Hereditas(Beijing) ›› 2023, Vol. 45 ›› Issue (11): 1007-1017.doi: 10.16288/j.yczz.23-119
• Review • Previous Articles Next Articles
Advances in the regulation of inflammasome activation by GBP family in infectious diseases
Shuting Quan1( ), Weiwei Jiao1,2(
), Weiwei Jiao1,2( ), Fang Xu3,2, Lin Sun1,2, Hui Qi1,2(
), Fang Xu3,2, Lin Sun1,2, Hui Qi1,2( ), Adong Shen1,2(
), Adong Shen1,2( )
)
- 1. Beijing Key Laboratory of Pediatric Respiratory Infection Diseases, MOE Key Laboratory of Major Diseases in Children, National Key Discipline of Pediatrics, National Clinical Research Center for Respiratory Diseases, Beijing Pediatric Research Institute, Beijing Children’s Hospital, Capital Medical University, National Center for Children’s Health, Beijing 100045, China
2. Baoding Key Laboratory for Precision Diagnosis and Treatment of Infectious Diseases in Children, Baoding Hospital of Beijing Children’s Hospital, Capital Medical University, Baoding 071000, China
3. Genetics and Birth Defects Control Center, Beijing Key Laboratory for Genetics of Birth Defects, MOE Key Laboratory of Major Diseases in Children, National Key Discipline of Pediatrics, Beijing Pediatric Research Institute, Beijing Children’s Hospital, Capital Medical University, National Center for Children’s Health, Beijing 100045, China
-
Received:2023-06-15Revised:2023-08-22Online:2023-11-20Published:2023-09-14 -
Contact:Hui Qi,Adong Shen E-mail:qst137@163.com;jiaowei310@163.com;qh20021983@163.com;shenad16@hotmail.com -
Supported by:National Natural Science Foundation of China(81871617);National Natural Science Foundation of China(81701971);National Natural Science Foundation of China(82172280);National Natural Science Foundation of China(82100010);CAS Key Laboratory of Pathogenic Microbiology and Immunology Open Project(CASPMI202201);Baoding Science and Technology Plan(2272P012);Training Plan for High-Level Public Health Technical Talents of Beijing Municipal Health Commission(2022-3-041)
Cite this article
Shuting Quan, Weiwei Jiao, Fang Xu, Lin Sun, Hui Qi, Adong Shen. Advances in the regulation of inflammasome activation by GBP family in infectious diseases[J]. Hereditas(Beijing), 2023, 45(11): 1007-1017.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
Table 1
The role of GBP in inflammasome activation"
| GBP家族成员 | 炎症小体活化类型 | 炎症小体活化途径 | 病原体 | 参考文献 |
|---|---|---|---|---|
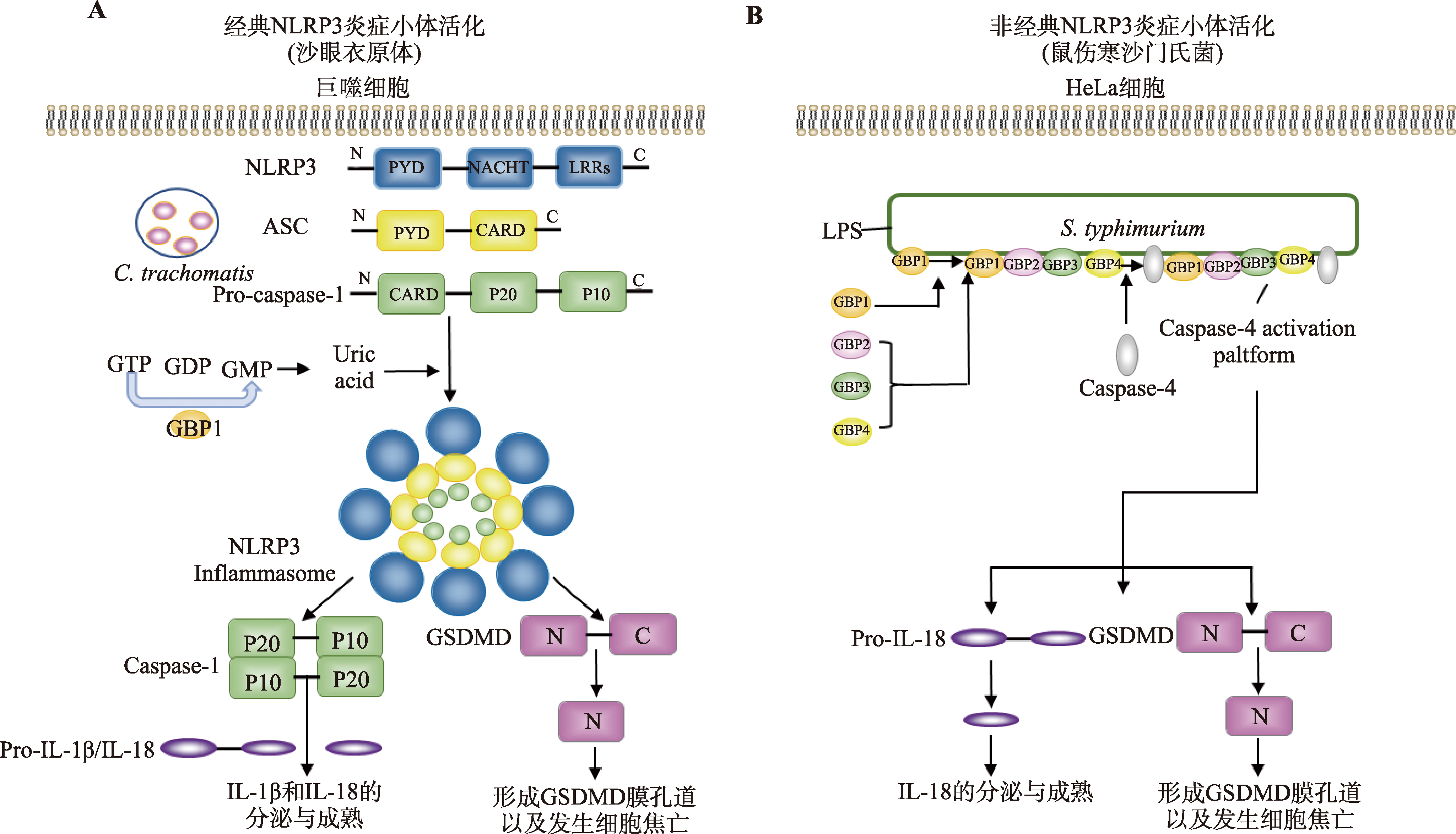
| GBP1 | 经典 | GBP1水解GTP后产生GMP,GMP被进一步分解代谢为尿酸,通过经典途径活化NLRP3炎症小体,引起成熟的caspase-1水平升高,促进IL-1β分泌以及细胞焦亡发生。 | 沙眼衣原体 | [ |
| 非经典 | (1)HeLa细胞中,GBP1作为胞质内的PRR结合细菌外膜的LPS,招募GBP2、GBP3和GBP4形成在细菌表面形成一个信号平台,进而招募caspase-4到此平台,GBP1、GBP3、caspase-4和LPS通过相互作用,引起caspase-4介导的非经典途径炎症小体活化、细胞焦亡以及IL-18分泌。其中,GBP1可能通过其GTPase结构域结合LPS的脂质A和/或内核,caspase-4的CARD与LPS的脂质A结合。 (2)巨噬细胞中,GBP1靶向于含有鼠伤寒沙门氏菌的液泡,并且招募caspase-4靶向液泡以及活化。 | 鼠伤寒沙门氏菌 | [ | |
| GBP2 | 经典 | GBP2在新凶手弗朗西丝菌感染中小鼠的巨噬细胞中促进经典途径AIM2炎症小体活化,并且促进大肠杆菌OMVs通过经典途径活化NLRP3炎症小体。 | 新凶手弗朗西丝菌、大肠杆菌 | [ |
| 非经典 | 受GBP1招募组成信号平台促进非经典途径炎症小体活化外(见GBP1非经典炎症小体活化)。 | 鼠伤寒沙门氏菌 | [ | |
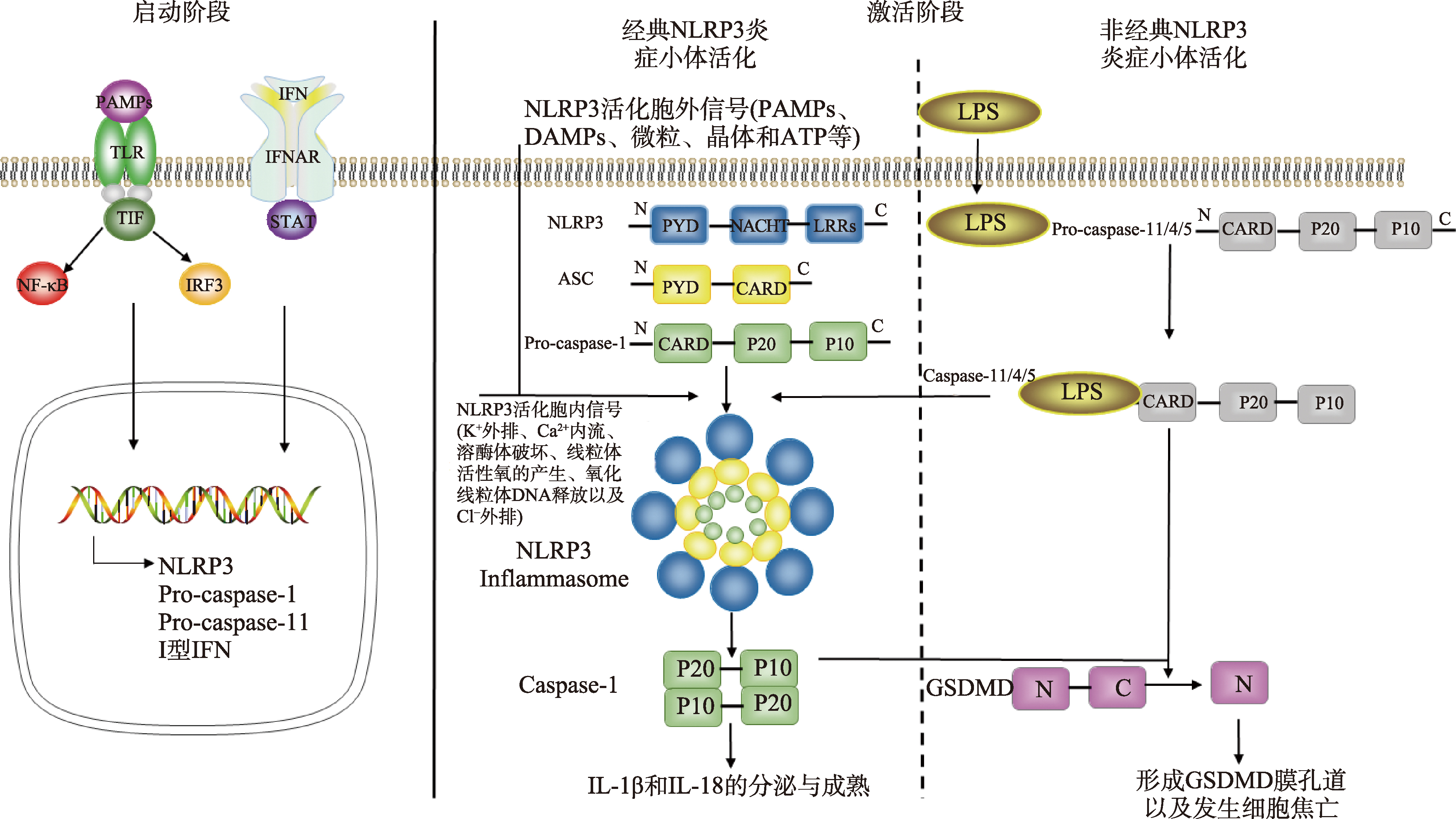
| GBP5 | 经典 | (1)LPS或鼠伤寒沙门氏菌作用后,GBP5可以促进巨噬细胞中NLRP3-ASC炎症小体的组装。其中,GBP5和NLRP3的pyrin 结构域之间的相互作用促进ASC聚合。 (2)GBP5是新凶手弗朗西丝菌感染小鼠巨噬细胞中重要的AIM2炎症小体激活剂,并促进胞浆细菌溶解。 | 鼠伤寒沙门氏菌、新凶手弗朗西丝菌 | [ |
| 非经典 | 布鲁氏杆菌感染中,GBP5是促进caspase-11对LPS识别的关键分子,可显著影响IL-1β分泌和LDH释放。 | 布鲁氏杆菌 | [ |
Table 2
The role of GBP played in infections induced by different pathogens"
| 病原体类型 | 病原体 | 具体机制 | 参考文献 |
|---|---|---|---|
| 细菌 | 嗜肺军团菌 | GBP蛋白可以使胞质内的菌体DNA释放到胞质中,通过非经典途径促进caspase-11活化,促进机体的免疫应答 | [ |
| BCG | mGBP1和mGBP10通过将吞噬细胞氧化酶或抗菌肽传递到含有BCG的吞噬体中,对胞内菌起到杀伤作用 | [ | |
| 鼠伤寒沙门氏菌 | GBP1靶向鼠伤寒沙门氏菌液泡,导致含沙门氏菌液泡破裂,沙门氏菌进入宿主细胞的胞质内,通过非经典途径促进caspase-4活化并诱导细胞焦亡 | [ | |
| 福氏志贺菌 | GBP1和GBP2均能在S. flexneri表面形成LPS-蛋白复合物,通过非经典途径促进caspase-4活化,进而促进宿主对病原菌的免疫应答。同时,限制S. flexneri的运动能力和菌在细胞间的播散 | [ | |
| 新凶手弗朗西斯菌 | 详见 | [ | |
| 单核细胞增生性李斯特单胞菌 | 单核细胞增生性李斯特单胞菌感染巨噬细胞中,GBP5可以通过经典途径促进NLRP3炎症小体活化 | [ | |
| 布鲁氏杆菌 | 详见 | [ | |
| 病毒 | SARS-CoV-2 | GBP2和GBP5通过抑制新型冠状病毒(severe acute respiratory syndrome coronavirus 2,SARS-CoV-2)分离株中的早期谱系的刺突蛋白的裂解,减弱病毒的传染性 | [ |
| HIV-1 | GBP5在HIV-1的靶细胞——巨噬细胞中表达,通过干扰病毒包膜糖蛋白(envelope glycoprotein,Env)的加工和病毒粒子掺入来减弱病毒的感染性 | [ | |
| 寄生虫 | 弓形虫 | 鼠型GBP2蛋白通过其C端结构域作用于弓形虫寄居的纳虫泡,进而抑制弓形虫的增殖 | [ |
| 其他 | 沙眼衣原体 | 详见 | [ |
| [1] |
Kutsch M, Coers J. Human guanylate binding proteins: nanomachines orchestrating host defense. FEBS J, 2021, 288(20): 5826-5849.
doi: 10.1111/febs.v288.20 |
| [2] |
Macmicking JD. IFN-inducible GTPases and immunity to intracellular pathogens. Trends Immunol, 2004, 25(11): 601-609.
doi: 10.1016/j.it.2004.08.010 pmid: 15489189 |
| [3] |
Martens S, Howard J. The interferon-inducible GTPases. Annu Rev Cell Dev Biol, 2006, 22: 559-589.
pmid: 16824009 |
| [4] |
Man SM, Place DE, Kuriakose T, Kanneganti TD. Interferon-inducible guanylate-binding proteins at the interface of cell-autonomous immunity and inflammasome activation. J Leukoc Biol, 2017, 101(1): 143-150.
doi: 10.1189/jlb.4MR0516-223R |
| [5] |
Krapp C, Hotter D, Gawanbacht A, Mclaren PJ, Kluge SF, Stürzel CM, Mack K, Reith E, Engelhart S, Ciuffi A, Hornung V, Sauter D, Telenti A, Kirchhoff F. Guanylate binding protein (GBP) 5 is an interferon-inducible inhibitor of HIV-1 infectivity. Cell Host Microbe, 2016, 19(4): 504-514.
doi: 10.1016/j.chom.2016.02.019 pmid: 26996307 |
| [6] |
Kim BH, Chee JD, Bradfield CJ, Park ES, Kumar P, Macmicking JD. Interferon-induced guanylate-binding proteins in inflammasome activation and host defense. Nat Immunol, 2016, 17(5): 481-489.
doi: 10.1038/ni.3440 |
| [7] |
Randow F, Macmicking JD, James LC. Cellular self-defense: how cell-autonomous immunity protects against pathogens. Science, 2013, 340(6133): 701-706.
doi: 10.1126/science.1233028 pmid: 23661752 |
| [8] |
Hagar JA, Powell DA, Aachoui Y, Ernst RK, Miao EA. Cytoplasmic LPS activates caspase-11: implications in TLR4-independent endotoxic shock. Science, 2013, 341(6151): 1250-1253.
doi: 10.1126/science.1240988 pmid: 24031018 |
| [9] |
Praefcke GJK. Regulation of innate immune functions by guanylate-binding proteins. Int J Med Microbiol, 2018, 308(1): 237-245.
doi: S1438-4221(17)30425-3 pmid: 29174633 |
| [10] |
Schwemmle M, Staeheli P. The interferon-induced 67-kDa guanylate-binding protein (hGBP1) is a GTPase that converts GTP to GMP. J Biol Chem, 1994, 269(15): 11299-11305.
pmid: 7512561 |
| [11] |
Ghosh A, Praefcke GJK, Renault L, Wittinghofer A, Herrmann C. How guanylate-binding proteins achieve assembly-stimulated processive cleavage of GTP to GMP. Nature, 2006, 440(7080): 101-104.
doi: 10.1038/nature04510 |
| [12] | Cui W, Braun E, Wang W, Tang JH, Zheng YY, Slater B, Li N, Chen C, Liu QX, Wang B, Li X, Duan YK, Xiao YJ, Ti RJ, Hotter D, Ji XY, Zhang L, Cui J, Xiong Y, Sauter D, Wang ZF, Kirchhoff F, Yang HT. Structural basis for GTP-induced dimerization and antiviral function of guanylate-binding proteins. Proc Natl Acad Sci USA, 2021, 118(15): e2022269118. |
| [13] |
Shenoy AR, Wellington DA, Kumar P, Kassa H, Booth CJ, Cresswell P, Macmicking JD. GBP5 promotes NLRP3 inflammasome assembly and immunity in mammals. Science, 2012, 336(6080): 481-485.
doi: 10.1126/science.1217141 pmid: 22461501 |
| [14] |
Resh MD. Trafficking and signaling by fatty-acylated and prenylated proteins. Nat Chem Biol, 2006, 2(11): 584-590.
doi: 10.1038/nchembio834 pmid: 17051234 |
| [15] |
Konstantinopoulos PA, Karamouzis MV, Papavassiliou AG. Post-translational modifications and regulation of the RAS superfamily of GTPases as anticancer targets. Nat Rev Drug Discov, 2007, 6(7): 541-555.
doi: 10.1038/nrd2221 pmid: 17585331 |
| [16] |
Tretina K, Park E S, Maminska A, Macmicking J D. Interferon-induced guanylate-binding proteins: guardians of host defense in health and disease. J Exp Med, 2019, 216(3): 482-500.
doi: 10.1084/jem.20182031 |
| [17] |
Britzen-Laurent N, Bauer M, Berton V, Fischer N, Syguda A, Reipschläger S, Naschberger E, Herrmann C, Stürzl M. Intracellular trafficking of guanylate-binding proteins is regulated by heterodimerization in a hierarchical manner. PLoS One, 2010, 5(12): e14246.
doi: 10.1371/journal.pone.0014246 |
| [18] |
Honkala AT, Tailor D, Malhotra SV. Guanylate-binding protein 1: an emerging target in inflammation and cancer. Front Immunol, 2019, 10: 3139.
doi: 10.3389/fimmu.2019.03139 pmid: 32117203 |
| [19] |
Syguda A, Bauer M, Benscheid U, Ostler N, Naschberger E, Ince S, Sturzl M, Herrmann C. Tetramerization of human guanylate-binding protein 1 is mediated by coiled-coil formation of the C-terminal alpha-helices. FEBS J, 2012, 279(14): 2544-2554.
doi: 10.1111/ejb.2012.279.issue-14 |
| [20] |
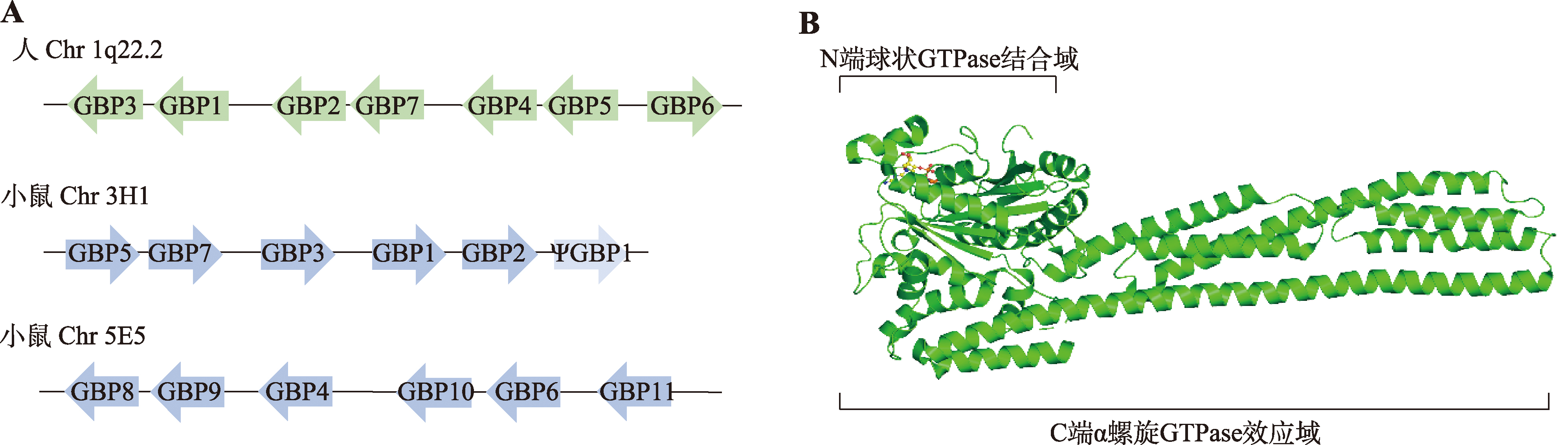
Olszewski MA, Gray J, Vestal DJ. In silico genomic analysis of the human and murine guanylate-binding protein (GBP) gene clusters. J Interferon Cytokine Res, 2006, 26(5): 328-352.
doi: 10.1089/jir.2006.26.328 |
| [21] |
Kresse A, Konermann C, Degrandi D, Beuter-Gunia C, Wuerthner J, Pfeffer K, Beer S. Analyses of murine GBP homology clusters based on in silico, in vitro and in vivo studies. BMC Genomics, 2008, 9: 158.
doi: 10.1186/1471-2164-9-158 pmid: 18402675 |
| [22] | EMBL-EBI.https://www.ebi.ac.uk/pdbe/pdbe-kb/proteins/P32455/structures. |
| [23] |
Nei M, Gu X, Sitnikova T. Evolution by the birth- and-death process in multigene families of the vertebrate immune system. Proc Natl Acad Sci USA, 1997, 94(15): 7799-7806.
doi: 10.1073/pnas.94.15.7799 pmid: 9223266 |
| [24] |
Wandel MP, Kim BH, Park ES, Boyle KB, Nayak K, Lagrange B, Herod A, Henry T, Zilbauer M, Rohde J, Macmicking JD, Randow F. Guanylate-binding proteins convert cytosolic bacteria into caspase-4 signaling platforms. Nat Immunol, 2020, 21(8): 880-891.
doi: 10.1038/s41590-020-0697-2 pmid: 32541830 |
| [25] |
Corte-Real J V, Baldauf H M, Abrantes J, Esteves P J. Evolution of the guanylate binding protein (GBP) genes: emergence of GBP7 genes in primates and further acquisition of a unique GBP3 gene in simians. Mol Immunol, 2021, 132: 79-81.
doi: 10.1016/j.molimm.2021.01.025 |
| [26] |
Li G, Zhang JY, Sun Y, Wang H, Wang YQ. The evolutionarily dynamic IFN-inducible GTPase proteins play conserved immune functions in vertebrates and cephalochordates. Mol Biol Evol, 2009, 26(7): 1619-1630.
doi: 10.1093/molbev/msp074 pmid: 19369598 |
| [27] |
Agostini L, Martinon F, Burns K, Mcdermott MF, Hawkins PN, Tschopp J. NALP3 forms an IL-1beta- processing inflammasome with increased activity in Muckle-Wells autoinflammatory disorder. Immunity, 2004, 20(3): 319-325.
doi: 10.1016/S1074-7613(04)00046-9 |
| [28] |
Lugrin J, Martinon F. The AIM2 inflammasome: sensor of pathogens and cellular perturbations. Immunol Rev, 2018, 281(1): 99-114.
doi: 10.1111/imr.12618 pmid: 29247998 |
| [29] |
Cai X, Chen JQ, Xu H, Liu SQ, Jiang QX, Halfmann R, Chen ZJJ. Prion-like polymerization underlies signal transduction in antiviral immune defense and inflammasome activation. Cell, 2014, 156(6): 1207-1222.
doi: S0092-8674(14)00199-8 pmid: 24630723 |
| [30] |
Swanson KV, Deng M, Ting JPY. The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat Rev Immunol, 2019, 19(8): 477-489.
doi: 10.1038/s41577-019-0165-0 pmid: 31036962 |
| [31] |
Sharma D, Kanneganti TD. The cell biology of inflammasomes: mechanisms of inflammasome activation and regulation. J Cell Biol, 2016, 213(6): 617-629.
doi: 10.1083/jcb.201602089 pmid: 27325789 |
| [32] | Yi YS. Dual roles of the caspase-11 non-canonical inflammasome in inflammatory bowel disease. Int Immunopharmacol, 2022, 108: 108739. |
| [33] |
Yi YS. Caspase-11 non-canonical inflammasome: a critical sensor of intracellular lipopolysaccharide in macrophage- mediated inflammatory responses. Immunology, 2017, 152(2): 207-217.
doi: 10.1111/imm.2017.152.issue-2 |
| [34] |
Shi JJ, Zhao Y, Wang YP, Gao WQ, Ding JJ, Li P, Hu LY, Shao F. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature, 2014, 514(7521): 187-192.
doi: 10.1038/nature13683 |
| [35] |
Yi YS. Functional crosstalk between non-canonical caspase-11 and canonical NLRP3 inflammasomes during infection-mediated inflammation. Immunology, 2020, 159(2): 142-155.
doi: 10.1111/imm.v159.2 |
| [36] |
Kayagaki N, Stowe IB, Lee BL, O'Rourke K, Anderson K, Warming S, Cuellar T, Haley B, Roose-Girma M, Phung QT, Liu PS, Lill JR, Li H, Wu JS, Kummerfeld S, Zhang J, Lee WP, Snipas SJ, Salvesen GS, Morris LX, Fitzgerald L, Zhang YF, Bertram EM, Goodnow CC, Dixit VM. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature, 2015, 526(7575): 666-671.
doi: 10.1038/nature15541 |
| [37] |
Shi JJ, Zhao Y, Wang K, Shi XY, Wang Y, Huang HW, Zhuang YH, Cai T, Wang FC, Shao F. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature, 2015, 526(7575): 660-665.
doi: 10.1038/nature15514 |
| [38] |
Kim BH, Shenoy AR, Kumar P, Das R, Tiwari S, Macmicking J D. A family of IFN-gamma-inducible 65-kD GTPases protects against bacterial infection. Science, 2011, 332(6030): 717-721.
doi: 10.1126/science.1201711 |
| [39] | Selleck EM, Fentress SJ, Beatty WL, Degrandi D, Pfeffer K, Virgin HW 4th, Macmicking JD, Sibley LD. Guanylate-binding protein 1 (Gbp1) contributes to cell-autonomous immunity against Toxoplasma gondii. PLoS Pathog, 2013, 9(4): e1003320. |
| [40] | Xavier A, Al-Zeer MA, Meyer TF, Daumke O. hGBP1 coordinates Chlamydia restriction and inflammasome activation through sequential GTP hydrolysis. Cell Rep, 2020, 31(7): 107667. |
| [41] |
Santos JC, Boucher D, Schneider LK, Demarco B, Dilucca M, Shkarina K, Heilig R, Chen KW, Lim RYH, Broz P. Human GBP1 binds LPS to initiate assembly of a caspase-4 activating platform on cytosolic bacteria. Nat Commun, 2020, 11(1): 3276.
doi: 10.1038/s41467-020-16889-z pmid: 32581219 |
| [42] |
Degrandi D, Kravets E, Konermann C, Beuter-Gunia C, Klümpers V, Lahme S, Rasch E, Mausberg AK, Beer- Hammer S, Pfeffer K. Murine guanylate binding protein 2 (mGBP2) controls Toxoplasma gondii replication. Proc Natl Acad Sci USA, 2013, 110(1): 294-299.
doi: 10.1073/pnas.1205635110 pmid: 23248289 |
| [43] |
Meunier E, Wallet P, Dreier RF, Costanzo S, Anton L, Rühl S, Dussurgey S, Dick MS, Kistner A, Rigard M, Degrandi D, Pfeffer K, Yamamoto M, Henry T, Broz P. Guanylate-binding proteins promote activation of the AIM2 inflammasome during infection with Francisella novicida. Nat Immunol, 2015, 16(5): 476-484.
doi: 10.1038/ni.3119 pmid: 25774716 |
| [44] |
Feng SY, Man SM. Captain GBP1: inflammasomes assemble, pyroptotic endgame. Nat Immunol, 2020, 21(8): 829-830.
doi: 10.1038/s41590-020-0727-0 pmid: 32699406 |
| [45] |
Man SM, Karki R, Malireddi RK, Neale G, Vogel P, Yamamoto M, Lamkanfi M, Kanneganti TD. The transcription factor IRF1 and guanylate-binding proteins target activation of the AIM2 inflammasome by Francisella infection. Nat Immunol, 2015, 16(5): 467-475.
doi: 10.1038/ni.3118 pmid: 25774715 |
| [46] |
Yang JM, Hwang I, Lee E, Shin SJ, Lee EJ, Rhee JH, Yu JW. Bacterial outer membrane vesicle-mediated cytosolic delivery of Flagellin triggers host NLRC4 canonical inflammasome signaling. Front Immunol, 2020, 11: 581165.
doi: 10.3389/fimmu.2020.581165 |
| [47] |
Virreira WS, Niedelman W, Jensen KD, Rosowski EE, Julien L, Spooner E, Caradonna K, Burleigh BA, Saeij JPJ, Ploegh HL, Frickel EM. Determinants of GBP recruitment to Toxoplasma gondii vacuoles and the parasitic factors that control it. PLoS One, 2011, 6(9): e24434.
doi: 10.1371/journal.pone.0024434 |
| [48] |
Sweeney TE, Braviak L, Tato CM, Khatri P. Genome-wide expression for diagnosis of pulmonary tuberculosis: a multicohort analysis. Lancet Respir Med, 2016, 4(3): 213-224.
doi: 10.1016/S2213-2600(16)00048-5 pmid: 26907218 |
| [49] |
Costa LLD, Delcroix M, Dalla Costa ER, Prestes IV, Milano M, Francis SS, Unis G, Silva DR, Riley LW, Rossetti ML. A real-time PCR signature to discriminate between tuberculosis and other pulmonary diseases. Tuberculosis (Edinb), 2015, 95(4): 421-425.
doi: 10.1016/j.tube.2015.04.008 |
| [50] |
Satproedprai N, Wichukchinda N, Suphankong S, Inunchot W, Kuntima T, Kumpeerasart S, Wattanapokayakit S, Nedsuwan S, Yanai H, Higuchi K, Harada N, Mahasirimongkol S. Diagnostic value of blood gene expression signatures in active tuberculosis in Thais: a pilot study. Genes Immun, 2015, 16(4): 253-260.
doi: 10.1038/gene.2015.4 pmid: 25764116 |
| [51] | Cerqueira DM, Gomes MTR, Silva ALN, Rungue M, Assis NRG, Guimaraes ES, Morais SB, Broz P, Zamboni DS, Oliveira SC. Guanylate-binding protein 5 licenses caspase-11 for Gasdermin-D mediated host resistance to Brucella abortus infection. PLoS Pathog, 2018, 14(12): e1007519. |
| [52] |
Liu BCY, Sarhan J, Panda A, Muendlein HI, Ilyukha V, Coers J, Yamamoto M, Isberg RR, Poltorak A. Constitutive interferon maintains GBP expression required for release of bacterial components upstream of pyroptosis and anti-DNA responses. Cell Rep, 2018, 24(1): 155-168.
doi: S2211-1247(18)30907-0 pmid: 29972777 |
| [53] | Fisch D, Bando H, Clough B, Hornung V, Yamamoto M, Shenoy AR, Frickel EM. Human GBP1 is a microbe-specific gatekeeper of macrophage apoptosis and pyroptosis. EMBO J, 2019, 38(13): e100926. |
| [54] | Dickinson MS, Kutsch M, Sistemich L, Hernandez D, Piro AS, Needham D, Lesser CF, Herrmann C, Coers J. LPS-aggregating proteins GBP1 and GBP2 are each sufficient to enhance caspase-4 activation both in cellulo and in vitro. Proc Natl Acad Sci USA, 2023, 120(15): e2078939176. |
| [55] |
Wandel MP, Pathe C, Werner EI, Ellison CJ, Boyle KB, von der Malsburg A, Rohde J, Randow F.GBPs inhibit motility of Shigella flexneri but are targeted for degradation by the bacterial ubiquitin Ligase IpaH9.8. Cell Host Microbe, 2017, 22(4): 507-518.
doi: S1931-3128(17)30395-5 pmid: 29024643 |
| [56] | Mesner D, Reuschl AK, Whelan MVX, Bronzovich T, Haider T, Thorne LG, Ragazzini R, Bonfanti P, Towers GJ, Jolly C. SARS-CoV-2 evolution influences GBP and IFITM sensitivity. Proc Natl Acad Sci USA, 2023, 120(5): e2082390176. |
| [1] | Zhenrong Yang, Gangqiao Zhou. The application of CRISPR genome editing technologies in the pathogenesis studies, diagnosis, prevention and treatment of infectious diseases [J]. Hereditas(Beijing), 2023, 45(11): 950-962. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||