Hereditas(Beijing) ›› 2025, Vol. 47 ›› Issue (8): 823-841.doi: 10.16288/j.yczz.24-378
• Review • Previous Articles Next Articles
Progress on nucleos(t)idyl lipid-based nanoparticles for nucleic acid drugs delivery
Jiamei Hong( ), Hongyi Liu(
), Hongyi Liu( ), Hua Guo, Jing Yu, Qi Zhang, Zhu Guan, Zhenjun Yang(
), Hua Guo, Jing Yu, Qi Zhang, Zhu Guan, Zhenjun Yang( )
)
- State Key Laboratory of Natural and Biomimetic Drugs, School of Pharmaceutical Sciences, Peking University, Beijing 100191, China
-
Received:2025-02-19Revised:2025-05-08Online:2025-05-09Published:2025-05-09 -
Contact:Zhenjun Yang E-mail:m17802277004@163.com;hongyiliu@stu.pku.edu.cn;yangzj@bjmu.edu.cn -
Supported by:Beijing Science and Technology Commission Beijing Science and Technology Plan Project(Z231100004823026)
Cite this article
Jiamei Hong, Hongyi Liu, Hua Guo, Jing Yu, Qi Zhang, Zhu Guan, Zhenjun Yang. Progress on nucleos(t)idyl lipid-based nanoparticles for nucleic acid drugs delivery[J]. Hereditas(Beijing), 2025, 47(8): 823-841.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
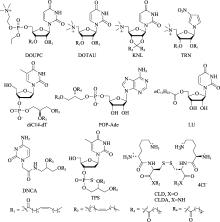
Table 1
The approved nucleic acid drugs"
| 种类 | 上市 年份 | 通用名/ 商品名 | 适应症 | 给药方式 | 包载/缀合 | 修饰 | 研发公司 |
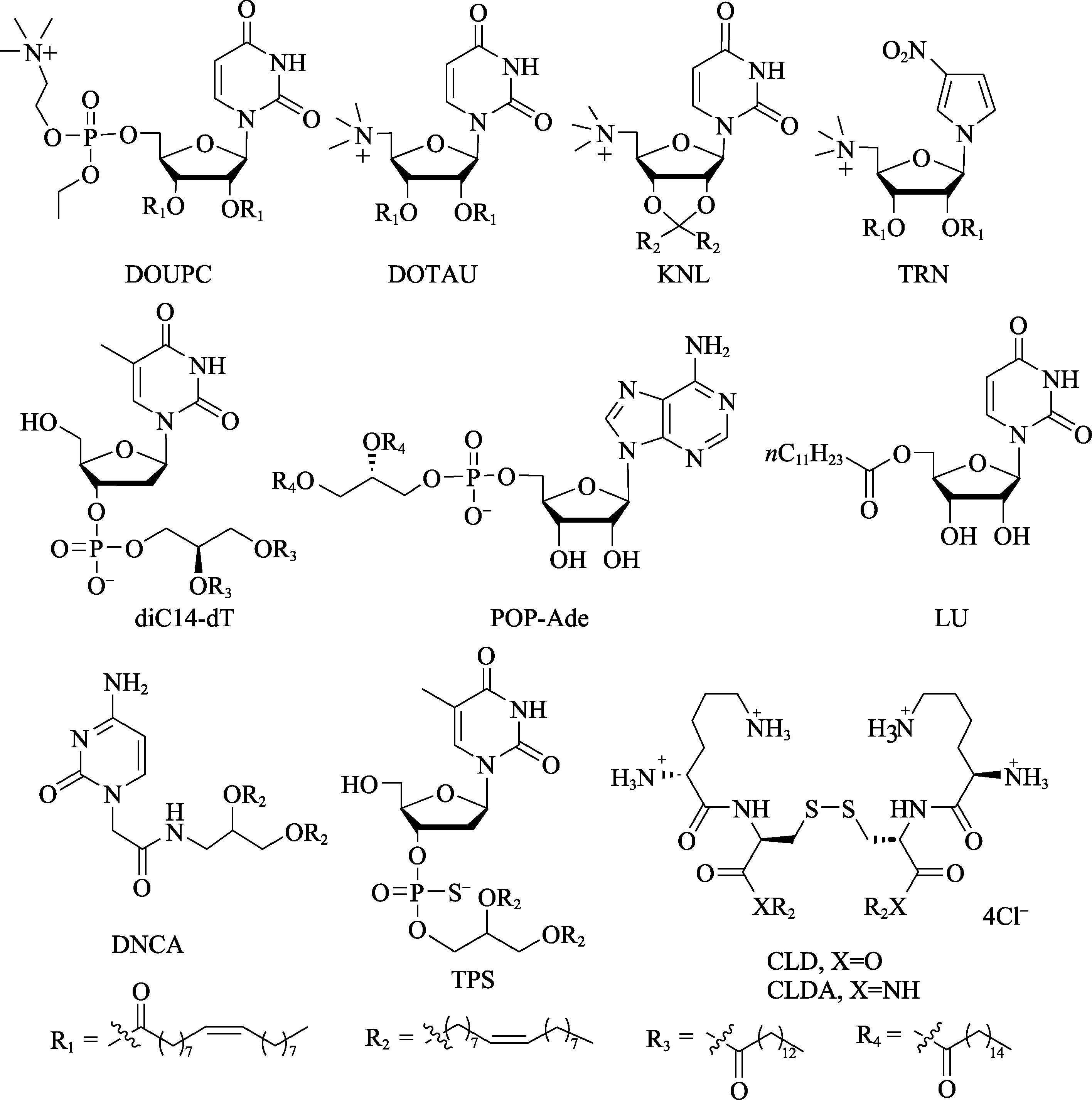
|---|---|---|---|---|---|---|---|
| 反义 核酸 | 1998 | Fomivirsen/ Vitraveve | 巨细胞病毒性视网膜炎 | 玻璃体注射 | Naked | PS | Ionis Pharmaceuticals (美国) |
| 2013 | Mipomersen/ Kynamro | 纯合子家族性高胆固醇 血症 | 皮下注射 | Naked | PS, 2′-OMOE | Ionis Pharmaceuticals (美国) | |
| 2016 | Eteplirsen/ Exongys 51 | 杜氏肌营养不良症 | 静脉注射 | Naked | PMO | Sarepta Therapeutics (美国) | |
| 2016 | Nusinersen/ Spinraza | 脊髓性肌萎缩症 | 鞘内注射 | Naked | PS, 2′-OMOE | Ionis Pharmaceuticals (美国)/ Biogen (美国) | |
| 2018 | Inotersen/ Tegsedi | 成人遗传性转甲状腺素 蛋白淀粉样变性 | 皮下注射 | Naked | PS, 2′-OMOE | Ionis Pharmaceuticals (美国)/ Akcea Therapeutics (美国) | |
| 2019 | Golodirsen/ Vyondys 53 | 杜氏肌营养不良症 | 静脉注射 | Naked | PMO | Sarepta Therapeutics (美国) | |
| 2019 | Volanesorsen/ Waylivra | 家族性高乳糜微粒血症 | 皮下注射 | Naked | PS, 2′-OMOE | Ionis Pharmaceuticals (美国)/ Akcea Therapeutics (美国) | |
| 2020 | Viltolarsen/ NS-065 | 杜氏肌营养不良症 | 静脉注射 | Naked | PMO | Nippon Shinyaku (日本) | |
| 2021 | Casimersen/ Amondys45 | 杜氏肌营养不良症 | 静脉注射 | Naked | PMO | Sarepta Therapeutics (美国) | |
| 2023 | Tofersen/ Qalsody | SOD1基因突变肌萎缩 侧索硬化症 | 静脉注射 | Naked | PS, 2′-OMOE | Ionis Pharmaceuticals (美国)/ Biogen (美国) | |
| 2023 | Eplontersen/ Wainua | 转甲状腺素蛋白淀粉样 变性 | 皮下注射 | GalNAc | PS, 2′-OMOE | Ionis Pharmaceuticals (美国)/ Akcea Therapeutics (美国) | |
| 2024 | Imetelstat/ Rytelo | 骨髓增生异常综合征 | 静脉注射 | C16 | NPS | Geron Corporation (美国) | |
| 2024 | Olezarsen/ Tryngolza | 家族性乳糜微粒血症 综合征 | 皮下注射 | GalNAc | PS, 2′-OMOE | Ionis Pharmaceuticals (美国) | |
| siRNA | 2018 | Patisiran/ Onpattro | 成人遗传性转甲状腺素 蛋白淀粉样变性 | 静脉注射 | LNP | 2′-OMe | Alnylam Pharmaceuticals (美国) |
| 2019 | Givosiran/ Givlaari | 成人急性肝卟啉病 | 皮下注射 | GalNAc | PS, 2′-OMe, 2′-F | Alnylam Pharmaceuticals (美国) | |
| 2020 | Lumasiran/ Oxlumo | 原发性高草酸尿症1型 | 皮下注射 | GalNAc | PS, 2′-OMe, 2′-F | Alnylam Pharmaceuticals (美国) | |
| 2021 | Inclisiran/ Leqvio | 高胆固醇血症血脂异常 | 皮下注射 | GalNAc | PS, 2′-OMe, 2′-F | Novartis (瑞士) | |
| 2022 | Vutrisiran/ Amvuttra | 成人遗传性转甲状腺素 蛋白淀粉样变性 | 皮下注射 | GalNAc | PS, 2′-OMe, 2′-F | Alnylam Pharmaceuticals (美国) | |
| 2023 | Nedosiran/ Rivfloza | 原发性高草酸尿症1型 | 皮下注射 | GalNAc | PS, 2′-OMe, 2′-F | Dicerna Pharmaceuticals (美国) | |
| 2025 | Fitusiran/ Qfitlia | A型或B型血友病 | 皮下注射 | GalNAc | PS, 2′-OMe, 2′-F | Sanofi (法国)/ Alnylam Pharmaceuticals (美国) | |
| mRNA | 2021 | Tozinameran/ Comirnaty | 新型冠状病毒感染 | 肌肉注射 | LNP | m1Ψ | BioNTech (德国)/Pfizer(美国) |
| 2020 | Elasomeran/ SpikeVax | 新型冠状病毒感染 | 肌肉注射 | LNP | m1Ψ | Moderna Therapeutics (美国) | |
| 2023 | SYS6006/ 度恩泰 | 新型冠状病毒感染 | 肌肉注射 | LNP | m1Ψ | 石药集团(中国) | |
| 2024 | mRNA-1345/ mRESVIA | 呼吸道合胞病毒感染 | 肌肉注射 | LNP | m1Ψ | Moderna Therapeutics (美国) | |
| 适配体 | 2004 | Pegaptanib/ Macugen | 年龄相关性黄斑变性 | 眼内注射 | PEG缀合 | 2′-OMe, 2′-F | Eyetech Pharmaceuticals (美国) |
| 2023 | Avacincaptad Pegol/Izervay | 年龄相关性黄斑变性 引起的地理萎缩 | 眼内注射 | PEG缀合 | 2′-OMe, 2′-F | Astellas Pharma (日本) |
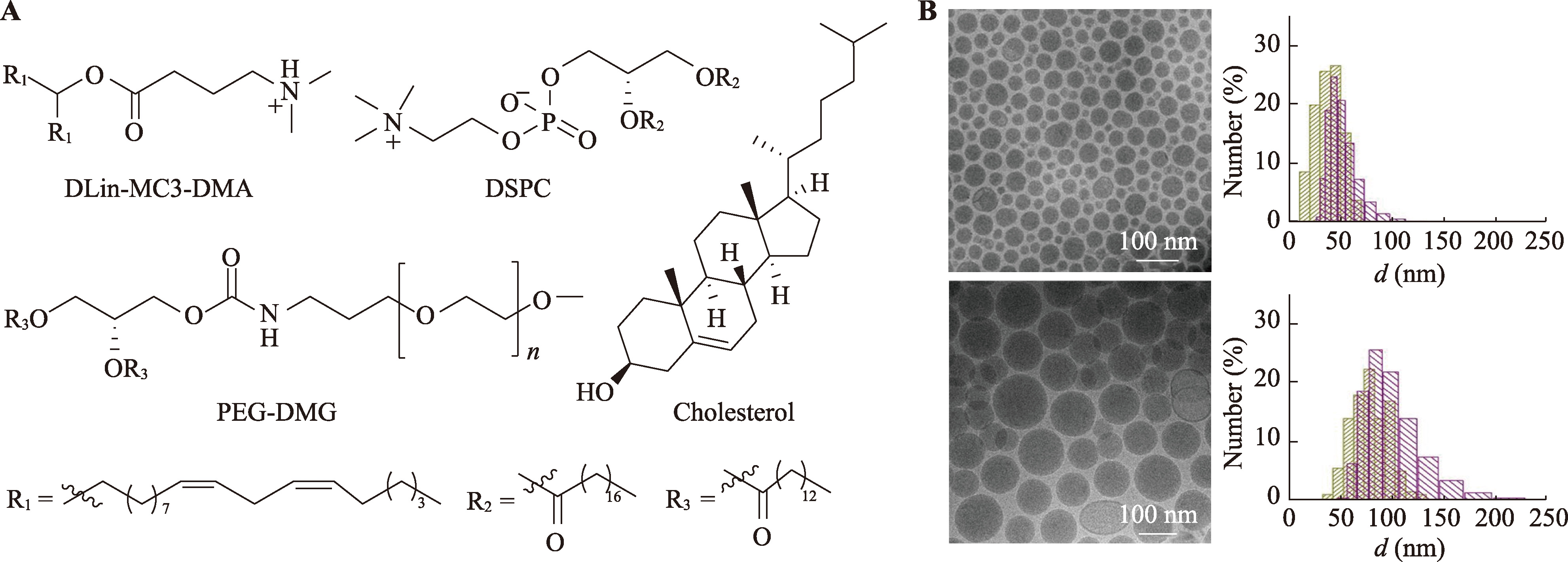
Table 3
Composition and ratio of SORT LNP"
| SORT LNP | 组成成分 | 摩尔比例 | 粒径(nm) | PDI | ζ-电位(mV) |
|---|---|---|---|---|---|
| mDLNP | 5A2-SC8:DOPE:Cholesterol:DMG-PEG2K | 15∶15∶30∶3 | 144.0 | 0.135 | -3.59 |
| 肝SORT | 5A2-SC8:DOPE:Cholesterol:DMG-PEG2K:DODAP | 15∶15∶30∶3∶15.75 | 154.6 | 0.115 | -3.82 |
| 脾SORT | 5A2-SC8:DOPE:Cholesterol:DMG-PEG2K:18PA | 15∶15∶30∶3∶27 | 167.8 | 0.144 | -1.84 |
| 肺SORT | 5A2-SC8:DOPE:Cholesterol:DMG-PEG2K:DOTAP | 15∶15∶30∶3∶63 | 114.8 | 0.159 | -0.89 |
Table 4
LNP-related candidates in phase III clinical trials"
| 名称 | 适应症 | 种类 | 临床试验号 |
|---|---|---|---|
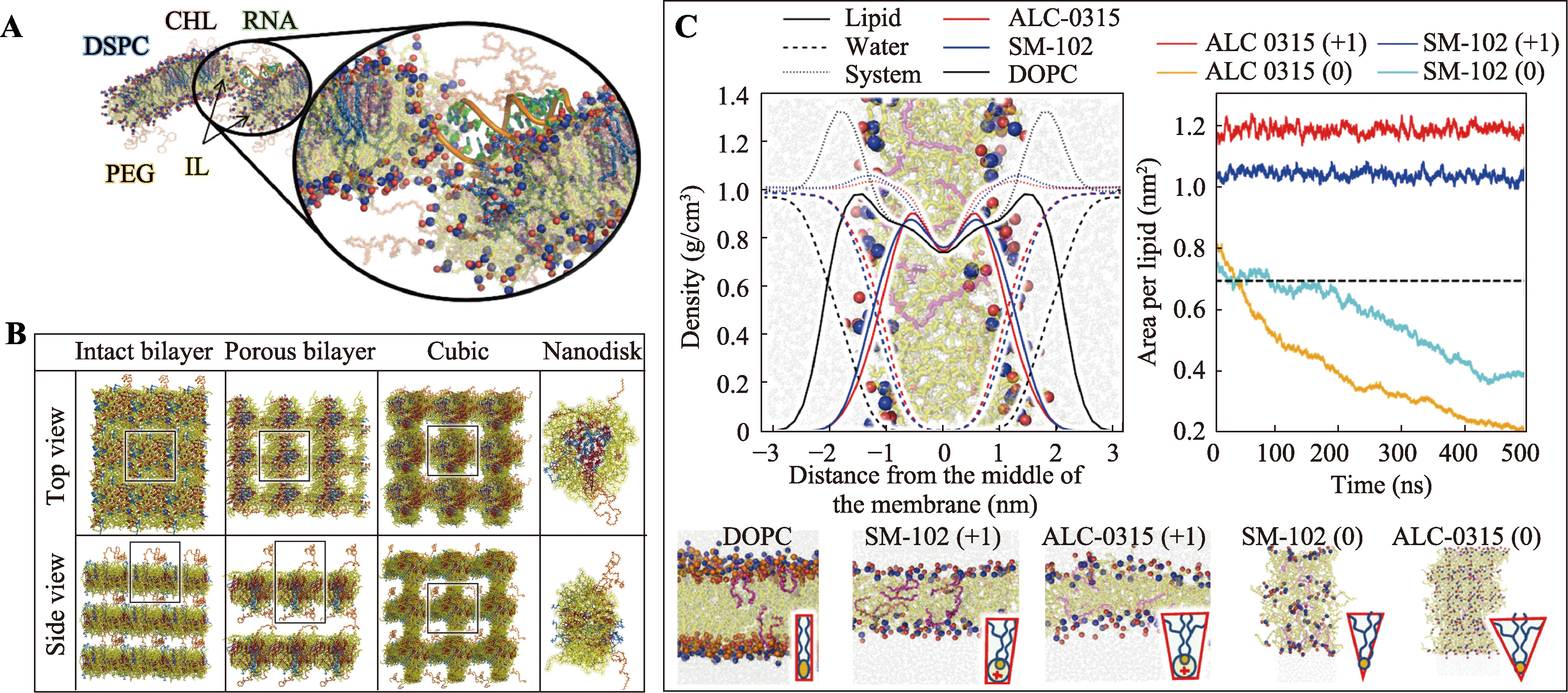
| mRNA-4157 | 黑色素瘤; 非小细胞肺癌 | mRNA肿瘤疫苗 | NCT05933577 NCT06077760 |
| mRNA-1273 | 带状疱疹 | mRNA病毒疫苗 | NCT05047770 |
| mRNA-1647 | 巨细胞病毒感染 | mRNA病毒疫苗 | NCT05085366 |
| mRNA-1010 | 季节性流感 | mRNA病毒疫苗 | NCT05415462 |
| qlRV | 流感 | mRNA病毒疫苗 | NCT05540522 |
| NTLA-2002 | 遗传性血管性 水肿 | sgRNA和 Cas9 mRNA | NCT06634420 |
Table 5
Comparison of loading efficiency and safety of nucleos(t)idyl lipid and LNP-encapsulated nucleic acid drugs"
| 核酸种类 | 100 g脂材载药量(g) | 安全性 | ||
|---|---|---|---|---|
| LNP | 核苷(酸)脂材制剂 | LNP | 核苷(酸)脂材制剂 | |
| siRNA | 8 | 26 | 炎症反应(Dlin-MC3-DMA<1 mg/kg); DSPE-PEG致产生抗体; 胆固醇含量高(~40%) | 大鼠最大耐受剂量:DNCA 300 mg/kg;CLD 15 mg/kg (未发表数据) |
| ASO | / | 22 | ||
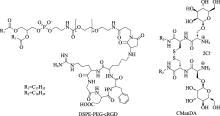
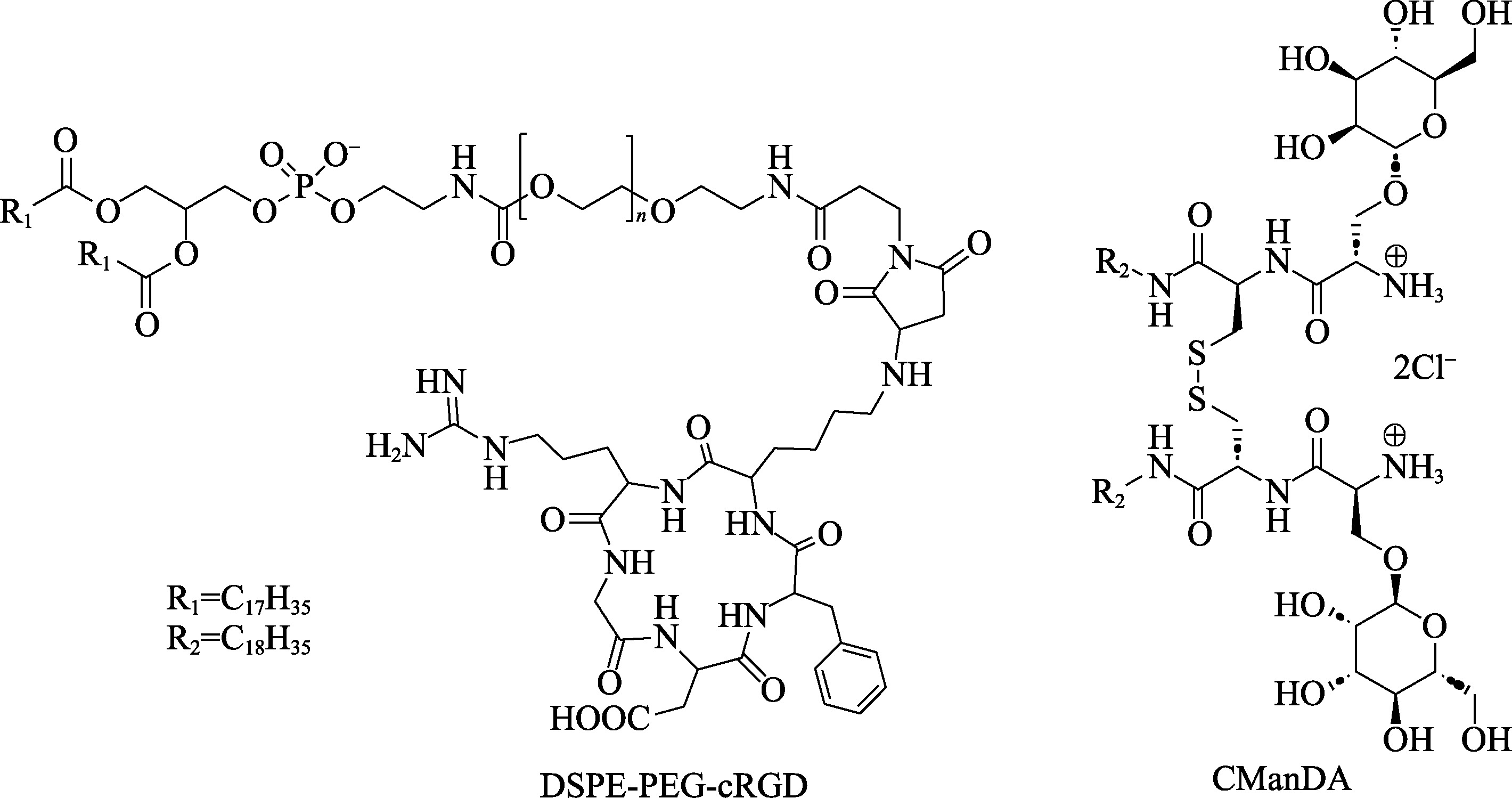
| 适配体 | / | 17 | ||
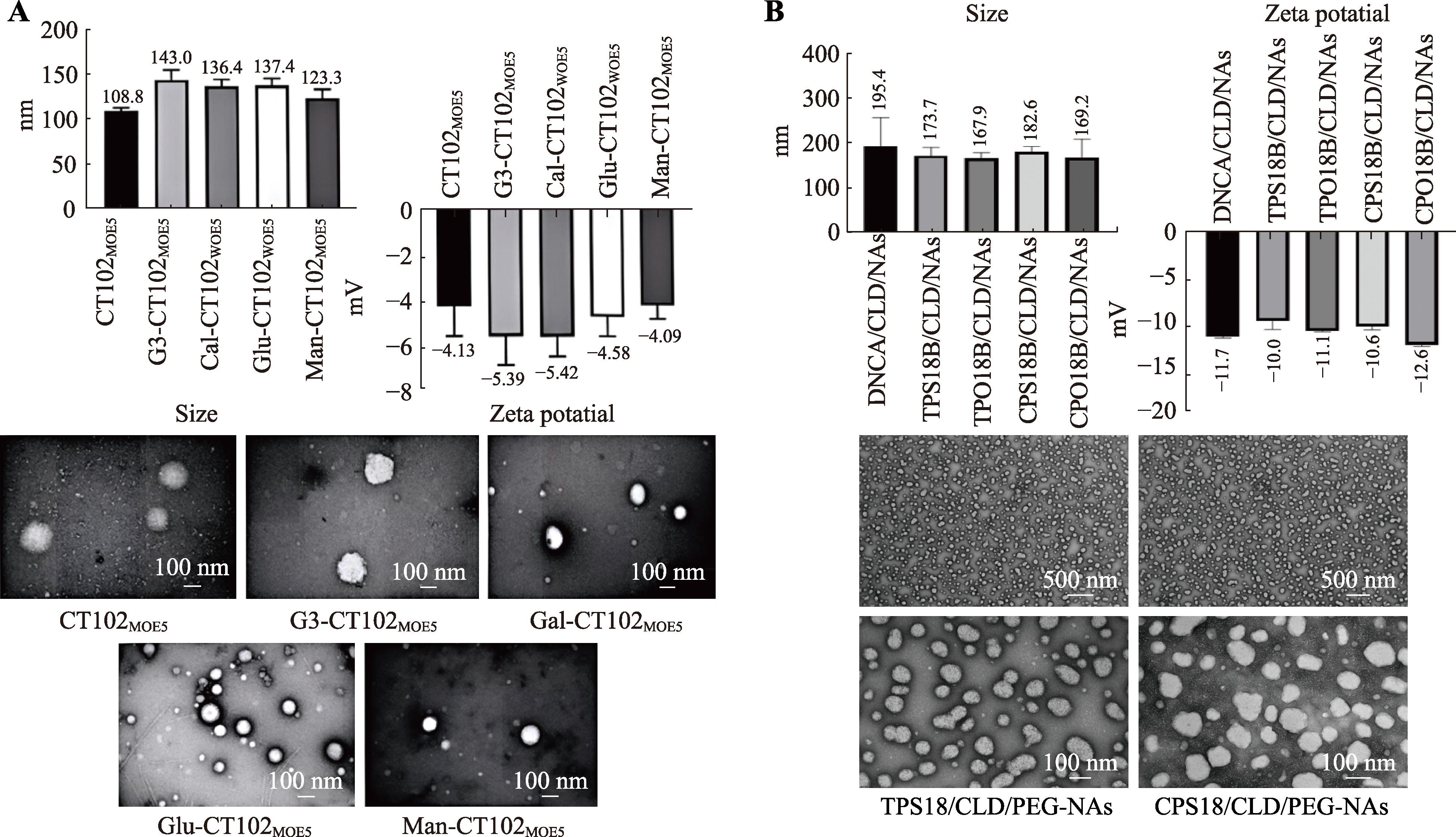
| 环二核苷酸 | / | 6 | ||
| mRNA疫苗 | 5~8 | 22 | ||
| [1] |
Sun XY, Setrerrahmane S, Li CC, Hu JL, Xu HM. Nucleic acid drugs: recent progress and future perspectives. Signal Transduct Target Ther, 2024, 9(1): 316.
pmid: 39609384 |
| [2] |
Hu B, Zhong LP, Weng YH, Peng L, Huang YY, Zhao YX, Liang XJ. Therapeutic siRNA: state of the art. Signal Transduct Target Ther, 2020, 5(1): 101.
pmid: 32561705 |
| [3] |
Zong Y, Lin Y, Wei T, Cheng Q. Lipid nanoparticle (LNP) enables mRNA delivery for cancer therapy. Adv Mater, 2023, 35(51): e2303261.
pmid: 37196221 |
| [4] |
Crooke ST, Baker BF, Crooke RM, Liang XH. Antisense technology: an overview and prospectus. Nat Rev Drug Discov, 2021, 20(6): 427-453.
pmid: 33762737 |
| [5] |
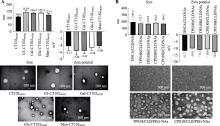
Santarpia G, Carnes E. Therapeutic applications of aptamers. Int J Mol Sci, 2024, 25(12): 6742.
pmid: 38928448 |
| [6] |
Dowdy SF. Overcoming cellular barriers for RNA therapeutics. Nat Biotechnol, 2017, 35(3): 222-229.
pmid: 28244992 |
| [7] |
Kanasty RL, Whitehead KA, Vegas AJ, Anderson DG. Action and reaction: the biological response to siRNA and its delivery vehicles. Mol Ther, 2012, 20(3): 513-524.
pmid: 22252451 |
| [8] |
Shen W, De Hoyos CL, Migawa MT, Vickers TA, Sun H, Low A, Bell A 3rd, Rahdar M, Mukhopadhyay S, Hart CE, Bell M, Riney S, Murray SF, Greenlee S, Crooke RM, Liang XH, Seth PP, Crooke ST. Chemical modification of PS-ASO therapeutics reduces cellular protein-binding and improves the therapeutic index. Nat Biotechnol, 2019, 37(6): 640-650.
pmid: 31036929 |
| [9] |
Khvorova A, Watts JK. The chemical evolution of oligonucleotide therapies of clinical utility. Nat Biotechnol, 2017, 35(3): 238-248.
pmid: 28244990 |
| [10] |
Springer AD, Dowdy SF. GalNAc-siRNA conjugates:leading the way for delivery of RNAi therapeutics. Nucleic Acid Ther, 2018, 28(3): 109-118.
pmid: 29792572 |
| [11] |
Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, Diemert D, Spector SA, Rouphael N, Creech CB, McGettigan J, Khetan S, Segall N, Solis J, Brosz A, Fierro C, Schwartz H, Neuzil K, Corey L, Gilbert P, Janes H, Follmann D, Marovich M, Mascola J, Polakowski L, Ledgerwood J, Graham BS, Bennett H, Pajon R, Knightly C, Leav B, Deng WP, Zhou HH, Han S, Ivarsson M, Miller J, Zaks T. COVE Study Group. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med, 2021, 384(5): 403-416.
pmid: 33378609 |
| [12] |
Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Moreira ED, Zerbini C, Bailey R, Swanson KA, Roychoudhury S, Koury K, Li P, Kalina WV, Cooper D, Frenck RW Jr, Hammitt LL, Türeci Ö, Nell H, Schaefer A, Ünal S, Tresnan DB, Mather S, Dormitzer PR, Şahin U, Jansen KU, Gruber WC, C4591001 Clinical Trial Group. Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine. N Engl J Med, 2020, 383(27): 2603-2615.
pmid: 33301246 |
| [13] |
Goswami J, Cardona JF, Hsu DC, Simorellis AK, Wilson L, Dhar R, Tomassini JE, Wang XW, Kapoor A, Collins A, Righi V, Lan L, Du JJ, Zhou HH, Stoszek SK, Shaw CA, Reuter C, Wilson E, Miller JM, Das R, study investigators. Safety and immunogenicity of mRNA-1345 RSV vaccine coadministered with an influenza or COVID-19 vaccine in adults aged 50 years or older: an observer-blinded, placebo-controlled, randomised, phase 3 trial. Lancet Infect Dis, 2024, 25(4): 411-423.
pmid: 39608389 |
| [14] |
Albertsen CH, Kulkarni JA, Witzigmann D, Lind M, Petersson K, Simonsen JB. The role of lipid components in lipid nanoparticles for vaccines and gene therapy. Adv Drug Deliv Rev, 2022, 188: 114416.
pmid: 35787388 |
| [15] |
Samaridou E, Heyes J, Lutwyche P. Lipid nanoparticles for nucleic acid delivery: current perspectives. Adv Drug Deliv Rev, 2020, 154-155: 37-63.
pmid: 32526452 |
| [16] |
Bitounis D, Jacquinet E, Rogers MA, Amiji MM. Strategies to reduce the risks of mRNA drug and vaccine toxicity. Nat Rev Drug Discov, 2024, 23(4): 281-300.
pmid: 38263456 |
| [17] |
Jiang ZW, Thayumanavan S. Non-cationic material design for nucleic acid delivery. Adv Ther (Weinh), 2020, 3(3): 1900206.
pmid: 34164572 |
| [18] |
Wang CR, Zhao CY, Wang WP, Liu XQ, Deng HZ. Biomimetic noncationic lipid nanoparticles for mRNA delivery. Proc Natl Acad Sci USA, 2023, 120(51): e2311276120.
pmid: 38079547 |
| [19] |
Gissot A, Camplo M, Grinstaff MW, Barthélémy P. Nucleoside, nucleotide and oligonucleotide based amphiphiles: a successful marriage of nucleic acids with lipids. Org Biomol Chem, 2008, 6(8): 1324-1333.
pmid: 18385837 |
| [20] |
Zhou XY, Wang SH, Zhu YJ, Pan YF, Zhang LH, Yang ZJ. Overcoming the delivery barrier of oligonucleotide drugs and enhancing nucleoside drug efficiency: the use of nucleolipids. Med Res Rev, 2020, 40(4): 1178-1199.
pmid: 31820472 |
| [21] |
Moreau L, Barthélémy P, Li YG, Luo D, Prata CAH, Grinstaff MW. Nucleoside phosphocholine amphiphile for in vitro DNA transfection. Mol Biosyst, 2005, 1(3): 260-264.
pmid: 16880990 |
| [22] |
Chabaud P, Camplo M, Payet D, Serin G, Moreau L, Barthélémy P, Grinstaff MW. Cationic nucleoside lipids for gene delivery. Bioconjug Chem, 2006, 17(2): 466-472.
pmid: 16536479 |
| [23] |
Luvino D, Khiati S, Oumzil K, Rocchi P, Camplo M, Barthélémy P. Efficient delivery of therapeutic small nucleic acids to prostate cancer cells using ketal nucleoside lipid nanoparticles. J Control Release, 2013, 172(3): 954-961.
pmid: 24041711 |
| [24] |
Tonelli G, Oumzil K, Nallet F, Gaillard C, Navailles L, Barthélémy P. Amino acid-nucleotide-lipids: effect of amino acid on the self-assembly properties. Langmuir, 2013, 29(18): 5547-5555.
pmid: 23565776 |
| [25] |
Montis C, Milani S, Berti D, Baglioni P. Complexes of nucleolipid liposomes with single-stranded and double- stranded nucleic acids. J Colloid Interface Sci, 2012, 373(1): 57-68.
pmid: 22138265 |
| [26] |
Cuomo F, Ceglie A, Colafemmina G, Germani R, Savelli G, Lopez F. Polyadenylic acid binding on cationic liposomes doped with the non-ionic nucleolipid lauroyl uridine. Colloids Surf B Biointerfaces, 2011, 82(2): 277-282.
pmid: 20884180 |
| [27] |
Zhou XY, Pan YF, Li Z, Li HT, Wu J, Ma Y, Guan Z, Yang ZJ. siRNA packaged with neutral cytidinyl/cationic/PEG lipids for enhanced antitumor efficiency and safety in vitro and in vivo. ACS Appl Bio Mater, 2020, 3(9): 6297-6309.
pmid: 35021760 |
| [28] |
Ma Y, Zhu YJ, Wang C, Pan DL, Liu S, Yang MY, Xiao ZP, Yang XT, Zhao WT, Zhou XY, Li YD, Pan YF, Sun J, Wang SH, Guan Z, Zhang LH, Yang ZJ. Annealing novel nucleobase-lipids with oligonucleotides or plasmid DNA based on H-bonding or π-π interaction: Assemblies and transfections. Biomaterials, 2018, 178: 147-157.
pmid: 29933101 |
| [29] |
Ma Y, Zhao WT, Li YD, Pan YF, Wang SH, Zhu YJ, Kong LX, Guan Z, Wang JC, Zhang LH, Yang ZJ. Structural optimization and additional targets identification of antisense oligonucleotide G3139 encapsulated in a neutral cytidinyl-lipid combined with a cationic lipid in vitro and in vivo. Biomaterials, 2019, 197: 182-193.
pmid: 30660994 |
| [30] |
Yu XT, Yu J, Dai H, Deng CY, Sun XD, Long SJ, Jiang ZJ, Jin HY, Guan Z, Yang ZJ. Novel formulation of c-di-GMP with cytidinyl/cationic lipid reverses T cell exhaustion and activates stronger anti-tumor immunity. Theranostics, 2022, 12(15): 6723-6739.
pmid: 36185614 |
| [31] |
Zhou JH, Shum KT, Burnett JC, Rossi JJ. Nanoparticle- based delivery of RNAi therapeutics: progress and challenges. Pharmaceuticals (Basel), 2013, 6(1): 85-107.
pmid: 23667320 |
| [32] |
Alexander MY, Akhurst RJ. Liposome-medicated gene transfer and expression via the skin. Hum Mol Genet, 1995, 4(12): 2279-2285.
pmid: 8634699 |
| [33] |
Lee TWR, Matthews DA, Blair GE. Novel molecular approaches to cystic fibrosis gene therapy. Biochem J, 2005, 387(Pt 1): 1-15.
pmid: 15656784 |
| [34] |
Remy JS, Sirlin C, Vierling P, Behr JP. Gene transfer with a series of lipophilic DNA-binding molecules. Bioconjugate Chem, 1994, 5(6): 647-654.
pmid: 7873668 |
| [35] |
Kulkarni JA, Witzigmann D, Leung J, Tam YYC, Cullis PR. On the role of helper lipids in lipid nanoparticle formulations of siRNA. Nanoscale, 2019, 11(45): 21733-21739.
pmid: 31713568 |
| [36] |
Yanez Arteta M, Kjellman T, Bartesaghi S, Wallin S, Wu XQ, Kvist AJ, Dabkowska A, Székely N, Radulescu A, Bergenholtz J, Lindfors L. Successful reprogramming of cellular protein production through mRNA delivered by functionalized lipid nanoparticles. Proc Natl Acad Sci USA, 2018, 115(15): E3351-E3360.
pmid: 29588418 |
| [37] |
Sarode A, Fan YC, Byrnes AE, Hammel M, Hura GL, Fu YG, Kou P, Hu C, Hinz FI, Roberts J, Koenig SG, Nagapudi K, Hoogenraad CC, Chen T, Leung D, Yen CW. Predictive high-throughput screening of PEGylated lipids in oligonucleotide-loaded lipid nanoparticles for neuronal gene silencing. Nanoscale Adv, 2022, 4(9): 2107-2123.
pmid: 36133441 |
| [38] |
Morille M, Passirani C, Vonarbourg A, Clavreul A, Benoit JP. Progress in developing cationic vectors for non-viral systemic gene therapy against cancer. Biomaterials, 2008, 29(24-25): 3477-3496.
pmid: 18499247 |
| [39] |
Schober GB, Story S, Arya DP. A careful look at lipid nanoparticle characterization: analysis of benchmark formulations for encapsulation of RNA cargo size gradient. Sci Rep, 2024, 14(1): 2403.
pmid: 38287070 |
| [40] |
Creusat G, Rinaldi AS, Weiss E, Elbaghdadi R, Remy JS, Mulherkar R, Zuber G. Proton sponge trick for pH-sensitive disassembly of polyethylenimine-based siRNA delivery systems. Bioconjugate Chem, 2010, 21(5): 994-1002.
pmid: 20481503 |
| [41] |
Pan YF, Zhu YJ, Ma Y, Hong JM, Zhao WT, Gao YJ, Guan J, Ren RN, Zhang Q, Yu J, Guan Z, Yang ZJ. Design and synthesis of nucleotidyl lipids and their application in the targeted delivery of siG12D for pancreatic cancer therapy. Biomed Pharmacother, 2024, 172: 116239.
pmid: 38325267 |
| [42] |
Li L, Long JR, Sang Y, Wang X, Zhou XY, Pan YF, Cao YM, Huang HY, Yang ZJ, Yang J, Wang SQ. Rational preparation and application of a mRNA delivery system with cytidinyl/cationic lipid. J Control Release, 2021, 340: 114-124.
pmid: 34699870 |
| [43] |
Pan YF, Guan J, Gao YJ, Zhu YJ, Li HT, Guo H, He QY, Guan Z, Yang ZJ. Modified ASO conjugates encapsulated with cytidinyl/cationic lipids exhibit more potent and longer-lasting anti-HCC effects. Mol Ther Nucleic Acids, 2023, 32: 807-821.
pmid: 37251692 |
| [44] |
Semple SC, Akinc A, Chen JX, Sandhu AP, Mui BL, Cho CK, Sah DWY, Stebbing D, Crosley EJ, Yaworski E, Hafez IM, Dorkin JR, Qin J, Lam K, Rajeev KG, Wong KF, Jeffs LB, Nechev L, Eisenhardt ML, Jayaraman M, Kazem M, Maier MA, Srinivasulu M, Weinstein MJ, Chen QM, Alvarez R, Barros SA, De S, Klimuk SK, Borland T, Kosovrasti V, Cantley WL, Tam YK, Manoharan M, Ciufolini MA, Tracy MA, de Fougerolles A, MacLachlan I, Cullis PR, Madden TD, Hope MJ. Rational design of cationic lipids for siRNA delivery. Nat Biotechnol, 2010, 28(2): 172-176.
pmid: 20081866 |
| [45] |
Crooke ST, Wang SY, Vickers TA, Shen W, Liang XH. Cellular uptake and trafficking of antisense oligonucleotides. Nat Biotechnol, 2017, 35(3): 230-237.
pmid: 28244996 |
| [46] | Zheng Y, Guo YJ, Li YT, Wu Y, Zhang LH, Yang ZJ. A novel gemini-like cationic lipid for the efficient delivery of siRNA. New J Chem, 2014, 38(10): 4952-4962. |
| [47] |
Yang XT, Zhu YJ, Wang C, Guan Z, Zhang LH, Yang ZJ. Alkylation of phosphorothioated thrombin binding aptamers improves the selectivity of inhibition of tumor cell proliferation upon anticoagulation. Biochim Biophys Acta, 2017, 1861(7): 1864-1869.
pmid: 28389332 |
| [48] | Zhang GP, Deng JL, Yang XT, Zhu YJ, Guan Z, Zhang LH, Yang ZJ. Selective alkylation and bioactivity of phosphorothioated nucleolin aptamer AS1411. J Chin Pharm Sci, 2017, 26(1): 23-30. |
| [49] |
Wu J, Wang SH, Li X, Zhang Q, Yang J, Ma Y, Guan Z, Yang ZJ. Selective anti-melanoma effect of phosphothioated aptamer encapsulated by neutral cytidinyl/cationic lipids. Front Cell Dev Biol, 2021, 9: 660233.
pmid: 34262898 |
| [50] |
Zhu YJ, Li X, Zhang Q, Yang XT, Sun XD, Pan Y, Yuan X, Ma Y, Xu B, Yang ZJ. Aptamer AS411 interacts with the KRAS promoter/hnRNP A1 complex and shows increased potency against drug-resistant lung cancer. RSC Med Chem, 2024, 15(5): 1515-1526.
pmid: 38784467 |
| [51] |
Zhou XY, Pan YF, Yu LJ, Wu J, Li Z, Li HT, Guan Z, Tang XJ, Yang ZJ. Feasibility of cRGD conjugation at 5′-antisense strand of siRNA by phosphodiester linkage extension. Mol Ther Nucleic Acids, 2021, 25: 603-612.
pmid: 34589281 |
| [52] |
Ceballos C, Prata CAH, Giorgio S, Garzino F, Payet D, Barthélémy P, Grinstaff MW, Camplo M. Cationic nucleoside lipids based on a 3-nitropyrrole universal base for siRNA delivery. Bioconjug Chem, 2009, 20(2): 193-196.
pmid: 19159294 |
| [53] |
Ceballos C, Khiati S, Prata CAH, Zhang XX, Giorgio S, Marsal P, Grinstaff MW, Barthélémy P, Camplo M. Cationic nucleoside lipids derived from universal bases: a rational approach for siRNA transfection. Bioconjug Chem, 2010, 21(6): 1062-1069.
pmid: 20481514 |
| [54] |
Fabre AL, Colotte M, Luis A, Tuffet S, Bonnet J. An efficient method for long-term room temperature storage of RNA. Eur J Hum Genet, 2014, 22: 379-385.
pmid: 23860045 |
| [55] |
Philipp J, Dabkowska A, Reiser A, Frank K, Krzysztoń R, Brummer C, Nickel B, Blanchet CE, Sudarsan A, Ibrahim M, Johansson S, Skantze P, Skantze U, Östman S, Johansson M, Henderson N, Elvevold K, Smedsrød B, Schwierz N, Lindfors L, Rädler JO. pH-dependent structural transitions in cationic ionizable lipid mesophases are critical for lipid nanoparticle function. Proc Natl Acad Sci USA, 2023, 120(50): e2310491120.
pmid: 38055742 |
| [56] |
Kulkarni JA, Darjuan MM, Mercer JE, Chen S, van der Meel R, Thewalt JL, Tam YYC, Cullis PR. On the formation and morphology of lipid nanoparticles containing ionizable cationic lipids and siRNA. ACS Nano, 2018, 12: 4787-4795.
pmid: 29614232 |
| [57] |
Ziller A, Nogueira SS, Hühn E, Funari SS, Brezesinski G, Hartmann H, Sahin U, Haas H, Langguth P. Incorporation of mRNA in lamellar lipid matrices for parenteral administration. Mol Pharm, 2018, 15(2): 642-651.
pmid: 29232147 |
| [58] |
Trollmann MFW, Böckmann RA. mRNA lipid nanoparticle phase transition. Biophys J, 2022, 121(20): 3927-3939.
pmid: 36045573 |
| [59] |
Li MY, Jia L, Xie YB, Ma WL, Yan ZH, Liu FF, Deng J, Zhu A, Siwei X, Su W, Liu XF, Li SQ, Wang HM, Yu P, Zhu T. Lyophilization process optimization and molecular dynamics simulation of mRNA-LNPs for SARS-CoV-2 vaccine. NPJ Vaccines, 2023, 8(1): 153.
pmid: 37813912 |
| [60] |
Meyer RA, Hussmann GP, Peterson NC, Santos JL, Tuesca AD. A scalable and robust cationic lipid/polymer hybrid nanoparticle platform for mRNA delivery. Int J Pharm, 2022, 611: 121314.
pmid: 34838950 |
| [61] |
Hajj KA, Melamed JR, Chaudhary N, Lamson NG, Ball RL, Yerneni SS, Whitehead KA. A potent branched-tail lipid nanoparticle enables multiplexed mRNA delivery and gene editing in vivo. Nano Lett, 2020, 20(7): 5167-5175.
pmid: 32496069 |
| [62] |
Paloncýová M, Čechová P, Šrejber M, Kührová P, Otyepka M. Role of ionizable lipids in SARS-CoV-2 vaccines as revealed by molecular dynamics simulations: from membrane structure to interaction with mRNA fragments. J Phys Chem Lett, 2021, 12(45): 11199-11205.
pmid: 34761943 |
| [63] |
Nakamura T, Sato Y, Yamada Y, Elwakil MMA, Kimura S, Younis MA, Harashima H. Extrahepatic targeting of lipid nanoparticles in vivo with intracellular targeting for future nanomedicines. Adv Drug Deliv Rev, 2022, 188: 114417.
pmid: 35787389 |
| [64] |
Akinc A, Maier MA, Manoharan M, Fitzgerald K, Jayaraman M, Barros S, Ansell S, Du XY, Hope MJ, Madden TD, Mui BL, Semple SC, Tam YK, Ciufolini M, Witzigmann D, Kulkarni JA, van der Meel R, Cullis PR. The Onpattro story and the clinical translation of nanomedicines containing nucleic acid-based drugs. Nat Nanotechnol, 2019, 14(12): 1084-1087.
pmid: 31802031 |
| [65] |
Cheng Q, Wei T, Farbiak L, Johnson LT, Dilliard SA, Siegwart DJ. Selective organ targeting (SORT) nanoparticles for tissue-specific mRNA delivery and CRISPR-Cas gene editing. Nat Nanotechnol, 2020, 15(4): 313-320.
pmid: 32251383 |
| [66] |
Dilliard SA, Cheng Q, Siegwart DJ. On the mechanism of tissue-specific mRNA delivery by selective organ targeting nanoparticles. Proc Natl Acad Sci USA, 2021, 118(52): e2109256118.
pmid: 34933999 |
| [67] |
LoPresti ST, Arral ML, Chaudhary N, Whitehead KA. The replacement of helper lipids with charged alternatives in lipid nanoparticles facilitates targeted mRNA delivery to the spleen and lungs. J Control Release, 2022, 345: 819-831.
pmid: 35346768 |
| [68] |
Kim M, Jeong M, Hur S, Cho Y, Park J, Jung H, Seo Y, Woo HA, Nam KT, Lee K, Lee H. Engineered ionizable lipid nanoparticles for targeted delivery of RNA therapeutics into different types of cells in the liver. Sci Adv, 2021, 7(9): eabf4398.
pmid: 33637537 |
| [69] |
Anthiya S, Öztürk SC, Yanik H, Tavukcuoglu E, Şahin A, Datta D, Charisse K, Álvarez DM, Loza MI, Calvo A, Sulheim E, Loevenich S, Klinkenberg G, Schmid R, Manoharan M, Esendağlı G, Alonso MJ. Targeted siRNA lipid nanoparticles for the treatment of KRAS-mutant tumors. J Control Release, 2023, 357: 67-83.
pmid: 36921725 |
| [70] |
Kim Y, Choi J, Kim EH, Park W, Jang H, Jang Y, Chi SG, Kweon DH, Lee K, Kim SH, Yang Y. Design of PD-L1-targeted lipid nanoparticles to turn on PTEN for efficient cancer therapy. Adv Sci (Weinh), 2024, 11(22): e2309917.
pmid: 38520717 |
| [71] |
Zhang YF, Li SX, Zhou XY, Sun J, Fan XM, Guan Z, Zhang LH, Yang ZJ. Construction of a targeting nanoparticle of 3′,3″-bis-peptide-siRNA conjugate/mixed lipid with postinserted DSPE-PEG2000-cRGD. Mol Pharm, 2019, 16(12): 4920-4928.
pmid: 31642677 |
| [72] |
Guo H, Hong JM, Zhu YJ, Gui HZ, Liu HY, Ren RN, Li Y, Shan SJ, Guan Z, Liu MZ, Yang ZJ. A Mannosylated peptidyl lipid CManDA doped into cytidinyl/cationic lipids efficiently delivers siG12Ss to lung cancer in vivo. J Control Release, 2025, 381: 113624.
pmid: 40073943 |
| [73] | Ma RP, Li YT, Wei Y, Zhou JJ, Ma JY, Zhang MK, Tu JY, Jiang JH, Xie ST, Tan WH, Liu XS. The dynamic process of mRNA delivery by lipid nanoparticles in vivo. Nano Today, 2024, 57: 102325. |
| [74] | Cullis PR, Felgner PL. The 60-year evolution of lipid nanoparticles for nucleic acid delivery. Nat Rev Drug Discov, 2024, 23(9): 709-722. |
| [75] |
Chen JJ, Ye ZF, Huang CF, Qiu M, Song DH, Li YM, Xu QB. Lipid nanoparticle-mediated lymph node-targeting delivery of mRNA cancer vaccine elicits robust CD8+ T cell response. Proc Natl Acad Sci USA, 2022, 119(34): e2207841119.
pmid: 35969778 |
| [76] |
Tahtinen S, Tong AJ, Himmels P, Oh J, Paler-Martinez A, Kim L, Wichner S, Oei Y, McCarron MJ, Freund EC, Amir ZA, de la Cruz CC, Haley B, Blanchette C, Schartner JM, Ye WL, Yadav M, Sahin U, Delamarre L, Mellman I. IL-1 and IL-1ra are key regulators of the inflammatory response to RNA vaccines. Nat Immunol, 2022, 23(4): 532-542.
pmid: 35332327 |
| [77] |
Ndeupen S, Qin Z, Jacobsen S, Bouteau A, Estanbouli H, Igyártó BZ. The mRNA-LNP platform's lipid nanoparticle component used in preclinical vaccine studies is highly inflammatory. iScience, 2021, 24(12): 103479.
pmid: 34841223 |
| [78] |
Lv K, Yu ZL, Wang J, Li N, Wang AP, Xue TZ, Wang QX, Shi YQ, Han L, Qin W, Gong JQ, Song HJ, Zhang TT, Chang CY, Chen H, Zhong XJ, Ding J, Chen R, Liu ML, Zhang WG, Cen S, Dong YJ. Discovery of ketal-ester ionizable lipid nanoparticle with reduced hepatotoxicity, enhanced spleen tropism for mRNA vaccine delivery. Adv Sci (Weinh), 2024, 11(45): e2404684.
pmid: 39387241 |
| [79] |
Judge A, McClintock K, Phelps JR, MacLachlan I. Hypersensitivity and loss of disease site targeting caused by antibody responses to PEGylated liposomes. Mol Ther, 2006, 13(2): 328-337.
pmid: 16275098 |
| [80] |
Back PI, Yu MZ, Modaresahmadi S, Hajimirzaei S, Zhang QS, Islam MR, Schwendeman AA, La-Beck NM. Immune implications of cholesterol-containing lipid nanoparticles. ACS Nano, 2024, 18(42): 28480-28501.
pmid: 39388645 |
| [81] |
Ma XF, Sun J, Qiu C, Wu YF, Zheng Y, Yu MZ, Pei XW, Wei L, Niu YJ, Pang WH, Yang ZJ, Wang JC, Zhang Q. The role of disulfide-bridge on the activities of H-shape gemini-like cationic lipid based siRNA delivery. J Control Release, 2016, 235: 99-111.
pmid: 27242198 |
| [82] |
Zhou ZY, Liu S, Zhang YF, Yang XT, Ma Y, Guan Z, Wu Y, Zhang LH, Yang ZJ. Reductive nanocomplex encapsulation of cRGD-siRNA conjugates for enhanced targeting to cancer cells. Inter J Nanomed, 2017, 12: 7255-7272.
pmid: 29042774 |
| [83] | Yang MY, Sun J, Wang C, Zhang YF, Zhang LH, Yang ZJ. Transfection of 3′,3″-bis-peptide-siRNA conjugate by cationic lipoplexes mixed with a neutral cytosin-1-yl-lipid. J Chin Pharm Sci, 2017, 26(10): 719-726. |
| [84] | Sun J, Qiu C, Diao YP, Wei W, Jin HW, Zheng Y, Wang JC, Zhang LH, Yang ZJ. Delivery pathway regulation of 3′,3′-bis-peptide-siRNA conjugate by nanocarrier architecture engineering. Mol Ther Nucleic Acids, 2018, 10: 75-90. |
| [85] | Gao YJ, Wang XX, Pan YF, Wang QX, Zhu YJ, Pan DL, Guan Z, Yang ZJ. Synthesis and anti-HCC activity of full 2ʹ-F/OMe-siRNA encapsulated with neutral cytidinyl/ cationic lipid. Acta Pharm Sin, 2023, 58(6): 1634-1640. |
| 高宇睛, 王玺贤, 潘宇飞, 汪全鑫, 朱月洁, 潘德林, 关注, 杨振军. 全2°-F/OMe-siRNA的设计合成及其新型混合脂材纳米制剂抗肝癌活性评价. 药学学报, 2023, 58(6): 1634-1640. [DOI] | |
| [86] |
Li Z, Wang XX, Zhou XY, Wang J, Guan Z, Yang ZJ. Optimization in chemical modification of single-stranded siRNA encapsulated by neutral cytidinyl/cationic lipids. Front Chem, 2022, 10: 843181.
pmid: 35345539 |
| [87] |
Guan J, Pan YF, Li HT, Zhu YJ, Gao YJ, Wang J, Zhou Y, Guan Z, Yang ZJ. Activity and tissue distribution of antisense oligonucleotide CT102 encapsulated with cytidinyl/cationic lipid against hepatocellular carcinoma. Mol Pharm, 2022, 19(12): 4552-4564.
pmid: 35508302 |
| [88] | Pu Y, Guan J, He QY, Zhu YJ, Pan DL, Guan Z, Yang ZJ. Investigation on efficacy against hepatocellular carcinoma of novel antisense oligonucleotide targeting IGF1R mRNA encapsulated with neutral cytidinyl/cationic lipid in vitro. Acta Pharm Sin, 2024, 59(5): 1441-1448. |
| 蒲洋, 管静, 何仟一, 朱月洁, 潘德林, 关注, 杨振军. 靶向IGF1R mRNA的反义寡核苷酸修饰物新型制剂体外抗肝癌活性研究. 药学学报, 2024, 59(5): 1441-1448. | |
| [89] |
Zhao XR, Xu JF, Liang XX, Wang ZY, Zhu YJ, Guo DY, Wang J, Amu G, Wang Q, Yang ZJ, Tang XJ. NQO1- activatable circular antisense oligonucleotides for tumor- cell-specific survivin gene silencing and antitumor therapy. J Med Chem, 2025, 68(4): 4466-4476.
pmid: 39921644 |
| [90] |
Li J, Yao PZ, Tang K, Zhao XY, Liu XY, Liu QG, Wei TX, Xuan H, Bian SQ, Guo Y, Yang ZJ, Zhang ZQ, Zhang LQ. Functional aptamers in vitro evolution for intranuclear blockage of RNA-protein interaction. J Am Chem Soc, 2024, 146(35): 24654-24662.
pmid: 39167715 |
| [91] |
Ni SJ, Zhuo ZJ, Pan YF, Yu YY, Li FF, Liu J, Wang LY, Wu XQ, Li DJ, Wan YY, Zhang LH, Yang ZJ, Zhang BT, Lu AP, Zhang G. Recent progress in aptamer discoveries and modifications for therapeutic applications. ACS Appl Mater Interfaces, 2021, 13(8): 8500-8519.
pmid: 32603135 |
| [92] | Wang SH, Zhu YJ, Wu J, Yang ZJ. Modifications and encapsulation for aptamers applications in the diseases targeting treatment and detection. Chin J Med Chem, 2019, 29(6): 456-468. |
| 王舒鹤, 朱月洁, 吴静, 杨振军. 核酸适配体修饰与包载及其在疾病靶向性治疗和检测中的应用. 中国药物化学杂志, 2019, 29(6): 456-468. | |
| [93] |
Sun XD, Yu XT, Zhao YQ, Xing L, Na LX, Chen Z, Xiao ZP, Dai H, Yu J, Long SJ, Wang QX, Shi XF, Guan Z, Lei M, Yang ZJ. Cyclic diguanylate analogues: Facile synthesis, STING binding mode and anti-tumor immunity delivered by cytidinyl/cationic lipid. Eur J Med Chem, 2023, 247: 115053.
pmid: 36587419 |
| [94] |
Yu J, Yu XT, Sun XD, Wang QX, Long SJ, Ren RN, Guan Z, Yang ZJ. Bis-2°-F-cGSASMP isomers encapsulated in cytidinyl/cationic lipids act as potent in situ autologous tumor vaccines. Mol Ther, 2024, 32(6): 1917-1933.
pmid: 38637990 |
| [95] |
Xu JF, Zhao XR, Liang XX, Guo DY, Wang J, Wang Q, Tang XJ. Development of miRNA-based PROTACs targeting Lin28 for breast cancer therapy. Sci Adv, 2024, 10(38): eadp0334.
pmid: 39292784 |
| No related articles found! |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||