Allard FD, Durairaj VD. Congenital ptosis. Surv Ophthalmol, 2014, 59(5):483-492.
doi: 10.1016/j.survophthal.2014.01.005 |
| [31] | Jones NC, Lynn ML, Gaudenz K, Sakai D, Aoto K, Rey JP, Glynn EF, Ellington L, Du CY, Dixon J, Dixon MJ, Trainor PA. Prevention of the neurocristopathy Treacher Collins syndrome through inhibition of p53 function. Nat Med, 2008, 14(2):125-133. |
| [32] |
Sakai D, Trainor PA. Face off against ROS: Tcof1/Treacle safeguards neuroepithelial cells and progenitor neural crest cells from oxidative stress during craniofacial development. Dev Growth Differ, 2016, 58(7):577-585.
doi: 10.1111/dgd.12305 |
| [33] |
Selleck MA, Bronner-Fraser M. Origins of the avian neural crest: the role of neural plate-epidermal interactions. Development, 1995, 121(2):525-538.
pmid: 7768190 |
| [34] |
Zhang YB, Hu JT, Zhang J, Zhou X, Li X, Gu CH, Liu T, Xie YC, Liu JQ, Gu ML, Wang PP, Wu TT, Qian J, Wang Y, Dong XQ, Yu J, Zhang QG. Genome-wide association study identifies multiple susceptibility loci for craniofacial microsomia. Nat Commun, 2016, 7:10605.
doi: 10.1038/ncomms10605 |
| [35] | Wilson DN, Doudna Cate JH. The structure and function of the eukaryotic ribosome. Cold Spring Harb Perspect Biol, 2012, 4(5):a011536. |
| [36] |
Narla A, Hurst SN, Ebert BL. Ribosome defects in disorders of erythropoiesis. Int J Hematol, 2011, 93(2):144-149.
doi: 10.1007/s12185-011-0776-0 |
| [37] |
Selvi R, Mukunda PA. Role of SOX9 in the etiology of Pierre-Robin syndrome. Iran J Basic Med Sci, 2013, 16(5):700-704.
pmid: 23826492 |
| [38] |
Boudjadi S, Chatterjee B, Sun WY, Vemu P, Barr FG. The expression and function of PAX3 in development and disease. Gene, 2018, 666:145-157.
doi: S0378-1119(18)30469-4 pmid: 29730428 |
| [39] |
Li H, Sheridan R, Williams T. Analysis of TFAP2A mutations in Branchio-Oculo-Facial Syndrome indicates functional complexity within the AP-2α DNA-binding domain. Hum Mol Genet, 2013, 22(16):3195-3206.
doi: 10.1093/hmg/ddt173 |
| [40] | Dixon J, Trainor PA, Dixon MJ. TCOF1 (treacle) and the treacher-collins syndrome. In: The Molecular Basis of Clinical Disorders of Morphogenesis. Oxford University Press. 2016. |
| [41] |
Dauwerse JG, Dixon J, Seland S, Ruivenkamp CAL, van Haeringen A, Hoefsloot LH, Peters DJM, Boers ACD, Daumer-Haas C, Maiwald R, Zweier C, Kerr B, Cobo AM, Toral JF, Hoogeboom AJM, Lohmann DR, Hehr U, Dixon MJ, Breuning MH, Wieczorek D. Mutations in genes encoding subunits of RNA polymerases I and III cause Treacher Collins syndrome. Nat Genet, 2011, 43(1):20-22.
doi: 10.1038/ng.724 |
| [42] | Engidaye G, Melku M, Enawgaw B. Diamond blackfan anemia: genetics, pathogenesis, diagnosis and treatment. EJIFCC, 2019, 30(1):67-81. |
| [43] | Chang CF, Schock EN, Billmire DA, Brugmann SA. Craniofacial syndromes: etiology, impact and treatment. In: Principles of Developmental Genetics (Second Edition). Oxford: Academic Press, 2015, 653-676. |
| [44] |
Cassina M, Cerqua C, Rossi S, Salviati L, Martini A, Clementi M, Trevisson E. A synonymous splicing mutation in the SF3B4 gene segregates in a family with highly variable Nager syndrome. Eur J Hum Genet, 2017, 25(3):371-375.
doi: 10.1038/ejhg.2016.176 pmid: 27966544 |
| [45] |
Shi JL, Severson C, Yang JX, Wedlich D, Klymkowsky MW. Snail2 controls mesodermal BMP/Wnt induction of neural crest. Development, 2011, 138(15):3135-3145.
doi: 10.1242/dev.064394 |
| [46] |
Sánchez-Mejías A, Fernández RM, López-Alonso M, Añtinolo G, Borrego S. New roles of EDNRB and EDN3 in the pathogenesis of Hirschsprung disease. Genet Med, 2010, 12(1):39-43.
doi: 10.1097/GIM.0b013e3181c371b0 pmid: 20009762 |
| [47] |
Fontanella B, Russolillo G, Meroni G. MID1 mutations in patients with X-linked Opitz G/BBB syndrome. Hum Mutat, 2008, 29(5):584-594.
doi: 10.1002/humu.20706 pmid: 18360914 |
| [48] |
Ghosh S, Li L, Tourtellotte WG. Retrograde nerve growth factor signaling abnormalities and the pathogenesis of familial dysautonomia. Neural Regen Res, 2021, 16(9):1795-1796.
doi: 10.4103/1673-5374.306081 |
| [49] |
Bachetti T, Ceccherini I. Causative and common PHOX2B variants define a broad phenotypic spectrum. Clin Genet, 2020, 97(1):103-113.
doi: 10.1111/cge.13633 pmid: 31444792 |
| [50] |
Lakhdar Y, El Houda HA, Mounji H, Elfakiri M, Rochdi Y, Moutaouakil A, Raji A. The Tietz syndrome associated with cardiac malformation: a case report with literature review. Egypt J Otolaryngol 2021, 37(1):112.
doi: 10.1186/s43163-021-00176-9 |
| [51] |
Pingault V, Zerad L, Bertani-Torres W, Bondurand N. SOX10: 20 years of phenotypic plurality and current understanding of its developmental function. J Med Genet, 2021, DOI: 10.1136/jmedgenet-2021-108105.
doi: 10.1136/jmedgenet-2021-108105 |
| [52] |
Agarwal S, Ojha A. Piebaldism: A brief report and review of the literature. Indian Dermatol Online J, 2012, 3(2):144-147.
doi: 10.4103/2229-5178.96722 |
| [53] |
Papakonstantinou E, Bacopoulou F, Brouzas D, Megalooikonomou V, D'Elia D, Bongcam-Rudloff E, Vlachakis D. NOTCH3 and CADASIL syndrome: a genetic and structural overview. EMBnet J, 2019, 24:e921.
doi: 10.14806/ej.24.0.921 |
| [54] |
Raivio T, Avbelj M, McCabe MJ, Romero CJ, Dwyer AA, Tommiska J, Sykiotis GP, Gregory LC, Diaczok D, Tziaferi V, Elting MW, Padidela R, Plummer L, Martin C, Feng B, Zhang CK, Zhou QY, Chen HB, Mohammadi M, Quinton R, Sidis Y, Radovick S, Dattani MT, Pitteloud N. Genetic overlap in Kallmann syndrome, combined pituitary hormone deficiency, and septo-optic dysplasia. J Clin Endocrinol Metab, 2012, 97(4):E694-E699.
doi: 10.1210/jc.2011-2938 |
| [55] |
Chacon-Camacho OF, Fuerte-Flores BI, Zenteno JC. TP63 mutation in a patient with acro-dermo-ungual- lacrimal- tooth syndrome: additional evidence of molecular overlap of the ADULT and EEC syndromes. Am J Med Genet A, 2016, 170(6):1635-1638.
doi: 10.1002/ajmg.a.37642 pmid: 27028492 |
| [56] |
Baggiolini A, Varum S, Mateos JM, Bettosini D, John N, Bonalli M, Ziegler U, Dimou L, Clevers H, Furrer R, Sommer L. Premigratory and migratory neural crest cells are multipotent in vivo. Cell Stem Cell, 2015, 16(3):314-322.
doi: 10.1016/j.stem.2015.02.017 pmid: 25748934 |
| [57] | Theveneau E, Mayor R. Neural crest cell migration: guidance, pathways, and cell-cell interactions. Neural Crest Cells, 2014, 73-88. |
| [58] |
Grill C, Bergsteinsdóttir K, Ogmundsdóttir MH, Pogenberg V, Schepsky A, Wilmanns M, Pingault V, Steingrímsson E. MITF mutations associated with pigment deficiency syndromes and melanoma have different effects on protein function. Hum Mol Genet, 2013, 22(21):4357-4367.
doi: 10.1093/hmg/ddt285 |
| [59] | Strobl-Mazzulla PH, Bronner ME. Epigenetic regulation of neural crest cells. Neural Crest Cells, 2014, 89-100. |
| [60] |
Hu N, Strobl-Mazzulla P, Sauka-Spengler T, Bronner ME. DNA methyltransferase3A as a molecular switch mediating the neural tube-to-neural crest fate transition. Genes Dev, 2012, 26(21):2380-2385.
doi: 10.1101/gad.198747.112 |
| [61] |
Jin BL, Tao Q, Peng JR, Soo HM, Wu W, Ying JM, Fields CR, Delmas AL, Liu XF, Qiu JX, Robertson KD. DNA methyltransferase 3B (DNMT3B) mutations in ICF syndrome lead to altered epigenetic modifications and aberrant expression of genes regulating development, neurogenesis and immune function. Hum Mol Genet, 2008, 17(5):690-709.
doi: 10.1093/hmg/ddm341 |
| [62] |
Yu C, Yao XM, Zhao JL, Wang P, Zhang Q, Zhao CJ, Yao SH, Wei YQ. Wolf-Hirschhorn syndrome candidate 1 (whsc1) functions as a tumor suppressor by governing cell differentiation. Neoplasia, 2017, 19(8):606-616.
doi: 10.1016/j.neo.2017.05.001 |
| [63] |
Kleefstra T, Brunner HG, Amiel J, Oudakker AR, Nillesen WM, Magee A, Geneviève D, Cormier-Daire V, van Esch H, Fryns JP, Hamel BCJ, Sistermans EA, de Vries BBA, van Bokhoven H. Loss-of-function mutations in euchromatin histone methyl transferase 1 (EHMT1) cause the 9q34 subtelomeric deletion syndrome. Am J Hum Genet, 2006, 79(2):370-377.
pmid: 16826528 |
| [64] |
Shpargel KB, Starmer J, Wang CC, Ge K, Magnuson T. UTX-guided neural crest function underlies craniofacial features of Kabuki syndrome. Proc Natl Acad Sci USA, 2017, 114(43):E9046-E9055.
doi: 10.1073/pnas.1705011114 |
| [65] |
Ignatius MS, Moose HE, El-Hodiri HM, Henion PD. colgate/hdac1 repression of foxd3 expression is required to permit mitfa-dependent melanogenesis. Dev Biol, 2008, 313(2):568-583.
doi: 10.1016/j.ydbio.2007.10.045 pmid: 18068699 |
| [66] |
Singh N, Trivedi CM, Lu MM, Mullican SE, Lazar MA, Epstein JA. Histone deacetylase 3 regulates smooth muscle differentiation in neural crest cells and development of the cardiac outflow tract. Circ Res, 2011, 109(11):1240-1249.
doi: 10.1161/CIRCRESAHA.111.255067 |
| [67] |
DeLaurier A, Nakamura Y, Braasch I, Khanna V, Kato H, Wakitani S, Postlethwait JH, Kimmel CB. Histone deacetylase-4 is required during early cranial neural crest development for generation of the zebrafish palatal skeleton. BMC Dev Biol, 2012, 12(1):16.
doi: 10.1186/1471-213X-12-16 |
| [68] |
Haberland M, Mokalled MH, Montgomery RL, Olson EN. Epigenetic control of skull morphogenesis by histone deacetylase 8. Genes Dev, 2009, 23(14):1625-1630.
doi: 10.1101/gad.1809209 |
| [69] |
Strobl-Mazzulla PH, Bronner ME. A PHD12-Snail2 repressive complex epigenetically mediates neural crest epithelial-to-mesenchymal transition. J Cell Biol, 2012, 198(6):999-1010.
doi: 10.1083/jcb.201203098 pmid: 22986495 |
| [70] |
Cox SG, Kim H, Garnett AT, Medeiros DM, An W, Crump JG. An essential role of variant histone H3. 3 for ectomesenchyme potential of the cranial neural crest. PLoS Genet, 2012, 8(9):e1002938.
doi: 10.1371/journal.pgen.1002938 |
| [71] |
Yoshimura K, Kitagawa H, Fujiki R, Tanabe M, Takezawa S, Takada I, Yamaoka I, Yonezawa M, Kondo T, Furutani Y, Yagi H, Yoshinaga S, Masuda T, Fukuda T, Yamamoto Y, Ebihara K, Li DY, Matsuoka R, Takeuchi JK, Matsumoto T, Kato S. Distinct function of 2 chromatin remodeling complexes that share a common subunit, Williams syndrome transcription factor (WSTF). Proc Natl Acad Sci USA, 2009, 106(23):9280-9285.
doi: 10.1073/pnas.0901184106 |
| [72] | Kosho T, Okamoto N, Coffin-Siris Syndrome International Collaborators. Genotype-phenotype correlation of Coffin- Siris syndrome caused by mutations in SMARCB1, SMARCA4, SMARCE1, and ARID1A. Am J Med Genet C Semin Med Genet, 2014, 166C(3):262-275. |
| [73] |
Wang DY, Weng YJ, Guo SY, Qin WH, Ni JL, Yu L, Zhang YX, Zhao QS, Ben JJ, Ma JQ. MicroRNA-1 regulates NCC migration and differentiation by targeting sec63. Int J Biol Sci, 2019, 15(12):2538-2547.
doi: 10.7150/ijbs.35357 |
| [74] | Wei AY, Zhao PH, Xia JL, Wang Q, Du XH. MicroRNA- 23a is required for the migration and differentiation of cranial neural crest cells of zebrafish. J Dev Med, 2017, 5(1):7-13. |
| 魏安瑶, 赵鹏辉, 夏景兰, 王强, 杜兴华. microRNA- 23a调控斑马鱼颅神经嵴细胞迁移和分化. 发育医学电子杂志, 2017, 5(1):7-13. | |
| [75] |
Torroglosa A, Alves MM, Fernández RM, Añtinolo G, Hofstra RM, Borrego S. Epigenetics in ENS development and Hirschsprung disease. Dev Biol, 2016, 417(2):209-216.
doi: 10.1016/j.ydbio.2016.06.017 pmid: 27321561 |
| [76] |
Eberhart JK, He XJ, Swartz ME, Yan YL, Song H, Boling TC, Kunerth AK, Walker MB, Kimmel CB, Postlethwait JH. MicroRNA Mirn140 modulates Pdgf signaling during palatogenesis. Nat Genet, 2008, 40(3):290-298.
doi: 10.1038/ng.82 pmid: 18264099 |
| [77] |
Gao S, Moreno M, Eliason S, Cao HJ, Li X, Yu WJ, Bidlack FB, Margolis HC, Baldini A, Amendt BA. TBX1 protein interactions and microRNA-96-5p regulation controls cell proliferation during craniofacial and dental development: implications for 22q11. 2 deletion syndrome. Hum Mol Genet, 2015, 24(8):2330-2348.
doi: 10.1093/hmg/ddu750 |
| [78] |
Itoh T, Nozawa Y, Akao Y. MicroRNA-141 and -200a are involved in bone morphogenetic protein-2-induced mouse pre-osteoblast differentiation by targeting distal-less homeobox 5. J Biol Chem, 2009, 284(29):19272-19279.
doi: 10.1074/jbc.M109.014001 |
| [79] | Yan B, Liu B, Zhu CD, Li KL, Yue LJ, Zhao JL, Gong XL, Wang CH. MicroRNA regulation of skin pigmentation in fish. J Cell Sci, 2013, 126(Pt 15):3401-3408. |
| [80] |
Sato TS, Handa A, Priya S, Watal P, Becker RM, Sato Y. Neurocristopathies: Enigmatic Appearances of Neural Crest Cell-derived Abnormalities. Radiographics, 2019, 39(7):2085-2102.
doi: 10.1148/rg.2019190086 |
| [81] |
Cerrizuela S, Vega-Lopez GA, Aybar MJ. The role of teratogens in neural crest development. Birth Defects Res, 2020, 112(8):584-632.
doi: 10.1002/bdr2.1644 pmid: 31926062 |
| [82] |
Fainsod A, Kot-Leibovich H. Xenopus embryos to study fetal alcohol syndrome, a model for environmental teratogenesis. Biochem Cell Biol, 2018, 96(2):77-87.
doi: 10.1139/bcb-2017-0219 |
| [83] |
Tolosa EJ, Fernández-Zapico ME, Battiato NL, Rovasio RA. Sonic hedgehog is a chemotactic neural crest cell guide that is perturbed by ethanol exposure. Eur J Cell Biol, 2016, 95(3/5):136-152.
doi: 10.1016/j.ejcb.2016.02.003 |
| [84] |
Flentke GR, Baulch JW, Berres ME, Garic A, Smith SM. Alcohol-mediated calcium signals dysregulate pro- survival Snai2/PUMA/Bcl2 networks to promote p53-mediated apoptosis in avian neural crest progenitors. Birth Defects Res, 2019, 111(12):686-699.
doi: 10.1002/bdr2.1508 pmid: 31021056 |
| [85] |
Mulder GB, Manley N, Grant J, Schmidt K, Zeng W, Eckhoff C, Maggio-Price L. Effects of excess vitamin A on development of cranial neural crest-derived structures: a neonatal and embryologic study. Teratology, 2000, 62(4):214-226.
pmid: 10992263 |
| [86] |
Mondal D, Shenoy SR, Mishra S. Retinoic acid embryopathy. Int J Appl Basic Med Res, 2017, 7(4):264-265.
doi: 10.4103/ijabmr.IJABMR_469_16 |
| [87] |
Zhao Y, Guo YJ, Tomac AC, Taylor NR, Grinberg A, Lee EJ, Huang S, Westphal H. Isolated cleft palate in mice with a targeted mutation of the LIM homeobox gene lhx8. Proc Natl Acad Sci USA, 1999, 96(26):15002-15006.
doi: 10.1073/pnas.96.26.15002 |
| [88] |
Niederreither K, Dollé P. Retinoic acid in development: towards an integrated view. Nat Rev Genet, 2008, 9(7):541-553.
doi: 10.1038/nrg2340 pmid: 18542081 |
| [89] |
Melnik BC. Overexpression of p53 explains isotretinoin's teratogenicity. Exp Dermatol, 2018, 27(1):91-93.
doi: 10.1111/exd.13420 pmid: 28833556 |
| [90] |
Melnik BC. Apoptosis may explain the pharmacological mode of action and adverse effects of isotretinoin, including teratogenicity. Acta Derm Venereol, 2017, 97(2):173-181.
doi: 10.2340/00015555-2535 |
| [91] |
Bronner ME, LeDouarin NM. Development and evolution of the neural crest: an overview. Dev Biol, 2012, 366(1):2-9.
doi: 10.1016/j.ydbio.2011.12.042 |
| [92] | Kennedy AE, Kandalam S, Olivares-Navarrete R, Dickinson AJG. E-cigarette aerosol exposure can cause craniofacial defects in Xenopus laevis embryos and mammalian neural crest cells. PLoS One, 2017, 12(9):e0185729. |
| [93] |
Sanbe A, Mizutani R, Miyauchi N, Yamauchi J, Nagase T, Yamamura KI, Tanoue A. Inhibitory effects of cigarette smoke extract on neural crest migration occur through suppression of R-spondin1 expression via aryl hydrocarbon receptor. Naunyn Schmiedebergs Arch Pharmacol, 2009, 380(6):569-576.
doi: 10.1007/s00210-009-0455-3 |
| [94] |
Esser C, Bargen I, Weighardt H, Haarmann-Stemmann T, Krutmann J. Functions of the aryl hydrocarbon receptor in the skin. Semin Immunopathol, 2013, 35(6):677-691.
doi: 10.1007/s00281-013-0394-4 |
| [95] |
Li JJ, Shi Y, Sun J, Zhang YF, Mao BY. Xenopus reduced folate carrier regulates neural crest development epigenetically. PLoS One, 2011, 6(11):e27198.
doi: 10.1371/journal.pone.0027198 |
| [96] |
Silveira AB, Laranjeira ABA, Rodrigues GOL, Leal PC, Cardoso BA, Barata JT, Yunes RA, Zanchin NIT, Brandalise SR, Yunes JA. PI3K inhibition synergizes with glucocorticoids but antagonizes with methotrexate in T-cell acute lymphoblastic leukemia. Oncotarget, 2015, 6(15):13105-13118.
doi: 10.18632/oncotarget.v6i15 |
| [97] |
Rajagopalan PTR, Zhang ZQ, McCourt L, Dwyer M, Benkovic SJ, Hammes GG. Interaction of dihydrofolate reductase with methotrexate: ensemble and single- molecule kinetics. Proc Natl Acad Sci USA, 2002, 99(21):13481-13486.
doi: 10.1073/pnas.172501499 |
| [98] |
Alata Jimenez N, Torres Pérez SA, Sánchez-Vásquez E, Fernandino JI, Strobl-Mazzulla PH. Folate deficiency prevents neural crest fate by disturbing the epigenetic Sox2 repression on the dorsal neural tube. Dev Biol, 2018, 444 Suppl 1: S193-S201.
doi: 10.1016/j.ydbio.2018.08.001 |
| [99] |
Zhang H, Chen HS, Luo HJ, An J, Sun L, Mei LY, He CF, Jiang L, Jiang W, Xia K, Li JD, Feng Y. Functional analysis of Waardenburg syndrome- associated PAX3 and SOX10 mutations: report of a dominant-negative SOX10 mutation in Waardenburg syndrome type II. Hum Genet, 2012, 131(3):491-503.
doi: 10.1007/s00439-011-1098-2 pmid: 21965087 |
| [100] |
Chen Q, Zhao Y, Shen G, Dai J. Etiology and pathogenesis of hemifacial microsomia. J Dent Res, 2018, 97(12):1297-1305.
doi: 10.1177/0022034518795609 pmid: 30205013 |
| [101] |
Gouignard N, Andrieu C, Theveneau E. Neural crest delamination and migration: looking forward to the next 150 years. Genesis, 2018, 56(6-7):e23107.
doi: 10.1002/dvg.23107 |
| [102] |
Zalc A, Sinha R, Gulati GS, Wesche DJ, Daszczuk P, Swigut T, Weissman IL, Wysocka J. Reactivation of the pluripotency program precedes formation of the cranial neural crest. Science, 2021, 371(6529): eabb4776.
doi: 10.1126/science.abb4776 |
| [103] |
Morarach K, Mikhailova A, Knoflach V, Memic F, Kumar R, Li W, Ernfors P, Marklund U. Diversification of molecularly defined myenteric neuron classes revealed by single-cell RNA sequencing. Nat Neurosci, 2021, 24(1):34-46.
doi: 10.1038/s41593-020-00736-x |
| [104] |
Artinger KB, Monsoro-Burq AH. Neural crest multipotency and specification: power and limits of single cell transcriptomic approaches. Fac Rev, 2021, 10:38.
doi: 10.12703/r/10-38 pmid: 34046642 |
| [1] | Yi Zhang, Zhi-Ying Wu. Pathogenesis and therapeutic advances of cerebral autosomal- dominant arteriopathy with subcortical infarcts and leukoencephalopathy [J]. Hereditas(Beijing), 2023, 45(7): 568-579. |
| [2] | XIE Zhao-Hui. A new target of antibacterial drugs: Bacterial mRNA degradation pathways [J]. HEREDITAS, 2013, 35(3): 324-332. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||