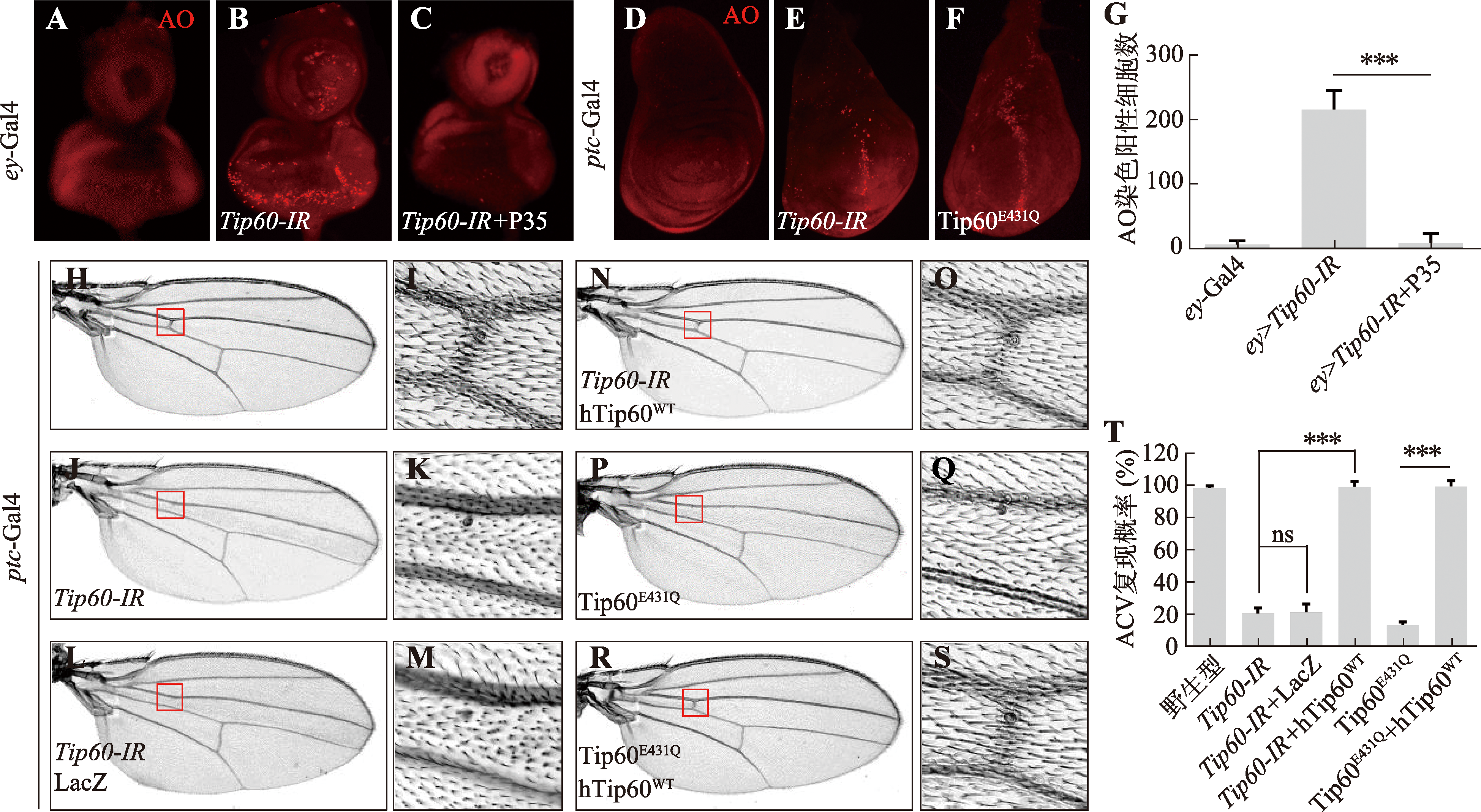
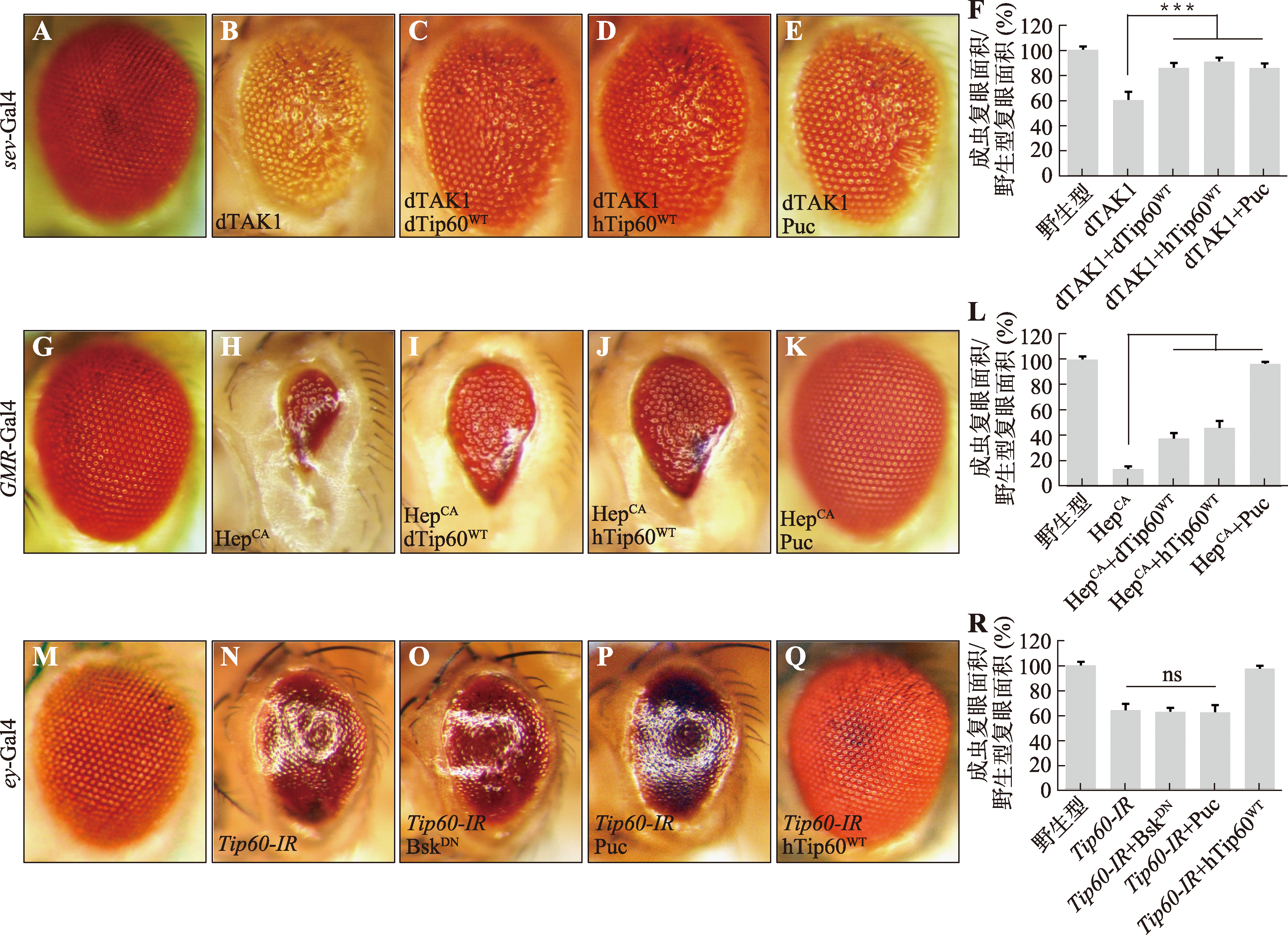
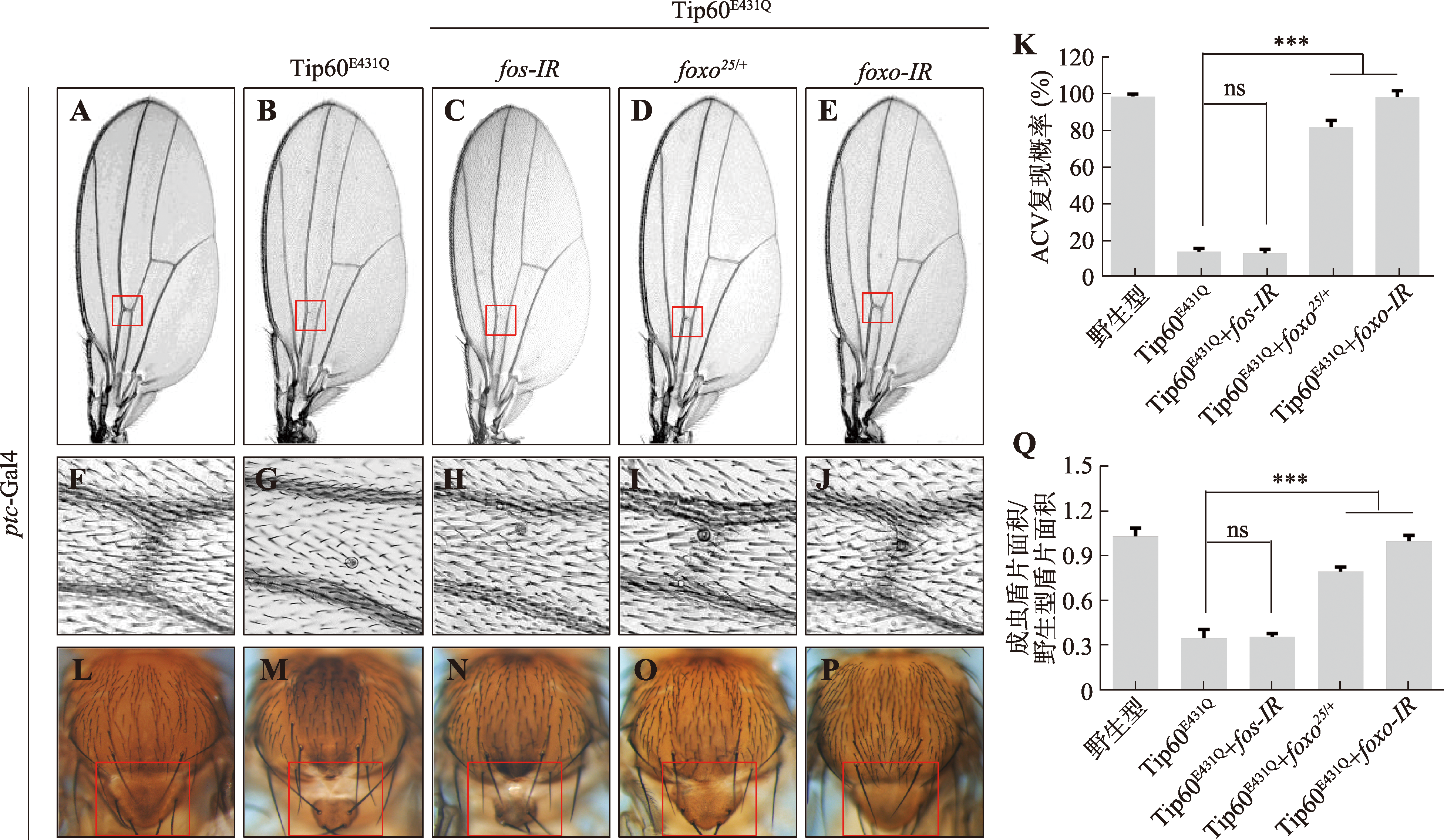
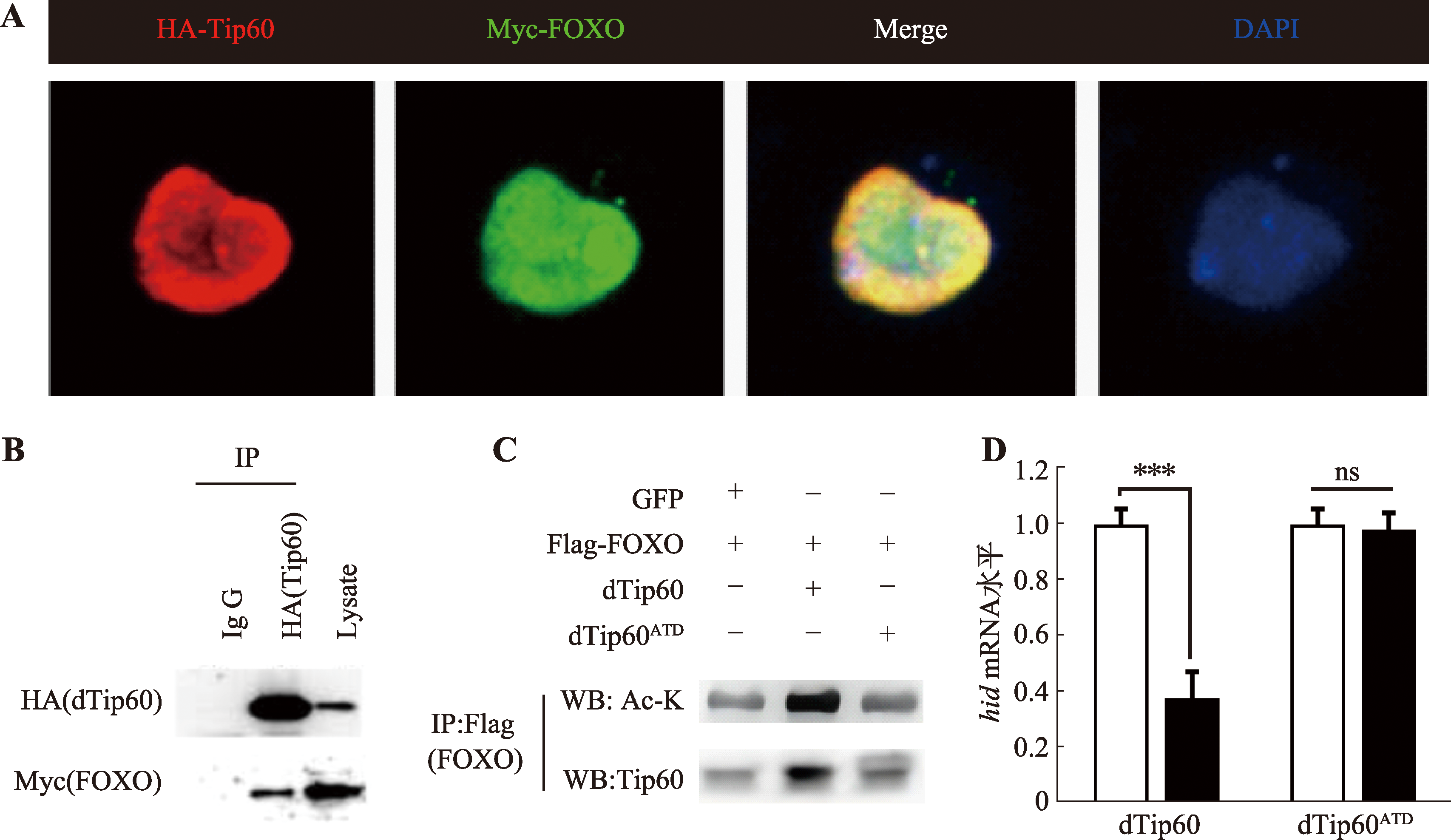
Hereditas(Beijing) ›› 2024, Vol. 46 ›› Issue (6): 490-501.doi: 10.16288/j.yczz.24-105
• Research Article • Previous Articles Next Articles
Tip60-FOXO regulates JNK signaling mediated apoptosis in Drosophila
Yang Jian( ), Shi Guojuan(
), Shi Guojuan( ), Peng Anghui, Xu Qingbo, Wang Ruiqi, Xue Lei, Yu Xinyang(
), Peng Anghui, Xu Qingbo, Wang Ruiqi, Xue Lei, Yu Xinyang( ), Sun Yihao(
), Sun Yihao( )
)
- Molecular Genetics Team of the Institute of Translational Medicine, Zhuhai People’s Hospital (Zhuhai Clinical Medical College of Jinan University), Zhuhai 519000, China
-
Received:2024-04-17Revised:2024-05-06Online:2024-06-20Published:2024-05-24 -
Supported by:National Natural Science Foundation of China(32100561);National Natural Science Foundation of China(32100447);Clinical Research Promotion Project of Zhuhai People’s Hospital(2023LCTS-36);Clinical Research Promotion Project of Zhuhai People’s Hospital(2023LCTS-41)
Cite this article
Yang Jian, Shi Guojuan, Peng Anghui, Xu Qingbo, Wang Ruiqi, Xue Lei, Yu Xinyang, Sun Yihao. Tip60-FOXO regulates JNK signaling mediated apoptosis in Drosophila[J]. Hereditas(Beijing), 2024, 46(6): 490-501.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
| [1] | Chen WQ, Xia CF, Zheng RS, Zhou MG, Lin CQ, Zeng HM, Zhang SW, Wang LJ, Yang ZX, Sun KX, Li H, Brown MD, Islami F, Bray F, Jemal A, He J. Disparities by province, age, and sex in site-specific cancer burden attributable to 23 potentially modifiable risk factors in China: a comparative risk assessment. Lancet Glob Health, 2019, 7(2): e257-e269. |
| [2] | Chen WQ, Zheng RS, Baade PD, Zhang SW, Zeng HM, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin, 2016, 66(2): 115-132. |
| [3] | Labi V, Erlacher M. How cell death shapes cancer. Cell Death Dis, 2015, 6(3): e1675. |
| [4] |
Valastyan S, Weinberg RA. Tumor metastasis: molecular insights and evolving paradigms. Cell, 2011, 147(2): 275-292.
doi: 10.1016/j.cell.2011.09.024 pmid: 22000009 |
| [5] | Dhanasekaran DN, Reddy EP. JNK-signaling: a multiplexing hub in programmed cell death. Genes Cancer, 2017, 8(9-10): 682-694. |
| [6] | Hu YM, Leo C, Yu S, Huang BCB, Wang H, Shen M, Luo Y, Daniel-Issakani S, Payan DG, Xu X. Identification and functional characterization of a novel human Misshapen/ Nck interacting kinase-related kinase, hMINKβ. J Biol Chem, 2004, 279(52): 54387-54397. |
| [7] |
Kamine J, Elangovan B, Subramanian T, Coleman D, Chinnadurai G. Identification of a cellular protein that specifically interacts with the essential cysteine region of the HIV-1 Tat transactivator. Virology, 1996, 216(2): 357-366.
pmid: 8607265 |
| [8] |
Squatrito M, Gorrini C, Amati B. Tip60 in DNA damage response and growth control: many tricks in one HAT. Trends Cell Biol, 2006, 16(9): 433-442.
doi: 10.1016/j.tcb.2006.07.007 pmid: 16904321 |
| [9] |
Ikura T, Ogryzko VV, Grigoriev M, Groisman R, Wang J, Horikoshi M, Scully R, Qin J, Nakatani Y. Involvement of the TIP60 histone acetylase complex in DNA repair and apoptosis. Cell, 2000, 102(4): 463-473.
pmid: 10966108 |
| [10] |
Doyon Y, Selleck W, Lane WS, Tan S, Côté J. Structural and functional conservation of the NuA4 histone acetyltransferase complex from yeast to humans. Mol Cell Biol, 2004, 24(5): 1884-1896.
doi: 10.1128/MCB.24.5.1884-1896.2004 pmid: 14966270 |
| [11] |
Kusch T, Florens L, Macdonald WH, Swanson SK, Yates JR 3rd, Abmayr SM, Washburn MP, Workman JL. Acetylation by Tip60 is required for selective histone variant exchange at DNA lesions. Science, 2004, 306(5704): 2084-2087.
pmid: 15528408 |
| [12] |
Yamamoto T, Horikoshi M. Novel substrate specificity of the histone acetyltransferase activity of HIV-1-Tat interactive protein Tip60. J Biol Chem, 1997, 272(49): 30595-30598.
doi: 10.1074/jbc.272.49.30595 pmid: 9388189 |
| [13] |
Patel JH, Du YP, Ard PG, Phillips C, Carella B, Chen CJ, Rakowski C, Chatterjee C, Lieberman PM, Lane WS, Blobel GA, Mcmahon SB. The c-MYC oncoprotein is a substrate of the acetyltransferases hGCN5/PCAF and TIP60. Mol Cell Biol, 2004, 24(24): 10826-10834.
pmid: 15572685 |
| [14] |
Legube G, Linares LK, Tyteca S, Caron C, Scheffner M, Chevillard-Briet M, Trouche D. Role of the histone acetyl transferase Tip60 in the p53 pathway. J Biol Chem, 2004, 279(43): 44825-44833.
doi: 10.1074/jbc.M407478200 pmid: 15310756 |
| [15] | Sapountzi V, Logan IR, Robson CN. Cellular functions of TIP60. Int J Biochem Cell Biol, 2006, 38(9): 1496-1509. |
| [16] |
Adamowicz M, Vermezovic J, D’Adda Di Fagagna F. NOTCH1 inhibits activation of ATM by impairing the formation of an ATM-FOXO3a-KAT5/Tip60 complex. Cell Rep, 2016, 16(8): 2068-2076.
doi: S2211-1247(16)30956-1 pmid: 27524627 |
| [17] | Khan C, Rusan NM. Using Drosophila to uncover the role of organismal physiology and the tumor microenvironment in cancer. Trends Cancer, 2024, 10(4): 289-311. |
| [18] | Tsintzas E, Niccoli T. Using Drosophila amyloid toxicity models to study Alzheimer's disease. Ann Hum Genet, 2024. |
| [19] |
Banerjee U, Girard JR, Goins LM, Spratford CM. Drosophila as a genetic model for hematopoiesis. Genetics, 2019, 211(2): 367-417.
doi: 10.1534/genetics.118.300223 pmid: 30733377 |
| [20] | Kahney EW, Snedeker JC, Chen X. Regulation of Drosophila germline stem cells. Curr Opin Cell Biol, 2019, 60: 27-35. |
| [21] |
Enomoto M, Siow C, Igaki T. Drosophila as a cancer model. Adv Exp Med Biol, 2018, 1076: 173-194.
doi: 10.1007/978-981-13-0529-0_10 pmid: 29951820 |
| [22] |
Weston CR, Davis RJ. The JNK signal transduction pathway. Curr Opin Cell Biol, 2007, 19(2): 142-149.
doi: 10.1016/j.ceb.2007.02.001 pmid: 17303404 |
| [23] | Gan T, Fan LX, Zhao L, Misra M, Liu M, Zhang M, Su Y. JNK signaling in Drosophila aging and longevity. Int J Mol Sci, 2021, 22(17): 9649. |
| [24] | Semba T, Sammons R, Wang XP, Xie XM, Dalby KN, Ueno NT. JNK signaling in stem cell self-renewal and differentiation. Int J Mol Sci, 2020, 21(7): 2613. |
| [25] |
Rana A, Rana B, Mishra R, Sondarva G, Rangasamy V, Das S, Viswakarma N, Kanthasamy A. Mixed lineage kinase-c-Jun N-terminal kinase axis: a potential therapeutic target in cancer. Genes Cancer, 2013, 4(9-10): 334-341.
doi: 10.1177/1947601913485415 pmid: 24349631 |
| [26] |
Solinas G, Becattini B. JNK at the crossroad of obesity, insulin resistance, and cell stress response. Mol Metab, 2017, 6(2): 174-184.
doi: S2212-8778(16)30244-7 pmid: 28180059 |
| [27] |
Weston CR, Davis RJ. The JNK signal transduction pathway. Curr Opin Genet Dev, 2002, 12(1): 14-21.
pmid: 11790549 |
| [28] |
Cavigelli M, Li WW, Lin A, Su B, Yoshioka K, Karin M. The tumor promoter arsenite stimulates AP-1 activity by inhibiting a JNK phosphatase. EMBO J, 1996, 15(22): 6269-6279.
pmid: 8947050 |
| [29] |
Kockel L, Homsy JG, Bohmann D. Drosophila AP-1: lessons from an invertebrate. Oncogene, 2001, 20(19): 2347-2364.
pmid: 11402332 |
| [30] |
Luo X, Puig O, Hyun J, Bohmann D, Jasper H. Foxo and Fos regulate the decision between cell death and survival in response to UV irradiation. EMBO J, 2007, 26(2): 380-390.
pmid: 17183370 |
| [31] |
Uhlirova M, Bohmann D. JNK- and Fos-regulated Mmp1 expression cooperates with Ras to induce invasive tumors in Drosophila. EMBO J, 2006, 25(22): 5294-5304.
doi: 10.1038/sj.emboj.7601401 pmid: 17082773 |
| [32] |
Essers MAG, Weijzen S, Saarloos I, de Ruiter ND, Bos JL, Burgering BMT. FOXO transcription factor activation by oxidative stress mediated by the small GTPase Ral and JNK. EMBO J, 2004, 23(24): 4802-4812.
doi: 10.1038/sj.emboj.7600476 pmid: 15538382 |
| [33] |
Lei K, Davis RJ. JNK phosphorylation of Bim-related members of the Bcl2 family induces Bax-dependent apoptosis. Proc Natl Acad Sci USA, 2003, 100(5): 2432-2437.
doi: 10.1073/pnas.0438011100 pmid: 12591950 |
| [34] |
Brown AK, Webb AE. Regulation of FOXO factors in mammalian cells. Curr Top Dev Biol, 2018, 127: 165-192.
doi: S0070-2153(17)30055-8 pmid: 29433737 |
| [35] |
Snigdha K, Singh A, Kango-Singh M. Yorkie-Cactus (IkappaBalpha)-JNK axis promotes tumor growth and progression in Drosophila. Oncogene, 2021, 40(24): 4124-4136.
doi: 10.1038/s41388-021-01831-4 pmid: 34017079 |
| [36] | Lam D, Shah S, de Castro IP, Loh SHY, Martins LM. Drosophila happyhour modulates JNK-dependent apoptosis. Cell Death Dis, 2010, 1(8): e66. |
| [37] | Camilleri-Robles C, Serras F, Corominas M. Role of D-GADD45 in JNK-dependent apoptosis and regeneration in Drosophila. Genes(Basel), 2019, 10(5): 378. |
| [38] | Igaki T. Correcting developmental errors by apoptosis: lessons from Drosophila JNK signaling. Apoptosis, 2009, 14(8): 1021-1028. |
| [39] | Sun YL, Jiang XF, Price BD. Tip60: connecting chromatin to DNA damage signaling. Cell Cycle, 2014, 9(5): 930-936. |
| [40] |
Tafesh-Edwards G, Eleftherianos I. JNK signaling in Drosophila immunity and homeostasis. Immunol Lett, 2020, 226: 7-11.
doi: S0165-2478(20)30348-5 pmid: 32598968 |
| [41] | Garg R, Kumariya S, Katekar R, Verma S, Goand UK, Gayen JR. JNK signaling pathway in metabolic disorders: an emerging therapeutic target. Eur J Pharmacol, 2021, 901: 174079. |
| [42] |
Saline M, Badertscher L, Wolter M, Lau R, Gunnarsson A, Jacso T, Norris T, Ottmann C, Snijder A. AMPK and AKT protein kinases hierarchically phosphorylate the N-terminus of the FOXO1 transcription factor, modulating interactions with 14-3-3 proteins. J Biol Chem, 2019, 294(35): 13106-13116.
doi: 10.1074/jbc.RA119.008649 pmid: 31308176 |
| [43] |
Drazic A, Myklebust LM, Ree R, Arnesen T. The world of protein acetylation. Biochim Biophys Acta, 2016, 1864(10): 1372-1401.
doi: 10.1016/j.bbapap.2016.06.007 pmid: 27296530 |
| [44] | Shen L, Lee S, Joo JC, Hong E, Cui ZY, Jo E, Park SJ, Jang HJ. Chelidonium majus induces apoptosis of human ovarian cancer cells via ATF3-mediated regulation of Foxo3a by Tip60. J Microbiol Biotechnol, 2022, 32(4): 493-503. |
| [45] | Tan KN, Avery VM, Carrasco-Pozo C. Metabolic roles of androgen receptor and Tip60 in androgen-dependent prostate cancer. Int J Mol Sci, 2020, 21(18): 6622. |
| [1] | Jinyi Zhang, Yumo He, Jingyu Zhou, Shufeng Weng, Huixia Ma, Taiyue Lin, Ying Xu. Hsa_circ_0007460 affects the survival of intracellular Mycobacterium tuberculosis by regulating autophagy and apoptosis of macrophages [J]. Hereditas(Beijing), 2023, 45(11): 1039-1051. |
| [2] | Mengxiao Wang, Margaret S. Ho. Recent advances on the role of glia in physiological behaviors: insights from Drosophila melanogaster [J]. Hereditas(Beijing), 2022, 44(4): 300-312. |
| [3] | Cuiling Wang, Xinyi Liu, Yahui Wang, Zheng Zhang, Zhidong Wang, Gangqiao Zhou. MCM2 promotes the proliferation, migration and invasion of cholangiocarcinoma cells by reducing the p53 signaling pathway [J]. Hereditas(Beijing), 2022, 44(3): 230-244. |
| [4] | Zhongyong Qin, Xiao Shi, Pingping Cao, Ying Chu, Wei Guan, Nan Yang, He Cheng, Yujie Sun. The NOXA promoter could function as an active enhancer to regulate the expression of BCL2 in the apoptosis response [J]. Hereditas(Beijing), 2020, 42(11): 1110-1121. |
| [5] | Wanyin Chen, Yidan Yan, Xiaojin Luan, Min Wang, Jie Fang. Functional analysis of CG8005 gene in Drosophila testis [J]. Hereditas(Beijing), 2020, 42(11): 1122-1132. |
| [6] | Tonglu YU,Dongliang Cai,Genfeng Zhu,Xiaojuan Ye,Taishan Min,Hongyan Chen,Daru Lu,Haoming Chen. Effects of CSN4 knockdown on proliferation and apoptosis of breast cancer MDA-MB-231 cells [J]. Hereditas(Beijing), 2019, 41(4): 318-326. |
| [7] | Maoliang Ran, Lianhua Dong, Bo Weng, Rong Cao, Fuzhi Peng, Hu Gao, Hui Luo, Bin Chen. miR-362 regulates the proliferation and apoptosis of porcine immature Sertoli cells through targeting the ZNF644 gene [J]. Hereditas(Beijing), 2018, 40(7): 572-584. |
| [8] | Jianyuan Zhao, Jiwei Ding, Zeyun Mi, Jinming Zhou, Tao Wei, Shan Cen. Genetic variance in the HIV-1 founder virus Vpr affects its ability to induce cell cycle G2 arrest and cell apoptosis [J]. HEREDITAS(Beijing), 2015, 37(5): 480-486. |
| [9] | Dan Bi,Yang Xu,Yue Pang,Qingwei Li. Molecular regulations of phosphatidylserine eversion in plasma membrane [J]. HEREDITAS(Beijing), 2015, 37(2): 140-147. |
| [10] | Lei Zhang, Yu Sui, Ting Wang, Lijian Li, Yuanjie Li, Caixia Jin, Fang Xu. Roles of hMMS2 gene in reversing the oxaliplatin tolerance of hu-man colon carcinoma cells [J]. HEREDITAS, 2014, 36(4): 346-353. |
| [11] | GUO Yun-Xue, MO De-Lin, CHEN Yao-Sheng, ZHANG Yue, ZHANG Yun, XIAO Shu-Qi, LIU Xiao-Hong. Effects of PI3K/AKT inhibitor wortmannin on proliferation and apoptosis of primary porcine preadipocytes [J]. HEREDITAS, 2012, 34(10): 1282-1290. |
| [12] | WANG Shi-Yao, JIN Wei-Na, WU Dan. Mechanisms of juvenile neuronal ceroid lipofuscinosis (JNCL) [J]. HEREDITAS, 2009, 31(8): 779-784. |
| [13] | MA Xiang-Dong, MA Xing, WU Xiao-Ming, CHEN Bi-Liang, WANG De-Tangx. Differentially expressed genes in diabetes-induced embryopathy [J]. HEREDITAS, 2009, 31(3): 280-284. |
| [14] | GUO Fen, LUO Zhi-Wen, LIU Zhao-Yu, LI Ru-Qin, LI Hong-Jian, ZHOU Tian-Hong. Studies of effect of prosaposin on cell proliferation, cell apoptosis and its possible molecular mechanism [J]. HEREDITAS, 2009, 31(12): 1226-1232. |
| [15] | FU Hao, ZHAO Dan-Yi, SUN Xiu-Ju, HUA Jun, YAN Yang, QIU Guang-Rong, SHANG Chao, FU Wei-Neng, SUN. Effect of B-RAF-specific RNA interference on gastric cancer BGC823 cell line [J]. HEREDITAS, 2007, 29(5): 537-537―540. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||