Hereditas(Beijing) ›› 2024, Vol. 46 ›› Issue (5): 398-407.doi: 10.16288/j.yczz.23-299
Previous Articles Next Articles
Effect of LRRC15 on autophagy in A549 cells
Qiwen Wang( ), Yanling Jia, Pan Li, Guoying Yu(
), Yanling Jia, Pan Li, Guoying Yu( )
)
- College of Life Science, Henan Normal University, State Key Laboratory of Cell Differentiation and Regulation, Henan International Joint Laboratory of Pulmonary Fibrosis, Henan Center for Outstanding Overseas Scientists of Organ Fibrosis, Xinxiang 453007, China
-
Received:2024-01-07Revised:2024-03-04Online:2024-03-12Published:2024-03-12 -
Contact:Guoying Yu E-mail:wangqiwen@htu.edu.cn;guoyingyu@htu.edu.cn -
Supported by:Key Scientific Research Projects of Henan Higher Education(22A180018);Henan Project of Science and Technology(232102310067);State Innovation Base for Pulmonary Fibrosis(“jl0”计划)
Cite this article
Qiwen Wang, Yanling Jia, Pan Li, Guoying Yu. Effect of LRRC15 on autophagy in A549 cells[J]. Hereditas(Beijing), 2024, 46(5): 398-407.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
| [1] | Lederer DJ, Martinez FJ. Idiopathic pulmonary fibrosis. N Engl J Med, 2018, 378(19): 1811-1823. |
| [2] | Miao Y, Li XH, Zhan YQ, Bai JK, Ma XY, Xi BR, Zhang JW, Zhou HG, Yang C. Research and development progress of antibody drugs for idiopathic pulmonary fibrosis. Acta Pharm Sin, 2021, 56(11): 2881-2886. |
| 苗洋, 李霄鹤, 翟芸芊, 白佳坤, 马晓阳, 希布日, 张建伟, 周红刚, 杨诚. 特发性肺纤维化抗体药物研发进展. 药学学报, 2021, 56(11): 2881-2886. | |
| [3] |
Richeldi L, Collard HR, Jones MG. Idiopathic pulmonary fibrosis. Lancet, 2017, 389(10082): 1941-1952.
doi: S0140-6736(17)30866-8 pmid: 28365056 |
| [4] |
Mi S, Li Z, Yang HZ, Liu H, Wang JP, Ma YG, Wang XX, Liu HZ, Sun W, Hu ZW. Blocking IL-17A promotes the resolution of pulmonary inflammation and fibrosis via TGF-β1-dependent and -independent mechanisms. J Immunol, 2011, 187(6): 3003-3014.
doi: 10.4049/jimmunol.1004081 pmid: 21841134 |
| [5] | Margaritopoulos GA, Tsitoura E, Tzanakis N, Spandidos DA, Siafakas NM, Sourvinos G, Antoniou KM. Self-eating: friend or foe? The emerging role of autophagy in idiopathic pulmonary fibrosis. Biomed Res Int, 2013, 2013: 420497. |
| [6] | Ray U, Pathoulas CL, Thirusangu P, Purcell JW, Kannan N, Shridhar V. Exploiting LRRC 15 as a novel therapeutic target in cancer. Cancer Res, 2022, 82(9): 1675-1681. |
| [7] |
O'Prey J, Wilkinson S, Ryan KM. Tumor antigen LRRC15 impedes adenoviral infection: implications for virus-based cancer therapy. J Virol, 2008, 82(12): 5933-5939.
doi: 10.1128/JVI.02273-07 pmid: 18385238 |
| [8] |
Purcell JW, Tanlimco SG, Hickson J, Fox M, Sho M, Durkin L, Uziel T, Powers R, Foster K, McGonigal T, Kumar S, Samayoa J, Zhang D, Palma JP, Mishra S, Hollenbaugh D, Gish K, Morgan-Lappe SE, Hsi ED, Chao DT. LRRC 15 Is a novel mesenchymal protein and stromal target for antibody-drug conjugates. Cancer Res, 2018, 78(14): 4059-4072.
doi: 10.1158/0008-5472.CAN-18-0327 pmid: 29764866 |
| [9] | Mariani A, Wang C, Oberg AL, Riska SM, Torres M, Kumka J, Multinu F, Sagar G, Roy D, Jung DB, Zhang Q, Grassi T, Visscher DW, Patel VP, Jin L, Staub JK, Cliby WA, Weroha SJ, Kalli KR, Hartmann LC, Kaufmann SH, Goode EL, Shridhar V. Genes associated with bowel metastases in ovarian cancer. Gynecol Oncol, 2019, 154(3): 495-504. |
| [10] | Sul OJ, Kim JH, Lee T, Seo KW, Cha HJ, Kwon B, Ahn JJ, Cho YS, Oh YM, Jegal Y, Ra SW. GSPE protects against bleomycin-induced pulmonary fibrosis in mice via ameliorating epithelial apoptosis through inhibition of oxidative stress. Oxid Med Cell Longev, 2022, 2022: 8200189. |
| [11] | Li L, Wang YY, Cheng MQ, Yin JB, Zhang X, Li L. Inhibitory effect of gentiopicroside on the apoptosis of alveolar epithelial cells induced by bleomycin. J Kunming Med Univ, 2019, 40(6): 11-15. |
| 李莉, 王永艳, 成梦群, 尹健彬, 张旋, 李丽. 龙胆苦苷对博来霉素诱导肺泡上皮细胞凋亡的抑制作用. 昆明医科大学学报, 2019, 40(6): 11-15. | |
| [12] | Thannickal VJ, Toews GB, White ES, Lynch JP 3rd, Martinez FJ. Mechanisms of pulmonary fibrosis. Annu Rev Med, 2004, 55: 395-417. |
| [13] | Zhang L, Wang Y, Wu GR, Xiong WN, Gu WK, Wang CY. Macrophages: friend or foe in idiopathic pulmonary fibrosis? Respir Res, 2018, 19(1): 170. |
| [14] |
Dominguez CX, Müller S, Keerthivasan S, Koeppen H, Hung J, Gierke S, Breart B, Foreman O, Bainbridge TW, Castiglioni A, Senbabaoglu Y, Modrusan Z, Liang YX, Junttila MR, Klijn C, Bourgon R, Turley SJ. Single-cell RNA sequencing reveals stromal evolution into LRRC15(+) myofibroblasts as a determinant of patient response to cancer immunotherapy. Cancer Discov, 2020, 10(2): 232-253.
doi: 10.1158/2159-8290.CD-19-0644 pmid: 31699795 |
| [15] |
Schuetz CS, Bonin M, Clare SE, Nieselt K, Sotlar K, Walter M, Fehm T, Solomayer E, Riess O, Wallwiener D, Kurek R, Neubauer HJ. Progression-specific genes identified by expression profiling of matched ductal carcinomas in situ and invasive breast tumors, combining laser capture microdissection and oligonucleotide microarray analysis. Cancer Res, 2006, 66(10): 5278-5286.
pmid: 16707453 |
| [16] |
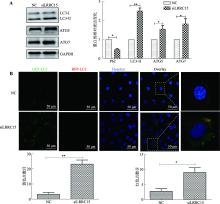
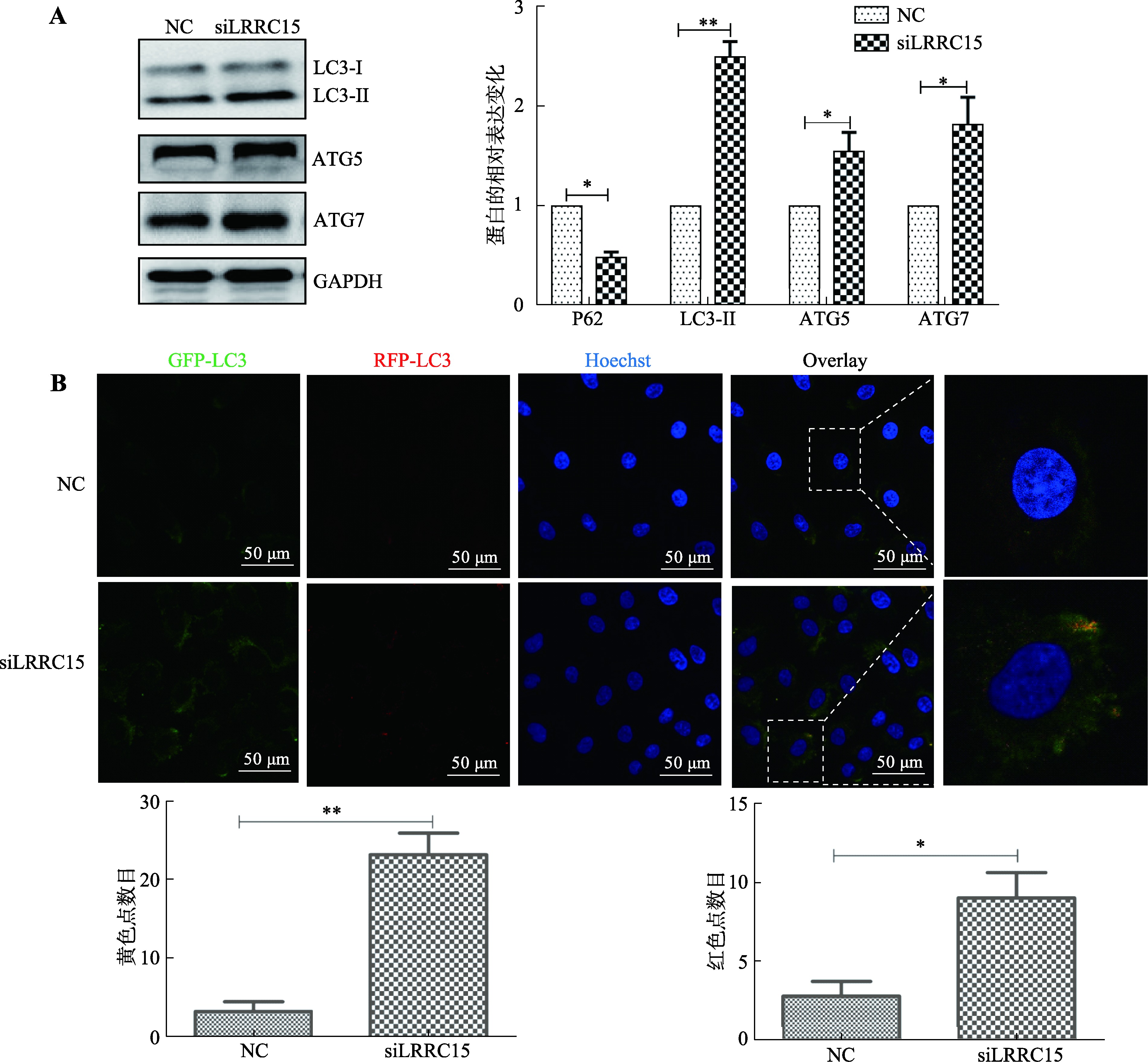
Kimura S, Fujita N, Noda T, Yoshimori T.Monitoring autophagy in mammalian cultured cells through the dynamics of LC3. Methods Enzymol, 2009, 452: 1-12.
doi: 10.1016/S0076-6879(08)03601-X pmid: 19200872 |
| [17] |
BenYounès A, Tajeddine N, Tailler M, Malik SA, Shen SS, Métivier D, Kepp O, Vitale I, Maiuri MC, Kroemer G.A fluorescence-microscopic and cytofluorometric system for monitoring the turnover of the autophagic substrate p62/SQSTM1. Autophagy, 2011, 7(8): 883-891.
pmid: 21460612 |
| [18] | Lu JL, Yu CX, Song LJ. Programmed cell death in hepatic fibrosis: current and perspectives. Cell Death Discov, 2023, 9(1): 449. |
| [19] | Wen JH, Li DY, Liang S, Yang C, Tang JX, Liu HF. Macrophage autophagy in macrophage polarization, chronic inflammation and organ fibrosis. Front Immunol, 2022, 13: 946832. |
| [20] | Wang QW, Chang CF, Gu NN, Pan CY, Xu CS. Effect of autophagy on liver regeneration. Hereditas (Beijing), 2015, 37(11): 1116-1124. |
| 王棋文, 常翠芳, 谷宁宁, 潘翠云, 徐存拴. 自噬在肝再生中的作用. 遗传, 2015, 37(11): 1116-1124. | |
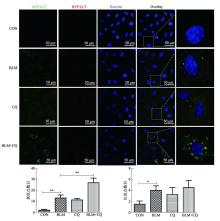
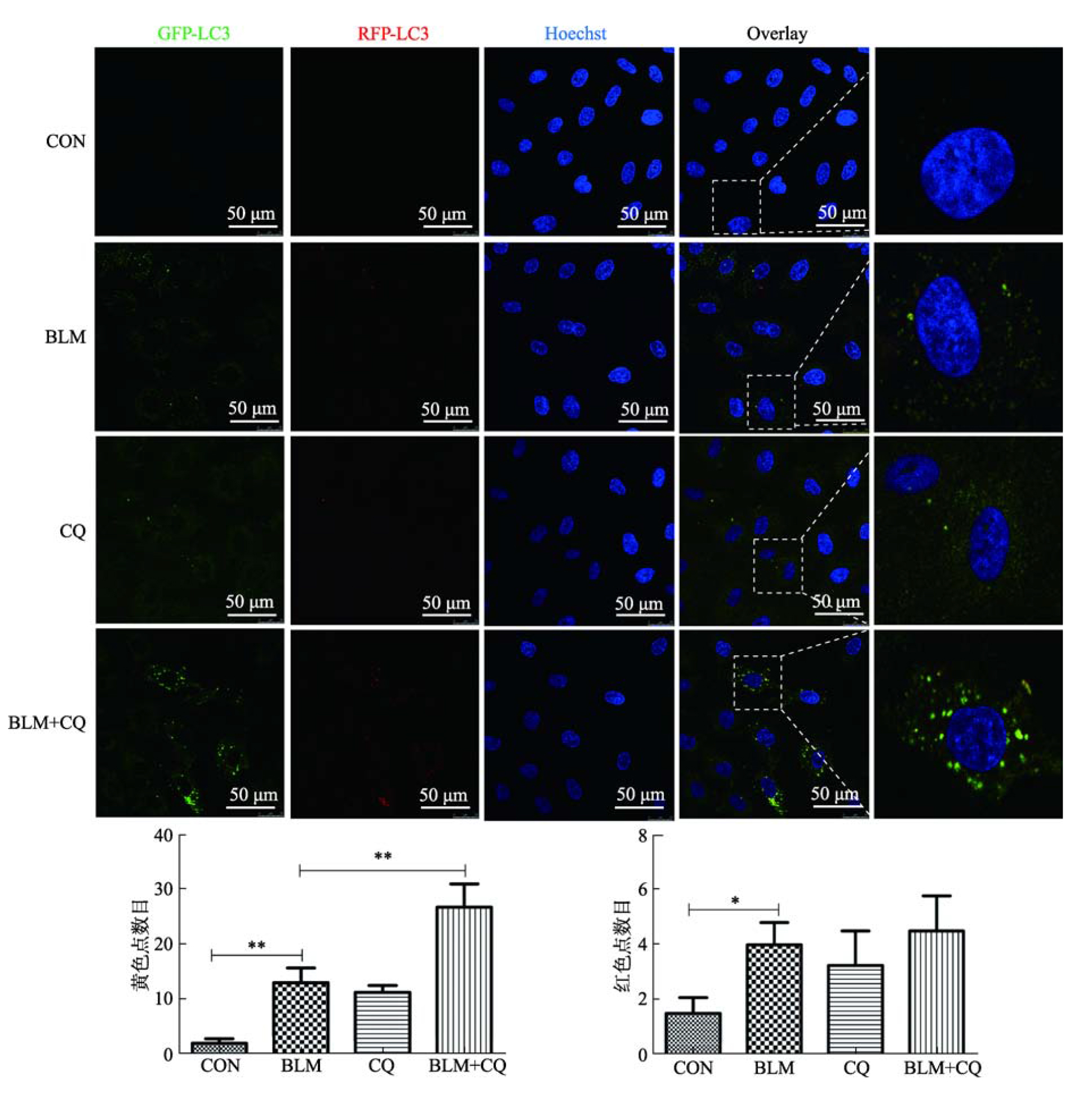
| [21] | Araya J, Kojima J, Takasaka N, Ito S, Fujii S, Hara H, Yanagisawa H, Kobayashi K, Tsurushige C, Kawaishi M, Kamiya N, Hirano J, Odaka M, Morikawa T, Nishimura SL, Kawabata Y, Hano H, Nakayama K, Kuwano K. Insufficient autophagy in idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol, 2013, 304(1): L56-69. |
| [22] |
Cabrera S, Maciel M, Herrera I, Nava T, Vergara F, Gaxiola M, López-Otín C, Selman M, Pardo A. Essential role for the ATG4B protease and autophagy in bleomycin- induced pulmonary fibrosis. Autophagy, 2015, 11(4): 670-684.
doi: 10.1080/15548627.2015.1034409 pmid: 25906080 |
| [23] |
Kobayashi K, Araya J, Minagawa S, Hara H, Saito N, Kadota T, Sato N, Yoshida M, Tsubouchi K, Kurita Y, Ito S, Fujita Y, Takasaka N, Utsumi H, Yanagisawa H, Hashimoto M, Wakui H, Kojima J, Shimizu K, Numata T, Kawaishi M, Kaneko Y, Asano H, Yamashita M, Odaka M, Morikawa T, Nakayama K, Kuwano K. Involvement of PARK2-mediated mitophagy in idiopathic pulmonary fibrosis pathogenesis. J Immunol, 2016, 197(2): 504-516.
doi: 10.4049/jimmunol.1600265 pmid: 27279371 |
| [24] |
Sosulski ML, Gongora R, Danchuk S, Dong CM, Luo FY, Sanchez CG.Deregulation of selective autophagy during aging and pulmonary fibrosis: the role of TGFβ1. Aging Cell, 2015, 14(5): 774-783.
doi: 10.1111/acel.12357 pmid: 26059457 |
| [25] | Yu GY, Tzouvelekis A, Wang R, Herazo-Maya JD, Ibarra GH, Srivastava A, DeIuliis G, Ahangari F, Woolard T, Aurelien N, Arrojo E Drigo R, Gan Y, Graham M, Liu XR, Homer RJ, Scanlan TS, Mannam P, Lee PJ, Herzog EL, Bianco AC, Kaminski N. Thyroid hormone inhibits lung fibrosis in mice by improving epithelial mitochondrial function. Nat Med, 2017, 24(1): 39-49. |
| [26] | Gui XH, Chen HW, Cai HR, Sun LY, Gu L. Leptin promotes pulmonary fibrosis development by inhibiting autophagy via PI3K/Akt/mTOR pathway. Biochem Biophys Res Commun, 2018, 498(3): 660-666. |
| [27] |
Xu G, Wang X, Yu H, Wang C, Liu Y, Zhao R, Zhang G. Beclin 1, LC3, and p62 expression in paraquat-induced pulmonary fibrosis. Hum Exp Toxicol, 2019, 38(7): 794-802.
doi: 10.1177/0960327119842633 pmid: 30977401 |
| [28] |
Zhao XY, Wei SS, Li ZJ, Lin C, Zhu ZF, Sun DS, Bai RP, Qian J, Gao XW, Chen GD, Xu ZP. Autophagic flux blockage in alveolar epithelial cells is essential in silica nanoparticle-induced pulmonary fibrosis. Cell Death Dis, 2019, 10(2): 127.
doi: 10.1038/s41419-019-1340-8 pmid: 30755584 |
| [29] | Wang YJ, Liu YS, Zhang M, Lv LW, Zhang X, Zhang P, Zhou YS. LRRC 15 promotes osteogenic differentiation of mesenchymal stem cells by modulating p65 cytoplasmic/ nuclear translocation. Stem Cell Res Ther, 2018, 9(1): 65. |
| [1] | Jinyi Zhang, Yumo He, Jingyu Zhou, Shufeng Weng, Huixia Ma, Taiyue Lin, Ying Xu. Hsa_circ_0007460 affects the survival of intracellular Mycobacterium tuberculosis by regulating autophagy and apoptosis of macrophages [J]. Hereditas(Beijing), 2023, 45(11): 1039-1051. |
| [2] | Jing Liu, Cong Yi, Shiming Xu. The regulatory effect of protein acetylation modification on autophagy [J]. Hereditas(Beijing), 2022, 44(1): 15-24. |
| [3] | Kexue Ma, Rui Li, Fangying Guo, Gege Song, Meng Wu, Guangwen Chen, Dezeng Liu. Functional analysis of autophagy-related gene Atg6 in planarian central nervous system regeneration [J]. Hereditas(Beijing), 2021, 43(8): 792-801. |
| [4] | Wang Shiming, Song Xiao, Zhao Xueying, Chen Hongyan, Wang Jiucun, Wu Junjie, Gao Zhiqiang, Qian Ji, Bai Chunxue, Li Qiang, Han Baohui, Lu Daru. Association between polymorphisms of autophagy pathway and responses in non-small cell lung cancer patients treated with platinum-based chemotherapy [J]. Hereditas(Beijing), 2017, 39(3): 250-262. |
| [5] | Xiaowei Zeng, Cuicui Liu, Ning Han, Hongwu Bian, Muyuan Zhu. Progress on the autophagic regulators and receptors in plants [J]. HEREDITAS(Beijing), 2016, 38(7): 644-650. |
| [6] | Qiwen Wang, Wei Jin, Cuifang Chang, Cunshuan Xu. Regulation of autophagy on dendritic cells during rat liver regeneration by IPA [J]. HEREDITAS(Beijing), 2015, 37(3): 276-282. |
| [7] | Qiwen Wang,Cuifang Chang,Ningning Gu,Cuiyun Pan,Cunshuan Xu. Effect of autophagy on liver regeneration [J]. HEREDITAS(Beijing), 2015, 37(11): 1116-1124. |
| [8] | Yuanyuan Chen, Hongyan Chen, Daru Lu. Molecular mechanisms of SNARE proteins in regulating autophagy [J]. HEREDITAS(Beijing), 2014, 36(6): 547-551. |
| [9] | Yuanchao Sun, Xunsi Qin, Hong Chen, Wei Shen. Epigenetic control of autophagy [J]. HEREDITAS(Beijing), 2014, 36(5): 447-455. |
| [10] | . Molecular mechanisms and functions of autophagy and the ubiq-uitin-proteasome pathway [J]. HEREDITAS, 2012, 34(1): 5-18. |
| [11] | WANG Shi-Yao, JIN Wei-Na, WU Dan. Mechanisms of juvenile neuronal ceroid lipofuscinosis (JNCL) [J]. HEREDITAS, 2009, 31(8): 779-784. |
| [12] | HE Jun-Lin, CAO Bo, WANG Ying-Xiong. Study on Centromeric Dots Variation of BGC823 Cells And A549 Cells [J]. HEREDITAS, 2005, 27(6): 877-881. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||