Hereditas(Beijing) ›› 2023, Vol. 45 ›› Issue (3): 187-197.doi: 10.16288/j.yczz.22-416
• Special Section:Excellent Doctoral Thesis • Previous Articles Next Articles
Inter-tissue communication of mitochondrial stress in aging
Qian Zhang1( ), Zihao Wang1,2(
), Zihao Wang1,2( ), Ye Tian1,2(
), Ye Tian1,2( )
)
- 1. State Key Laboratory of Molecular Developmental Biology, Institute of Genetics and Developmental Biology, Chinese Academy of Sciences, Beijing 100101, China
2. University of Chinese Academy of Sciences, Beijing 100093, China
-
Received:2022-12-21Revised:2023-01-30Online:2023-03-20Published:2023-02-24 -
Contact:Tian Ye E-mail:zhangqian@genetics.ac.cn;wangzihao@genetics.ac.cn;ytian@genetics.ac.cn -
Supported by:the National Natural Science Foundation of China(32200624);the China National Postdoctoral Program for Innovative Talents(BX2021356);the China Postdoctoral Science Foundation Funded Project(2021M703474)
Cite this article
Qian Zhang, Zihao Wang, Ye Tian. Inter-tissue communication of mitochondrial stress in aging[J]. Hereditas(Beijing), 2023, 45(3): 187-197.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
| [1] |
Van Der Bliek AM, Sedensky MM, Morgan PG.Cell biology of the mitochondrion. Genetics, 2017, 207(3): 843-871.
doi: 10.1534/genetics.117.300262 pmid: 29097398 |
| [2] |
Pellegrino MW, Nargund AM, Kirienko NV, Gillis R, Fiorese CJ, Haynes CM. Mitochondrial UPR-regulated innate immunity provides resistance to pathogen infection. Nature, 2014, 516(7531): 414-417.
doi: 10.1038/nature13818 |
| [3] |
Kurland CG, Andersson SGE. Origin and evolution of the mitochondrial proteome. Microbiol Mol Biol Rev, 2000, 64(4): 786-820.
doi: 10.1128/MMBR.64.4.786-820.2000 |
| [4] |
Friedman JR, Nunnari J. Mitochondrial form and function. Nature, 2014, 505(7483): 335-343.
doi: 10.1038/nature12985 |
| [5] | Shpilka T, Haynes CM. The mitochondrial UPR: mechanisms, physiological functions and implications in ageing. Nat Rev Mol, 2018, 19(2): 109-120. |
| [6] |
Pickles S, Vigié P, Youle RJ. Mitophagy and quality control mechanisms in mitochondrial maintenance. Curr Biol, 2018, 28(4): R170-R185.
doi: 10.1016/j.cub.2018.01.004 |
| [7] |
Bock FJ, Tait SWG. Mitochondria as multifaceted regulators of cell death. Nat Rev Mol Cell Biol, 2020, 21(2): 85-100.
doi: 10.1038/s41580-019-0173-8 |
| [8] |
Copeland JM, Cho J, Lo Jr T, Hur JH, Bahadorani S, Arabyan T, Rabie J, Soh J, Walker DW. Extension of Drosophila life span by RNAi of the mitochondrial respiratory chain. Curr Biol, 2009, 19(19): 1591-1598.
doi: 10.1016/j.cub.2009.08.016 pmid: 19747824 |
| [9] |
Dillin A, Hsu AL, Arantes-Oliveira N, Lehrer-Graiwer J, Hsin H, Fraser AG, Kamath RS, Ahringer J, Kenyon C. Rates of behavior and aging specified by mitochondrial function during development. Science, 2002, 298(5602): 2398-2401.
pmid: 12471266 |
| [10] |
Zhu D, Li XY, Tian Y. Mitochondrial-to-nuclear communication in aging: an epigenetic perspective. Trends Biochem Sci, 2022, 47(8): 645-659.
doi: 10.1016/j.tibs.2022.03.008 pmid: 35397926 |
| [11] |
Liu XX, Jiang N, Hughes B, Bigras E, Shoubridge E, Hekimi S. Evolutionary conservation of the clk-1- dependent mechanism of longevity: loss of mclk1 increases cellular fitness and lifespan in mice. Genes Dev, 2005, 19(20): 2424-2434.
doi: 10.1101/gad.1352905 |
| [12] |
Feng J, Bussière F, Hekimi S. Mitochondrial electron transport is a key determinant of life span in Caenorhabditis elegans. Dev Cell, 2001, 1(5): 633-644.
pmid: 11709184 |
| [13] |
Durieux J, Wolff S, Dillin A. The cell-non-autonomous nature of electron transport chain-mediated longevity. Cell, 2011, 144(1): 79-91.
doi: 10.1016/j.cell.2010.12.016 pmid: 21215371 |
| [14] |
Kang GM, Min SH, Lee CH, Kim JY, Lim HS, Choi MJ, Jung SB, Park JW, Kim S, Park CB, Dugu H, Choi JH, Jang WH, Park SE, Cho YM, Kim JG, Kim KG, Choi CS, Kim YB, Lee C, Shong M, Kim MS. Mitohormesis in hypothalamic POMC neurons mediates regular exercise- induced high-turnover metabolism. Cell Metab, 2021, 33(2): 334-349.e6.
doi: 10.1016/j.cmet.2021.01.003 |
| [15] |
Gómez-Valadés AG, Pozo M, Varela L, Boudjadja MB, Ramírez S, Chivite I, Eyre E, Haddad-Tóvolli R, Obri A, Milà-Guasch M, Altirriba J, Schneeberger M, Imbernón M, Garcia-Rendueles AR, Gama-Perez P, Rojo-Ruiz J, Rácz B, Alonso MT, Gomis R, Zorzano A, D'Agostino G, Alvarez CV, Nogueiras R, Garcia-Roves PM, Horvath TL, Claret M. Mitochondrial cristae-remodeling protein OPA1 in POMC neurons couples Ca2+ homeostasis with adipose tissue lipolysis. Cell Metab, 2021, 33(9): 1820-1835.e9.
doi: 10.1016/j.cmet.2021.07.008 pmid: 34343501 |
| [16] |
Su B, Wang XL, Zheng L, Perry G, Smith MA, Zhu XW. Abnormal mitochondrial dynamics and neurodegenerative diseases. Biochim Biophys Acta, 2010, 1802(1): 135-142.
doi: 10.1016/j.bbadis.2009.09.013 pmid: 19799998 |
| [17] |
Dunham-Snary KJ, Ballinger SW. Mitochondrial-nuclear DNA mismatch matters. Science, 2015, 349(6255): 1449-1450.
doi: 10.1126/science.aac5271 pmid: 26404813 |
| [18] |
Matilainen O, Quirós PM, Auwerx J. Mitochondria and epigenetics—crosstalk in homeostasis and stress. Trends Cell Biol, 2017, 27(6): 453-463.
doi: S0962-8924(17)30028-4 pmid: 28274652 |
| [19] |
Saki M, Prakash A. DNA damage related crosstalk between the nucleus and mitochondria. Free Radic Biol Med, 2017, 107: 216-227.
doi: 10.1016/j.freeradbiomed.2016.11.050 |
| [20] |
Hetz C, Zhang KZ, Kaufman RJ. Mechanisms, regulation and functions of the unfolded protein response. Nat Rev Mol Cell Biol, 2020, 21(8): 421-438.
doi: 10.1038/s41580-020-0250-z |
| [21] |
Anderson NS, Haynes CM. Folding the mitochondrial UPR into the integrated stress response. Trends Cell Biol, 2020, 30(6): 428-439.
doi: S0962-8924(20)30055-6 pmid: 32413314 |
| [22] |
Martinus RD, Garth GP, Webster TL, Cartwright P, Naylor DJ, Høj PB, Hoogenraad NJ. Selective induction of mitochondrial chaperones in response to loss of the mitochondrial genome. Eur J Biochem, 1996, 240(1): 98-103.
pmid: 8797841 |
| [23] |
Zhao Q, Wang JH, Levichkin IV, Stasinopoulos S, Ryan MT, Hoogenraad NJ. A mitochondrial specific stress response in mammalian cells. EMBO J, 2002, 21(17): 4411-4419.
pmid: 12198143 |
| [24] |
Haynes CM, Petrova K, Benedetti C, Yang Y, Ron D. ClpP mediates activation of a mitochondrial unfolded protein response in C. elegans. Dev Cell, 2007, 13(4): 467-480.
pmid: 17925224 |
| [25] |
Haynes CM, Yang Y, Blais SP, Neubert TA, Ron D.The matrix peptide exporter HAF-1 signals a mitochondrial UPR by activating the transcription factor ZC376.7 in C. elegans. Mol Cell, 2010, 37(4): 529-540.
doi: 10.1016/j.molcel.2010.01.015 pmid: 20188671 |
| [26] |
Yoneda T, Benedetti C, Urano F, Clark SG, Harding HP, Ron D. Compartment-specific perturbation of protein handling activates genes encoding mitochondrial chaperones. J Cell Sci, 2004, 117 (Pt 18): 4055-4066.
pmid: 15280428 |
| [27] |
Houtkooper RH, Mouchiroud L, Ryu D, Moullan N, Katsyuba E, Knott G, Williams RW, Auwerx J. Mitonuclear protein imbalance as a conserved longevity mechanism. Nature, 2013, 497(7450): 451-457.
doi: 10.1038/nature12188 |
| [28] |
Lee SJ, Hwang AB, Kenyon C. Inhibition of respiration extends C. elegans life span via reactive oxygen species that increase HIF-1 activity. Curr Biol, 2010, 20(23): 2131-2136.
doi: 10.1016/j.cub.2010.10.057 |
| [29] |
Mouchiroud L, Houtkooper RH, Moullan N, Katsyuba E, Ryu D, Cantó C, Mottis A, Jo YS, Viswanathan M, Schoonjans K, Guarente L, Auwerx J. The NAD+/sirtuin pathway modulates longevity through activation of mitochondrial UPR and FOXO signaling. Cell, 2013, 154(2): 430-441
doi: 10.1016/j.cell.2013.06.016 pmid: 23870130 |
| [30] |
Van Raamsdonk JM, Hekimi S.Deletion of the mitochondrial superoxide dismutase sod-2 extends lifespan in Caenorhabditis elegans. PLoS Genet, 2009, 5(2): e1000361.
doi: 10.1371/journal.pgen.1000361 |
| [31] |
Tian Y, Garcia G, Bian Q, Steffen KK, Joe L, Wolff S, Meyer BJ, Dillin A. Mitochondrial stress induces chromatin reorganization to promote longevity and UPRmt. Cell, 2016, 165(5): 1197-1208.
doi: S0092-8674(16)30402-0 pmid: 27133166 |
| [32] |
Deng P, Naresh NU, Du YG, Lamech LT, Yu J, Zhu LJ, Pukkila-Worley R, Haynes CM.Mitochondrial UPR repression during Pseudomonas aeruginosa infection requires the bZIP protein ZIP-3. Proc Natl Acad Sci USA, 2019, 116(13): 6146-6151.
doi: 10.1073/pnas.1817259116 pmid: 30850535 |
| [33] |
Benedetti C, Haynes CM, Yang Y, Harding HP, Ron D. Ubiquitin-like protein 5 positively regulates chaperone gene expression in the mitochondrial unfolded protein response. Genetics, 2006, 174(1): 229-239.
pmid: 16816413 |
| [34] | Merkwirth C, Jovaisaite V, Durieux J, Matilainen O, Jordan SD, Quiros PM, Steffen KK, Williams EG, Mouchiroud L, Tronnes SU, Murillo V, Wolff SC, Shaw RJ, Auwerx J, Dillin A. Two conserved histone demethylases regulate mitochondrial stress-induced longevity. Cell Genetics, 2016, 165(5): 1209-1223. |
| [35] |
Zhu D, Wu XY, Zhou J, Li XY, Huang XH, Li JS, Wu JB, Bian Q, Wang YC, Tian Y. NuRD mediates mitochondrial stress-induced longevity via chromatin remodeling in response to acetyl-CoA level. Sci Adv, 2020, 6(31): eabb2529.
doi: 10.1126/sciadv.abb2529 |
| [36] |
Li TY, Sleiman MB, Li H, Gao AW, Mottis A, Bachmann AM, El Alam G, Li X, Goeminne LJE, Schoonjans K, Auwerx J. The transcriptional coactivator CBP/p300 is an evolutionarily conserved node that promotes longevity in response to mitochondrial stress. Nat Aging, 2021, 1(2): 165-178.
doi: 10.1038/s43587-020-00025-z pmid: 33718883 |
| [37] |
Nargund AM, Pellegrino MW, Fiorese CJ, Baker BM, Haynes CM. Mitochondrial import efficiency of ATFS-1 regulates mitochondrial UPR activation. Science, 2012, 337(6094): 587-590.
doi: 10.1126/science.1223560 pmid: 22700657 |
| [38] |
Shao L-W, Peng Q, Dong MY, Gao KY, Li YM, Li Y, Li CY, Liu Y. Histone deacetylase HDA-1 modulates mitochondrial stress response and longevity. Nat Commun, 2020, 11(1): 4639.
doi: 10.1038/s41467-020-18501-w pmid: 32934238 |
| [39] |
Nargund AM, Fiorese CJ, Pellegrino MW, Deng P, Haynes CM. Mitochondrial and nuclear accumulation of the transcription factor ATFS-1 promotes OXPHOS recovery during the UPRmt. Mol Cell, 2015, 58(1): 123-133.
doi: 10.1016/j.molcel.2015.02.008 pmid: 25773600 |
| [40] |
Liu Y, Samuel BS, Breen PC, Ruvkun G. Caenorhabditis elegans pathways that surveil and defend mitochondria. Nature, 2014, 508(7496): 406-410.
doi: 10.1038/nature13204 |
| [41] |
Estes KA, Dunbar TL, Powell JR, Ausubel FM, Troemel ER.bZIP transcription factor zip-2 mediates an early response to Pseudomonas aeruginosa infection in Caenorhabditis elegans. Proc Natl Acad Sci USA, 2010, 107(5):2153-2158.
doi: 10.1073/pnas.0914643107 |
| [42] |
Fiorese CJ, Schulz AM, Lin YF, Rosin N, Pellegrino MW, Haynes CM. The transcription factor ATF5 mediates a mammalian mitochondrial UPR. Curr Biol, 2016, 26(15): 2037-2043.
doi: S0960-9822(16)30614-5 pmid: 27426517 |
| [43] |
Münch C, Harper JW. Mitochondrial unfolded protein response controls matrix pre-RNA processing and translation. Nature, 2016, 534(7609): 710-713.
doi: 10.1038/nature18302 |
| [44] |
Hinnebusch AG, Ivanov IP, Sonenberg N. Translational control by 5′-untranslated regions of eukaryotic mRNAs. Science, 2016, 352(6292): 1413-1416.
doi: 10.1126/science.aad9868 pmid: 27313038 |
| [45] |
Fessler E, Eckl E-M, Schmitt S, Mancilla IA, Meyer- Bender MF, Hanf M, Philippou-Massier J, Krebs S, Zischka H, Jae LT. A pathway coordinated by DELE1 relays mitochondrial stress to the cytosol. Nature, 2020, 579(7799): 433-437.
doi: 10.1038/s41586-020-2076-4 |
| [46] |
Guo XY, Aviles G, Liu Y, Tian RL, Unger BA, Lin Y-HT, Wiita AP, Xu K, Correia MA, Kampmann M. Mitochondrial stress is relayed to the cytosol by an OMA1- DELE1-HRI pathway. Nature, 2020, 579(7799): 427-432.
doi: 10.1038/s41586-020-2078-2 |
| [47] |
Samluk L, Urbanska M, Kisielewska K, Mohanraj K, Kim MJ, Machnicka K, Liszewska E, Jaworski J, Chacinska A. Cytosolic translational responses differ under conditions of severe short-term and long-term mitochondrial stress. Mol Biol Cell, 2019, 30(15): 1864-1877.
doi: 10.1091/mbc.E18-10-0628 pmid: 31116686 |
| [48] |
Kim Y, Park J, Kim S, Kim MA, Kang MG, Kwak C, Kang M, Kim B, Rhee HW, Kim VN. PKR senses nuclear and mitochondrial signals by interacting with endogenous double-stranded RNAs. Mol Cell, 2018, 71(6): 1051- 1063.e6.
doi: S1097-2765(18)30598-7 pmid: 30174290 |
| [49] |
Rath E, Berger E, Messlik A, Nunes T, Liu B, Kim SC, Hoogenraad N, Sans M, Sartor RB, Haller D. Induction of dsRNA-activated protein kinase links mitochondrial unfolded protein response to the pathogenesis of intestinal inflammation. Gut, 2012, 61(9): 1269-1278.
doi: 10.1136/gutjnl-2011-300767 pmid: 21997551 |
| [50] |
Michel S, Canonne M, Arnould T, Renard P. Inhibition of mitochondrial genome expression triggers the activation of CHOP-10 by a cell signaling dependent on the integrated stress response but not the mitochondrial unfolded protein response. Mitochondrion, 2015, 21: 58-68.
doi: 10.1016/j.mito.2015.01.005 pmid: 25643991 |
| [51] |
Naresh NU, Haynes CM. Signaling and regulation of the mitochondrial unfolded protein response. Cold Spring Harb Perspect Biol, 2019, 11(6): a033944.
doi: 10.1101/cshperspect.a033944 |
| [52] |
Owusu-Ansah E, Song W, Perrimon N. Muscle mitohormesis promotes longevity via systemic repression of insulin signaling. Cell, 2013, 155(3): 699-712.
doi: 10.1016/j.cell.2013.09.021 pmid: 24243023 |
| [53] |
Berendzen KM, Durieux J, Shao LW, Tian Y, Kim HE, Wolff S, Liu Y, Dillin A. Neuroendocrine coordination of mitochondrial stress signaling and proteostasis. Cell, 2016, 166(6): 1553-1563.e10.
doi: S0092-8674(16)31139-4 pmid: 27610575 |
| [54] |
Shao LW, Niu R, Liu YW. Neuropeptide signals cell non-autonomous mitochondrial unfolded protein response. Cell Res, 2016, 26(11): 1182-1196.
doi: 10.1038/cr.2016.118 pmid: 27767096 |
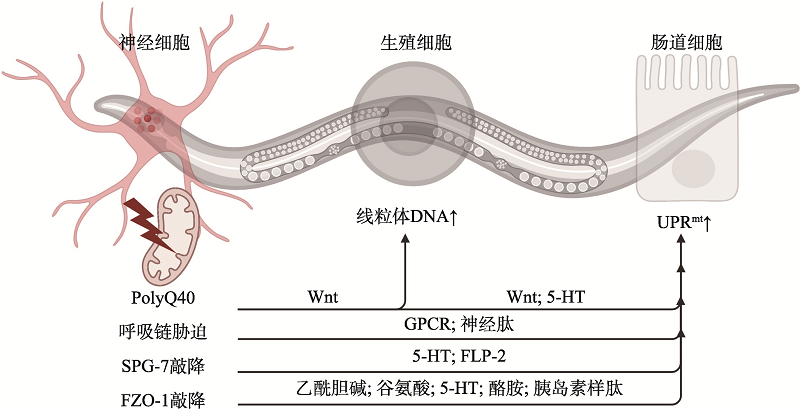
| [55] |
Zhang Q, Wu XY, Chen P, Liu LM, Xin N, Tian Y, Dillin A. The mitochondrial unfolded protein response is mediated cell-non-autonomously by retromer-dependent Wnt signaling. Cell, 2018, 174(4): 870-883.e17.
doi: S0092-8674(18)30797-9 pmid: 30057120 |
| [56] |
Chen LT, Lin CT, Lin LY, Hsu JM, Wu YC, Pan CL. Neuronal mitochondrial dynamics coordinate systemic mitochondrial morphology and stress response to confer pathogen resistance in C. elegans. Dev Cell, 2021, 56(12): 1770-1785.e12.
doi: 10.1016/j.devcel.2021.04.021 pmid: 33984269 |
| [57] |
Liu YL, Zhou J, Zhang N, Wu XY, Zhang Q, Zhang WF, Li XY, Tian Y. Two sensory neurons coordinate the systemic mitochondrial stress response via GPCR signaling in C. elegans. Dev Cell, 2022, 57(21): 2469-2482.e5.
doi: 10.1016/j.devcel.2022.10.001 |
| [58] |
Srinivasan S, Sadegh L, Elle IC, Christensen AGL, Faergeman NJ, Ashrafi K. Serotonin regulates C. elegans fat and feeding through independent molecular mechanisms. Cell Metab, 2008, 7(6): 533-544.
doi: 10.1016/j.cmet.2008.04.012 pmid: 18522834 |
| [59] |
Chen D, Taylor KP, Hall Q, Kaplan JM. The neuropeptides FLP-2 and PDF-1 act in concert to arouse Caenorhabditis elegans locomotion. Genetics, 2016, 204(3): 1151-1159.
doi: 10.1534/genetics.116.192898 |
| [60] |
Waterson MJ, Horvath TL. Neuronal regulation of energy homeostasis: beyond the hypothalamus and feeding. Cell Metab, 2015, 22(6): 962-970.
doi: 10.1016/j.cmet.2015.09.026 pmid: 26603190 |
| [61] |
Nusse R, Clevers H. Wnt/β-catenin signaling, disease, and emerging therapeutic modalities. Cell, 2017, 169(6): 985-999.
doi: S0092-8674(17)30547-0 pmid: 28575679 |
| [62] |
Baron R, Kneissel M. WNT signaling in bone homeostasis and disease: from human mutations to treatments. Nat Med, 2013, 19(2):179-192.
doi: 10.1038/nm.3074 pmid: 23389618 |
| [63] |
Shang S, Hua F, Hu ZW. The regulation of β-catenin activity and function in cancer: therapeutic opportunities. Oncotarget, 2017, 8(20): 33972-33989.
doi: 10.18632/oncotarget.15687 pmid: 28430641 |
| [64] |
Bänziger C, Soldini D, Schütt C, Zipperlen P, Hausmann G, Basler K. Wntless, a conserved membrane protein dedicated to the secretion of Wnt proteins from signaling cells. Cell, 2006, 125(3): 509-522.
doi: 10.1016/j.cell.2006.02.049 pmid: 16678095 |
| [65] |
Christiane N-V, Eric W. Mutations affecting segment number and polarity in Drosophila. Nature, 1980, 287(5785): 795-801.
doi: 10.1038/287795a0 |
| [66] |
Feige MJ, Hendershot LM. Disulfide bonds in ER protein folding and homeostasis. Curr Opin Cell Biol, 2011, 23(2): 167-175.
doi: 10.1016/j.ceb.2010.10.012 pmid: 21144725 |
| [67] |
Li XY, Li JS, Zhu D, Zhang N, Hao XS, Zhang WF, Zhang Q, Liu YL, Wu XY, Tian Y.Protein disulfide isomerase PDI-6 regulates Wnt secretion to coordinate inter-tissue UPRmt activation and lifespan extension in C. elegans. Cell Rep, 2022, 39(10): 110931.
doi: 10.1016/j.celrep.2022.110931 |
| [68] |
Zhang Q, Wang ZH, Zhang WF, Wen QB, Li XY, Zhou J, Wu XY, Guo YQ, Liu YL, Wei CS, Qian WF, Tian Y. The memory of neuronal mitochondrial stress is inherited transgenerationally via elevated mitochondrial DNA levels. Nat Cell Biol, 2021, 23(8): 870-880.
doi: 10.1038/s41556-021-00724-8 pmid: 34341532 |
| [69] |
Zhang Q, Tian Y. Molecular insights into the transgenerational inheritance of stress memory. J Genet Genomics, 2021, 49(2): 89-95.
doi: 10.1016/j.jgg.2021.11.015 |
| [70] |
Calculli G, Lee HJ, Shen K, Pham U, Herholz M, Trifunovic A, Dillin A, Vilchez D. Systemic regulation of mitochondria by germline proteostasis prevents protein aggregation in the soma of C. elegans. Sci Adv, 2021, 7(26): eabg3012.
doi: 10.1126/sciadv.abg3012 |
| [71] |
Lan JF, Rollins JA, Zang X, Wu D, Zou LN, Wang Z, Ye C, Wu ZX, Kapahi P, Rogers AN, Chen D. Translational regulation of non-autonomous mitochondrial stress response promotes longevity. Cell Rep, 2019, 28(4): 1050-1062.e6.
doi: S2211-1247(19)30858-7 pmid: 31340143 |
| [72] |
Kharitonenkov A, Shiyanova TL, Koester A, Ford AM, Micanovic R, Galbreath EJ, Sandusky GE, Hammond LJ, Moyers JS, Owens RA, Gromada J, Brozinick JT, Hawkins ED, Wroblewski VJ, Li DS, Mehrbod F, Jaskunas SR, Shanafelt AB. FGF-21 as a novel metabolic regulator. J Clin Invest, 2005, 115(6): 1627-1635.
doi: 10.1172/JCI23606 pmid: 15902306 |
| [73] |
Inagaki T, Dutchak P, Zhao GX, Ding XS, Gautron L, Parameswara V, Li Y, Goetz R, Mohammadi M, Esser V, Elmquist JK, Gerard RD, Burgess SC, Hammer RE, Mangelsdorf DJ, Kliewer SA.Endocrine regulation of the fasting response by PPARα-mediated induction of fibroblast growth factor 21. Cell Metab, 2007, 5(6): 415-425.
doi: 10.1016/j.cmet.2007.05.003 |
| [74] |
Kim KH, Jeong YT, Oh H, Kim SH, Cho JM, Kim YN, Kim SS, Kim DH, Hur KY, Kim HK, Ko T, Han J, Kim HL, Kim J, Back SH, Komatsu M, Chen H, Chan DC, Konishi M, Itoh N, Choi CS, Lee MS. Autophagy deficiency leads to protection from obesity and insulin resistance by inducing Fgf21 as a mitokine. Nat Med, 2013, 19(1): 83-92.
doi: 10.1038/nm.3014 pmid: 23202295 |
| [75] |
Zhang Y, Xie Y, Berglund ED, Coate KC, He TT, Katafuchi T, Xiao GH, Potthoff MJ, Wei W, Wan YH, Yu RT, Evans RM, Kliewer SA, Mangelsdorf DJ. The starvation hormone, fibroblast growth factor-21, extends lifespan in mice. Elife, 2012, 1: e00065.
doi: 10.7554/eLife.00065 |
| [76] |
Suomalainen A, Elo JM, Pietiläinen KH, Hakonen AH, Sevastianova K, Korpela M, Isohanni P, Marjavaara SK, Tyni T, Kiuru-Enari S, Pihko H, Darin N, Õunap K, Kluijtmans LA, Paetau A, Buzkova J, Bindoff LA, Annunen-Rasila J, Uusimaa J, Rissanen A, Yki-Järvinen H, Hirano M, Tulinius M, Smeitink J, Tyynismaa H. FGF-21 as a biomarker for muscle-manifesting mitochondrial respiratory chain deficiencies: a diagnostic study. Lancet Neurol, 2011, 10(9): 806-818.
doi: 10.1016/S1474-4422(11)70155-7 pmid: 21820356 |
| [77] |
Li JS, Cui JM, Tian Y. Neuron-periphery mitochondrial stress communication in aging and diseases. Life Med, 2022, 1(2): 168-178.
doi: 10.1093/lifemedi/lnac051 |
| [78] |
Hauser AS, Chavali S, Masuho I, Jahn LJ, Martemyanov KA, Gloriam DE, Babu MM. Pharmacogenomics of GPCR drug targets. Cell, 2018, 172(1-2): 41-54.e19.
doi: S0092-8674(17)31384-3 pmid: 29249361 |
| [79] |
Wettschureck N, Offermanns S. Mammalian G proteins and their cell type specific functions. Physiol Rev, 2005, 85(4): 1159-1204.
pmid: 16183910 |
| [1] | Shan He, Jian Zhao, Xiaofeng Song. Effects of N6-methyladenosine modification on the function of the female reproductive system [J]. Hereditas(Beijing), 2023, 45(6): 472-487. |
| [2] | Xiaokang Shang, Simeng Zhang, Junjun Ni. Research progress of cathepsin B in brain aging and Alzheimer’s diseases [J]. Hereditas(Beijing), 2023, 45(3): 212-220. |
| [3] | Jiali Li, Jin Li, Hu Wang. Age-associated proteostasis collapse [J]. Hereditas(Beijing), 2022, 44(9): 733-744. |
| [4] | Jie Yuan, Shiqing Cai. The regulatory mechanisms of behavioral and cognitive aging [J]. Hereditas(Beijing), 2021, 43(6): 545-570. |
| [5] | Ziyan Liu, Ai Gao. Progress on inflamm-aging in hematologic diseases [J]. Hereditas(Beijing), 2021, 43(12): 1132-1141. |
| [6] | Xuewen Liu, Hongmei Wu, Ying Bai, Qun Zeng, Zemin Cao, Xiushan Wu, Min Tang. Potassium channel Shaker play a protective role against cardiac aging in Drosophila [J]. Hereditas(Beijing), 2021, 43(1): 94-99. |
| [7] | Chuanming Liu,Lijun Ding,Jiayin Li,Jianwu Dai,Haixiang Sun. Advances in the study of ovarian dysfunction with aging [J]. Hereditas(Beijing), 2019, 41(9): 816-826. |
| [8] | Yunfeng Pan, Yanyi Wang, Jingwen Chen, Yimei Fan. Mitochondrial metabolism’s effect on epigenetic change and aging [J]. Hereditas(Beijing), 2019, 41(10): 893-904. |
| [9] | Hongdong Li,Guini Hong,Zheng Guo. Age-related DNA methylation changes in peripheral whole blood [J]. HEREDITAS(Beijing), 2015, 37(2): 165-173. |
| [10] | RUAN Qing-Wei YU Zhuo-Wei BAO Zhi-Jun MA Yong-Xing. The relationship between the polymorphism of immunity genes and both aging and age-related diseases [J]. HEREDITAS, 2013, 35(7): 813-822. |
| [11] | JIA Shu-Ting, YANG Shi-Hua, LUO Ying. Utilization of Werner syndrome mouse model in studying premature aging and tumor [J]. HEREDITAS, 2009, 31(8): 785-790. |
| [12] | ZHANG Xiu-Feng, TANG Wen-Ru, LUO Ying. Aging or tumor: the crosstalk between telomerase and p53 [J]. HEREDITAS, 2009, 31(5): 451-456. |
| [13] | LI Hong, BAI Xiao-Juan, LIU Qiang, WANG Ning-Fu. The study on inhabiting endothelial cell aging by targeted silencing of p22phox [J]. HEREDITAS, 2008, 30(9): 1175-1181. |
| [14] | YU Jian-Ning, WANG Meng, WANG Dan-Qiu, LI Shao-Hua, SHAO Gen-Bao, WU Cai-Feng, LIU Hong-Lin . Chromosome changes of aged oocytes after ovulation [J]. HEREDITAS, 2007, 29(2): 225-225―229. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||