Hereditas(Beijing) ›› 2024, Vol. 46 ›› Issue (6): 478-489.doi: 10.16288/j.yczz.24-083
• Research Article • Previous Articles Next Articles
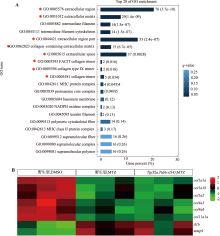
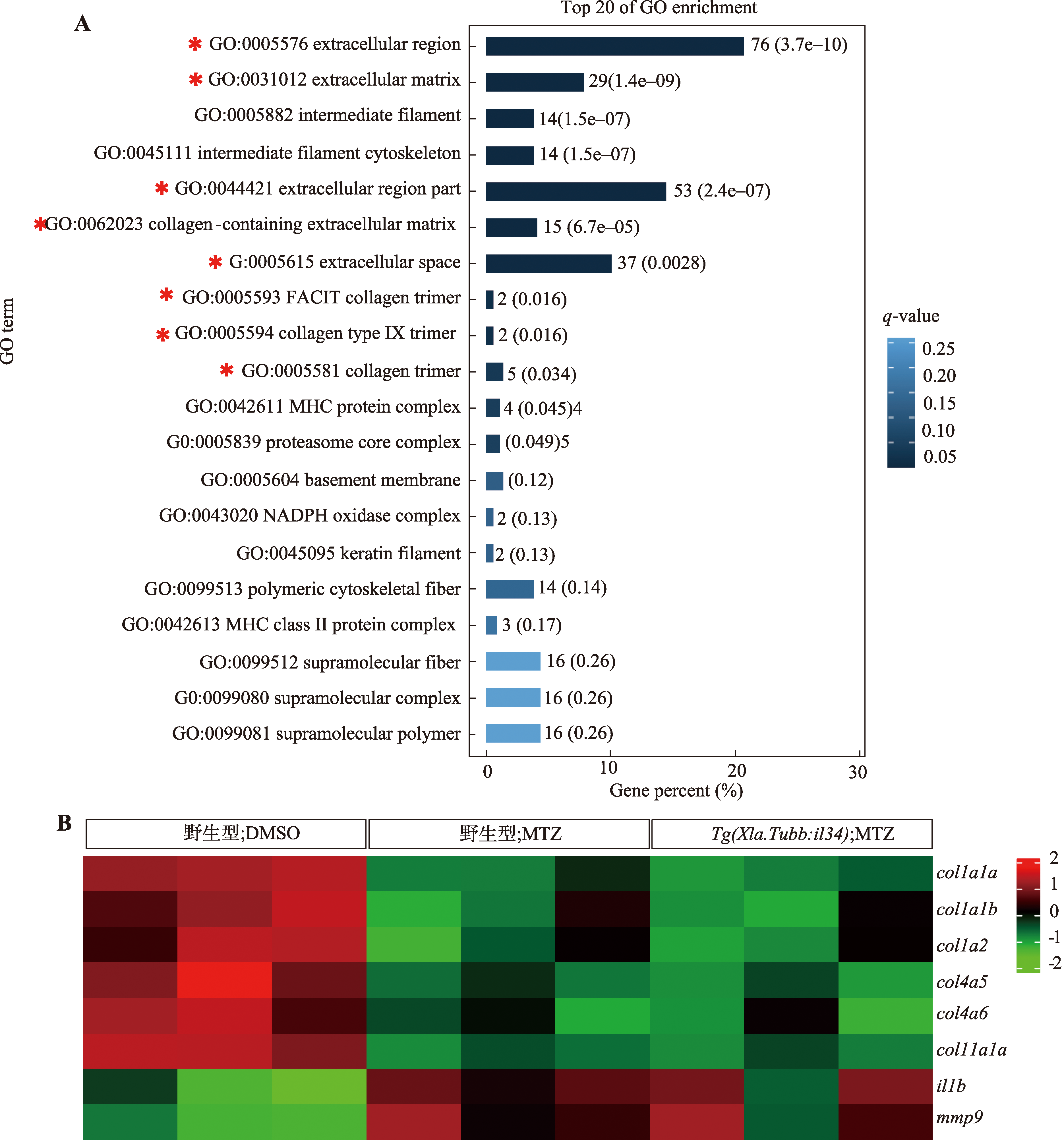
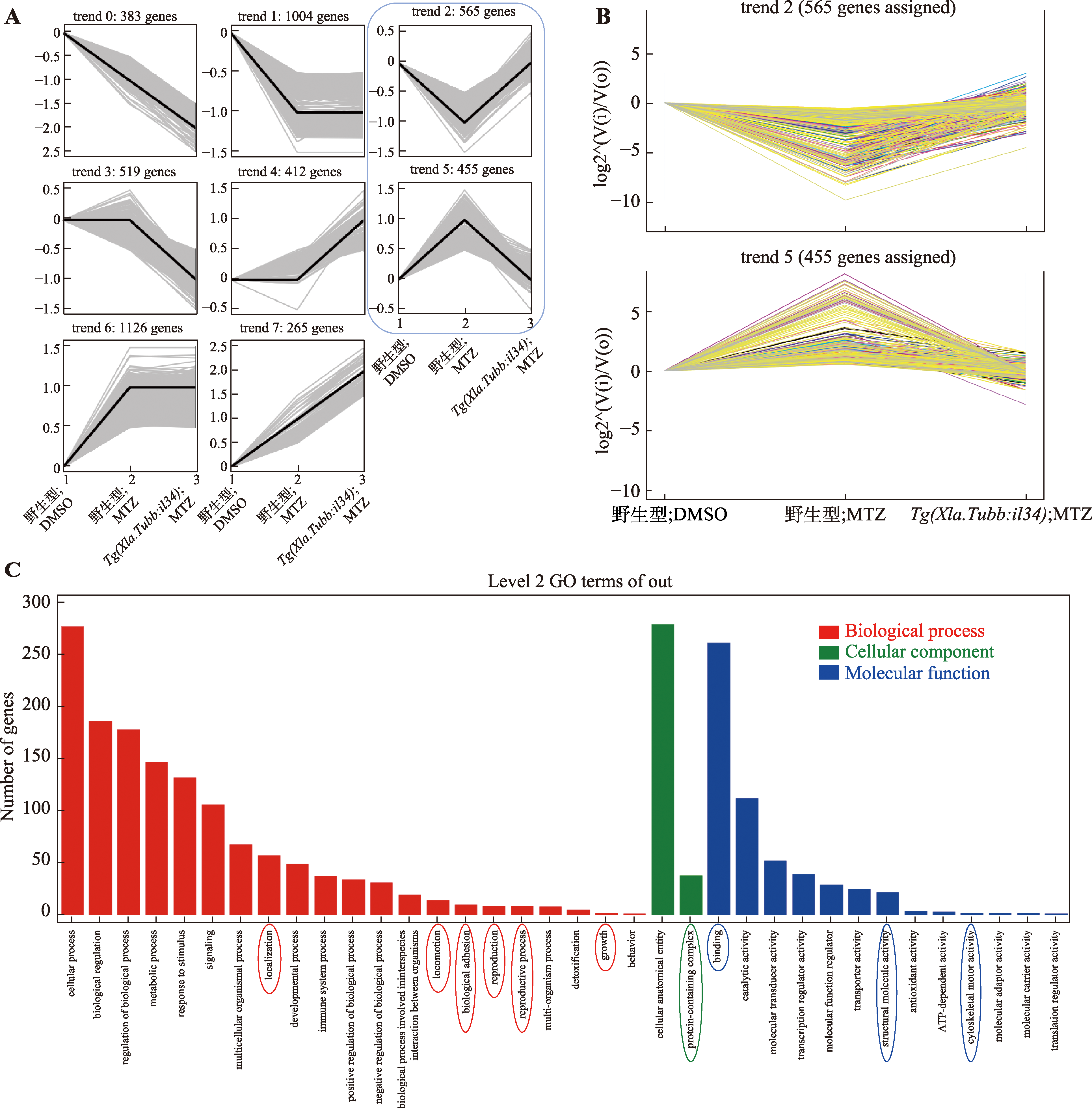
Il34 rescues metronidazole-induced impairment of spinal cord regeneration in zebrafish central nervous system
Liu Jixiang( ), Lai Siting, Bai Jing, Xu Jin(
), Lai Siting, Bai Jing, Xu Jin( )
)
- Division of Development Biology & Regenerative Medicine, South China University of Technology, Guangzhou 510006, China
-
Received:2024-03-25Revised:2024-05-02Online:2024-06-20Published:2024-05-21 -
Supported by:National Natural Science Foundation of China(32170827);National Natural Science Foundation of China(32370886)
Cite this article
Liu Jixiang, Lai Siting, Bai Jing, Xu Jin. Il34 rescues metronidazole-induced impairment of spinal cord regeneration in zebrafish central nervous system[J]. Hereditas(Beijing), 2024, 46(6): 478-489.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
| [1] | Cosar C, Julou L. The activity of 1-(2-hydroxyethyl)-2- methyl-5-nitroimidazole (R. P. 8823) against experimental Trichomonas vaginalis infections. Ann Inst Pasteur (Paris), 1959, 96(2): 238-241. |
| [2] |
Freeman CD, Klutman NE, Lamp KC. Metronidazole. A therapeutic review and update. Drugs, 1997, 54(5): 679-708.
doi: 10.2165/00003495-199754050-00003 pmid: 9360057 |
| [3] | Jokipii L, Jokipii AM. In vitro susceptibility of Giardia lamblia trophozoites to metronidazole and tinidazole. Infect Dis, 1980, 141(3): 317-325. |
| [4] | Farthing MJ, Inge PM. Antigiardial activity of the bile salt-like antibiotic sodium fusidate. J Antimicrob Chemother, 1986, 17(2): 165-171. |
| [5] |
Vinayak VK, Mahajan RC, Chitkara NL. In-vitro activity of methronidazole on the cysts of Entamoeba histolytica. Indian J Pathol Bacteriol, 1975, 18(1): 61-64.
pmid: 166039 |
| [6] |
Mahajan RC, Chitkara NL, Vinayak VK, Dutta DV. In vitro comparative evaluation of tinidazole and metronidazole on strains of Entamoeba histolytica. Indian J Pathol Bacteriol, 1974, 17(4): 226-228.
pmid: 4376127 |
| [7] |
Tally FP, Sutter VL, Finegold SM. Treatment of anaerobic infections with metronidazole. Antimicrob Agents Chemother, 1975, 7(5): 672-675.
doi: 10.1128/AAC.7.5.672 pmid: 1096810 |
| [8] |
Kusumi RK, Plouffe JF, Wyatt RH, Fass RJ. Central nervous system toxicity associated with metronidazole therapy. Ann Intern Med, 1980, 93(1): 59-60.
pmid: 7396319 |
| [9] |
Alvarez RS, Richardson DA, Bent AE, Ostergard DR. Central nervous system toxicity related to prolonged metronidazole therapy. Am J Obstet Gynecol, 1983, 145(5): 640-641.
pmid: 6829642 |
| [10] |
Guglielmo BJ. Metronidazole neurotoxicity: suspicions confirmed. Clin Infect Dis, 2021, 72(12): 2101-2102.
doi: 10.1093/cid/ciaa400 pmid: 32266372 |
| [11] | Quickfall D, Daneman N, Dmytriw AA, Juurlink DN. Metronidazole-induced neurotoxicity. CMAJ, 2021, 193(42): E1630. |
| [12] |
Kuriyama A, Jackson JL, Doi A, Kamiya T. Metronidazole- induced central nervous system toxicity: a systematic review. Clin Neuropharmacol, 2011, 34(6): 241-247.
doi: 10.1097/WNF.0b013e3182334b35 pmid: 21996645 |
| [13] | Patel K, Green-Hopkins I, Lu S, Tunkel AR. Cerebellar ataxia following prolonged use of metronidazole: case report and literature review. Int J Infect Dis, 2008, 12(6): e111-e114. |
| [14] |
White DT, Mumm JS. The nitroreductase system of inducible targeted ablation facilitates cell-specific regenerative studies in zebrafish. Methods, 2013, 62(3): 232-240.
doi: 10.1016/j.ymeth.2013.03.017 pmid: 23542552 |
| [15] | Lai ST, Kumari A, Liu JX, Zhang YY, Zhang WQ, Yen KY, Xu J. Chemical screening reveals Ronidazole is a superior prodrug to Metronidazole for nitroreductase-induced cell ablation system in zebrafish larvae. J Genet Genomics, 2021, 48(12): 1081-1090. |
| [16] |
Gürcü B, Koca YB, Özkut M, Tuğlu Mİ. Matrix changes due to the toxic effects of metronidazole in intestinal tissue of fish (Onchorhynchus mykiss). Chemosphere, 2016, 144: 1605-1610.
doi: 10.1016/j.chemosphere.2015.10.043 pmid: 26517388 |
| [17] |
Salter MW, Beggs S. Sublime microglia: expanding roles for the guardians of the CNS. Cell, 2014, 158(1): 15-24.
doi: 10.1016/j.cell.2014.06.008 pmid: 24995975 |
| [18] | Hu XM, Leak RK, Shi YJ, Suenaga J, Gao YQ, Zheng P, Chen J. Microglial and macrophage polarization-new prospects for brain repair. Nat Rev Neurol, 2015, 11(1): 56-64. |
| [19] |
Prinz M, Jung S, Priller J. Microglia biology: one century of evolving concepts. Cell, 2019, 179(2): 292-311.
doi: S0092-8674(19)31004-9 pmid: 31585077 |
| [20] |
Colonna M, Butovsky O. Microglia function in the central nervous system during health and neurodegeneration. Annu Rev Immunol, 2017, 35: 441-468.
doi: 10.1146/annurev-immunol-051116-052358 pmid: 28226226 |
| [21] |
Wright-Jin EC, Gutmann DH. Microglia as dynamic cellular mediators of brain function. Trends Mol Med, 2019, 25(11): 967-979.
doi: S1471-4914(19)30236-9 pmid: 31597593 |
| [22] |
Davies LC, Jenkins SJ, Allen JE, Taylor PR. Tissue- resident macrophages. Nat Immunol, 2013, 14(10): 986-995.
doi: 10.1038/ni.2705 pmid: 24048120 |
| [23] |
Zhou X, Wahane S, Friedl MS, Kluge M, Friedel CC, Avrampou K, Zachariou V, Guo L, Zhang B, He XJ, Friedel RH, Zou HY. Microglia and macrophages promote corralling, wound compaction and recovery after spinal cord injury via Plexin-B2. Nat Neurosci, 2020, 23(3): 337-350.
doi: 10.1038/s41593-020-0597-7 pmid: 32112058 |
| [24] | Li Y, He XL, Kawaguchi R, Zhang Y, Wang Q, Monavarfeshani A, Yang ZY, Chen B, Shi ZJ, Meng HY, Zhou SL, Zhu JJ, Jacobi A, Swarup V, Popovich PG, Geschwind DH, He ZG. Microglia-organized scar-free spinal cord repair in neonatal mice. Nature, 2020, 587(7835): 613-618. |
| [25] |
Wu ST, Xue RT, Hassan S, Nguyen TML, Wang TN, Pan HR, Xu J, Liu QF, Zhang WQ, Wen ZL. Il34-Csf1r pathway regulates the migration and colonization of microglial precursors. Dev Cell, 2018, 46(5): 552-563.e4.
doi: S1534-5807(18)30641-5 pmid: 30205037 |
| [26] | Baghdadi M, Umeyama Y, Hama N, Kobayashi T, Han N, Wada H, Seino KI. Interleukin-34, a comprehensive review. J Leukoc Biol, 2018, 104(5): 931-951. |
| [27] | Chitramuthu BP, Bennett HP. High resolution whole mount in situ hybridization within zebrafish embryos to study gene expression and function. J Vis Exp, 2013, (80): e50644. |
| [28] |
Zhou WB, Hildebrandt F. Inducible podocyte injury and proteinuria in transgenic zebrafish. J Am Soc Nephrol, 2012, 23(6): 1039-1047.
doi: 10.1681/ASN.2011080776 pmid: 22440901 |
| [29] |
Croese T, Castellani G, Schwartz M. Immune cell compartmentalization for brain surveillance and protection. Nat Immunol, 2021, 22(9): 1083-1092.
doi: 10.1038/s41590-021-00994-2 pmid: 34429552 |
| [30] |
Borst K, Dumas AA, Prinz M. Microglia: immune and non-immune functions. Immunity, 2021, 54(10): 2194-2208.
doi: 10.1016/j.immuni.2021.09.014 pmid: 34644556 |
| [31] |
Li QY, Barres BA. Microglia and macrophages in brain homeostasis and disease. Nat Rev Immunol, 2018, 18(4): 225-242.
doi: 10.1038/nri.2017.125 pmid: 29151590 |
| [32] |
Li L, Jin H, Xu J, Shi YY, Wen ZL. Irf8 regulates macrophage versus neutrophil fate during zebrafish primitive myelopoiesis. Blood, 2011, 117(4): 1359-1369.
doi: 10.1182/blood-2010-06-290700 pmid: 21079149 |
| [33] |
Wehner D, Tsarouchas TM, Michael A, Haase C, Weidinger G, Reimer MM, Becker T, Becker CG. Wnt signaling controls pro-regenerative Collagen XII in functional spinal cord regeneration in zebrafish. Nat Commun, 2017, 8(1): 126.
doi: 10.1038/s41467-017-00143-0 pmid: 28743881 |
| [34] | Tsata V, Möllmert S, Schweitzer C, Kolb J, Möckel C, Böhm B, Rosso G, Lange C, Lesche M, Hammer J, Kesavan G, Beis D, Guck J, Brand M, Wehner D. A switch in pdgfrb(+) cell-derived ECM composition prevents inhibitory scarring and promotes axon regeneration in the zebrafish spinal cord. Dev Cell, 2021, 56(4): 509-524.e9. |
| [35] |
O'Shea TM, Burda JE, Sofroniew MV. Cell biology of spinal cord injury and repair. J Clin Invest, 2017, 127(9): 3259-3270.
doi: 10.1172/JCI90608 pmid: 28737515 |
| [36] | Bloom O, Herman PE, Spungen AM. Systemic inflammation in traumatic spinal cord injury. Exp Neurol, 2020, 325: 113143. |
| [37] |
Morales RA, Allende ML. Peripheral macrophages promote tissue regeneration in zebrafish by fine-tuning the inflammatory response. Front Immunol, 2019, 10: 253.
doi: 10.3389/fimmu.2019.00253 pmid: 30891030 |
| [38] |
Benowitz LI, Popovich PG. Inflammation and axon regeneration. Curr Opin Neurol, 2011, 24(6): 577-583.
doi: 10.1097/WCO.0b013e32834c208d pmid: 21968547 |
| [39] | Jing X, Wang S, Tang H, Li DZ, Zhou FY, Xin LJ, He QQ, Hu SS, Zhang TW, Chen T, Song JL. Dynamically bioresponsive DNA hydrogel incorporated with dual-functional stem cells from apical papilla-derived exosomes promotes diabetic bone regeneration. ACS Appl Mater Interfaces, 2022, 14(14): 16082-16099. |
| [40] |
Tsarouchas TM, Wehner D, Cavone L, Munir T, Keatinge M, Lambertus M, Underhill A, Barrett T, Kassapis E, Ogryzko N, Feng Y, van Ham TJ, Becker T, Becker CG. Dynamic control of proinflammatory cytokines Il-1β and Tnf-α by macrophages in zebrafish spinal cord regeneration. Nat Commun, 2018, 9(1): 4670.
doi: 10.1038/s41467-018-07036-w pmid: 30405119 |
| [41] |
Eva R, Fawcett J. Integrin signalling and traffic during axon growth and regeneration. Curr Opin Neurobiol, 2014, 27: 179-185.
doi: 10.1016/j.conb.2014.03.018 pmid: 24793179 |
| [42] |
Nieuwenhuis B, Haenzi B, Andrews MR, Verhaagen J, Fawcett JW. Integrins promote axonal regeneration after injury of the nervous system. Biol Rev Camb Philos Soc, 2018, 93(3): 1339-1362.
doi: 10.1111/brv.12398 pmid: 29446228 |
| [43] |
Blanquie O, Bradke F. Cytoskeleton dynamics in axon regeneration. Curr Opin Neurobiol, 2018, 51: 60-69.
doi: S0959-4388(18)30008-4 pmid: 29544200 |
| [1] | Jiaxin Hong, Song’en Xu, Wenqing Zhang, Wei Liu. The interaction of Pu.1 and cMyb in zebrafish neutrophil development [J]. Hereditas(Beijing), 2024, 46(4): 319-332. |
| [2] | Piao Sun, Ying Li, Fan Liu, Lu Wang. Generation and analysis of TPI deficiency zebrafish model [J]. Hereditas(Beijing), 2024, 46(3): 232-241. |
| [3] | Kailun Li, Jingao Lu, Xiaohui Chen, Wenqing Zhang, Wei Liu. The role of the allantoin in promoting fracture healing in osteoclast-deficient zebrafish [J]. Hereditas(Beijing), 2023, 45(4): 341-353. |
| [4] | Shuyu Mao, Changrui Zhao, Chang Liu. The nuclear receptor REV-ERBα integrates circadian clock and energy metabolism [J]. Hereditas(Beijing), 2023, 45(2): 99-114. |
| [5] | Jing’ao Lu, Chunyan Huang, Zhiyin Lin, Zheng Tang, Ning Ma, Zhibin Huang. The role of the cd99l2 gene on leukocyte interstitial migration in zebrafish [J]. Hereditas(Beijing), 2022, 44(9): 798-809. |
| [6] | Pengfei Zheng, Haibo Xie, Panpan Zhu, Chengtian Zhao. Distribution pattern of floor plate neurons in zebrafish [J]. Hereditas(Beijing), 2022, 44(6): 510-520. |
| [7] | Hongyu Dai, Dong Ji, Cheng Tan, Jie Sun, Hao Yao. Research progress on the role and regulatory mechanism of pathogenic Th17 cells in neuroinflammation [J]. Hereditas(Beijing), 2022, 44(4): 289-299. |
| [8] | Tingting Zhang, Feng Liu. Study on a detection method of protein tyrosine sulfation modification in zebrafish [J]. Hereditas(Beijing), 2022, 44(2): 178-186. |
| [9] | Tingting Jia, Lei Lei, Xinyuan Wu, Shunyou Cai, Yixuan Chen, Yu Xue. Study on the mechanism of metformin on zebrafish skeletal development and damage repair [J]. Hereditas(Beijing), 2022, 44(1): 68-79. |
| [10] | Yajie Wang, Shuangshuang Wu, Jiang Chu, Xiangyang Kong. Lung microbiome mediates the progression from chronic obstructive pulmonary disease to lung cancer through inflammation [J]. Hereditas(Beijing), 2021, 43(1): 30-39. |
| [11] | Jiani Guo, Fan Liu, Lu Wang. Zebrafish blood disease models and applications [J]. Hereditas(Beijing), 2020, 42(8): 725-738. |
| [12] | Feng Xiong,Xunwei Xie,Luyuan Pan,Kuoyu Li,Liyue Liu,Yun Zhang,Linglu Li,Yonghua Sun. Development of resources, technologies and services at the China Zebrafish Resource Center [J]. Hereditas(Beijing), 2018, 40(8): 683-692. |
| [13] | Xin Zhou,Weiyun Li,Hongyan Wang. The roles and mechanisms of MST1/2 in the innate immune response [J]. Hereditas(Beijing), 2017, 39(7): 642-649. |
| [14] | Jingjin Xu, Wenjuan Zhang, Jingyi Wang, Liyun Yao, Yutian Pan, Yixin Ou, Yu Xue. The active component screening of Anoectochilus roxburghii and the functional study on inhibition of melanogenesis in zebrafish [J]. Hereditas(Beijing), 2017, 39(12): 1178-1187. |
| [15] | Shanshan Liu, Cuizhen Zhang, Gang Peng. Effects of starvation on the expression of feeding related neuropeptides in the larval zebrafish hypothalamus [J]. Hereditas(Beijing), 2016, 38(9): 821-830. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||