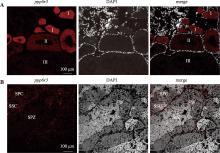
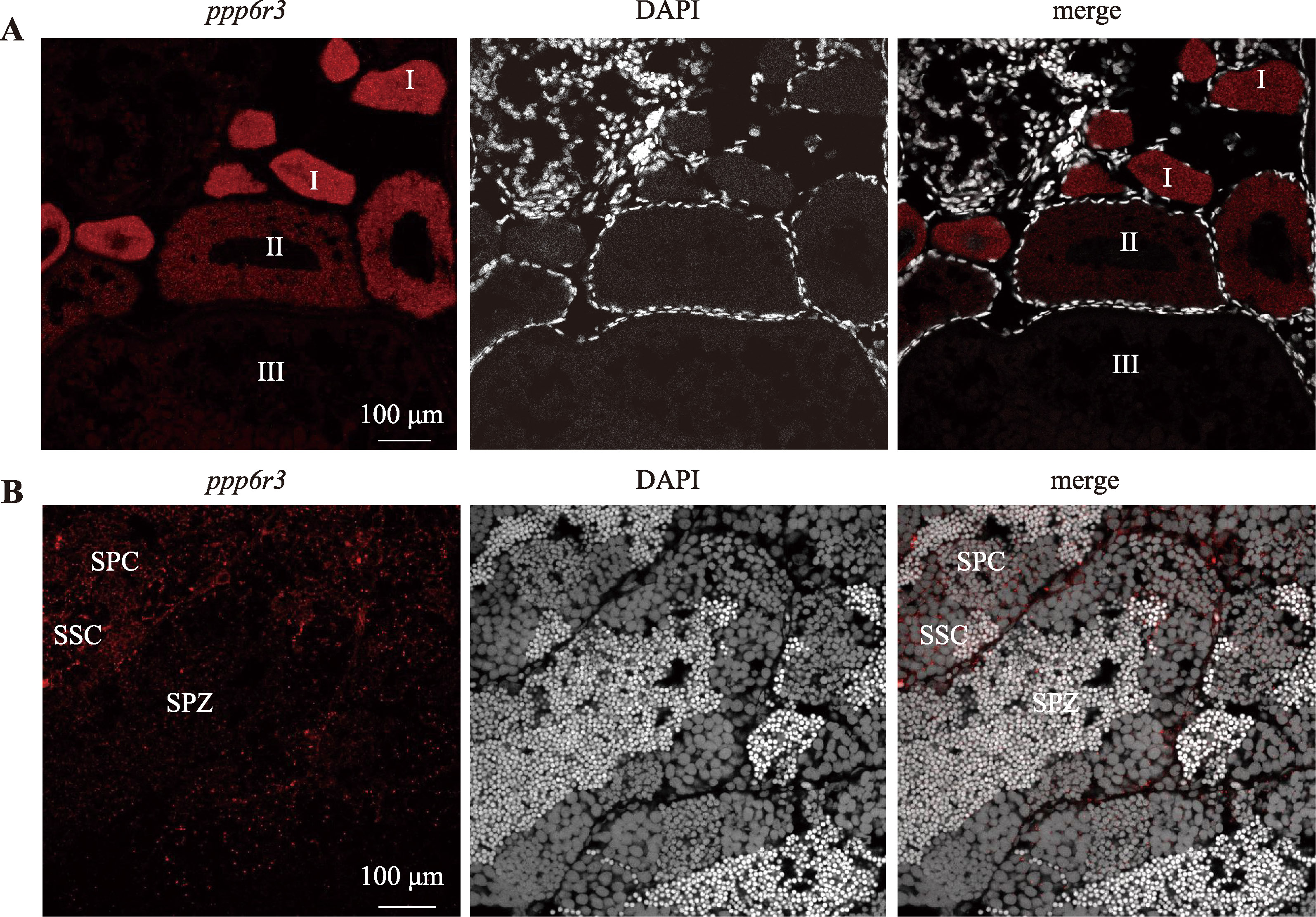
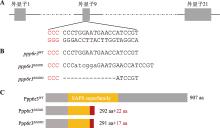
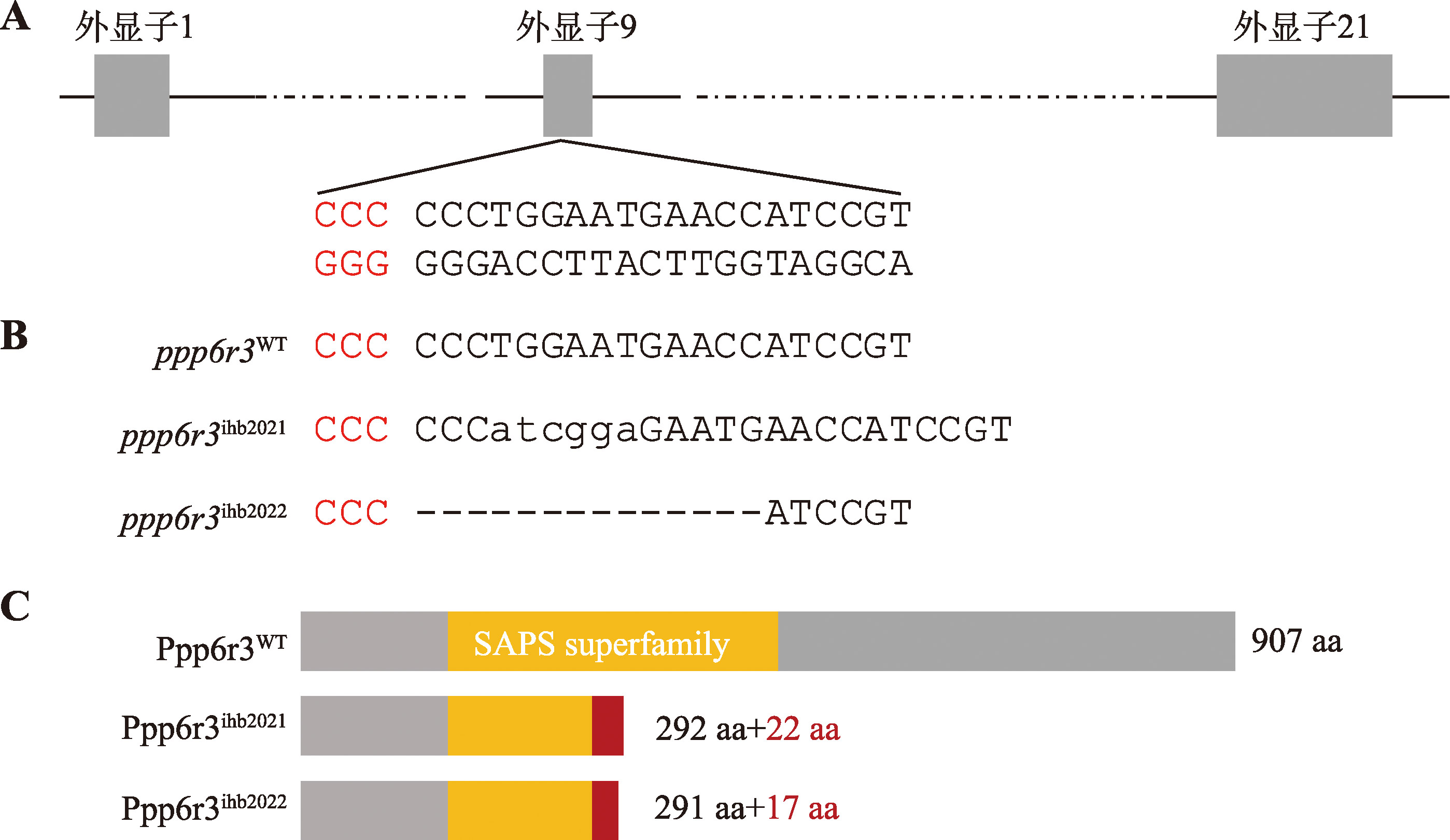
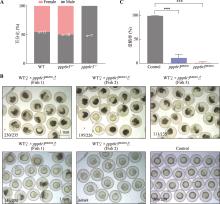
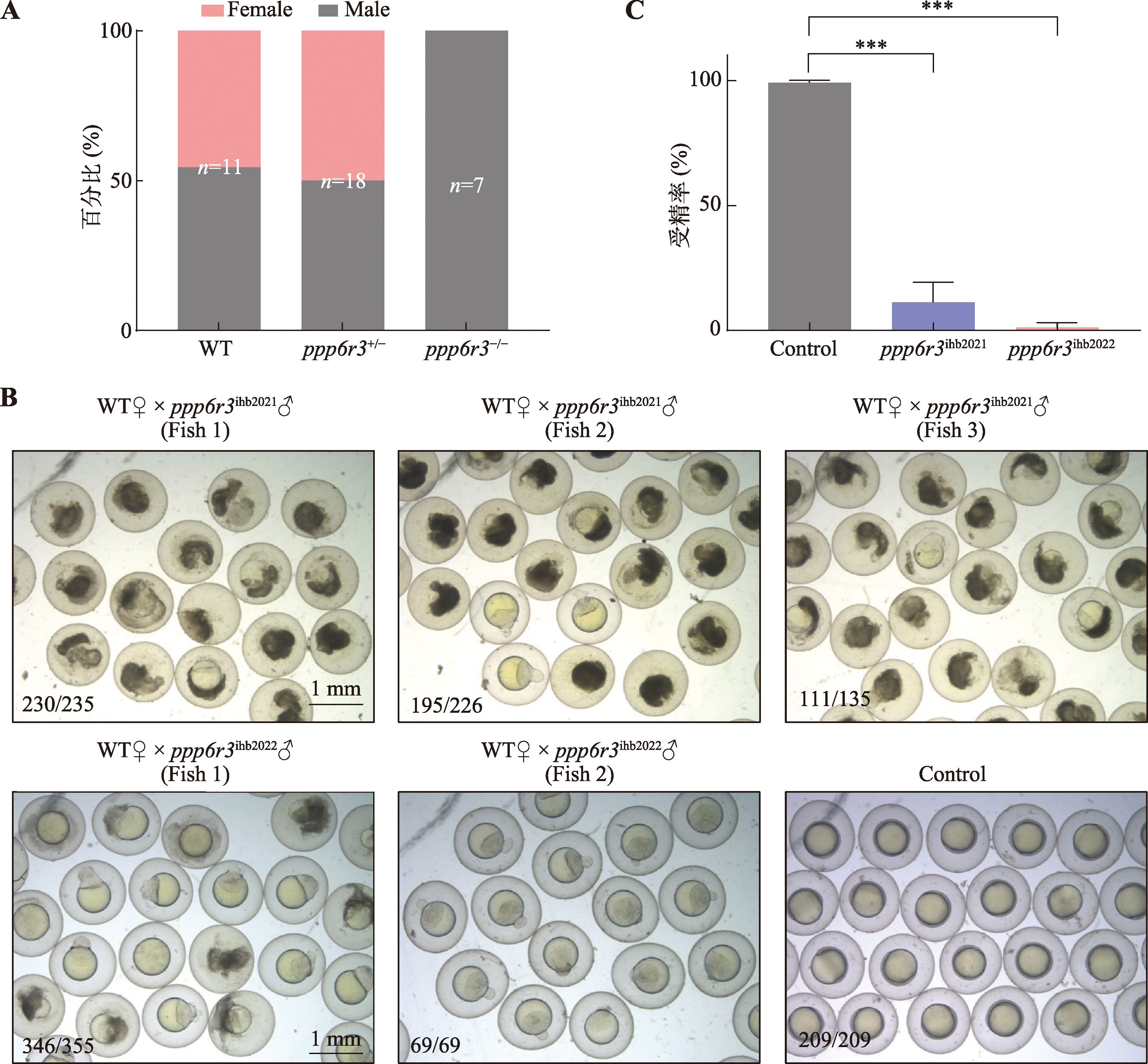
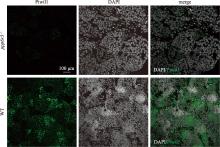
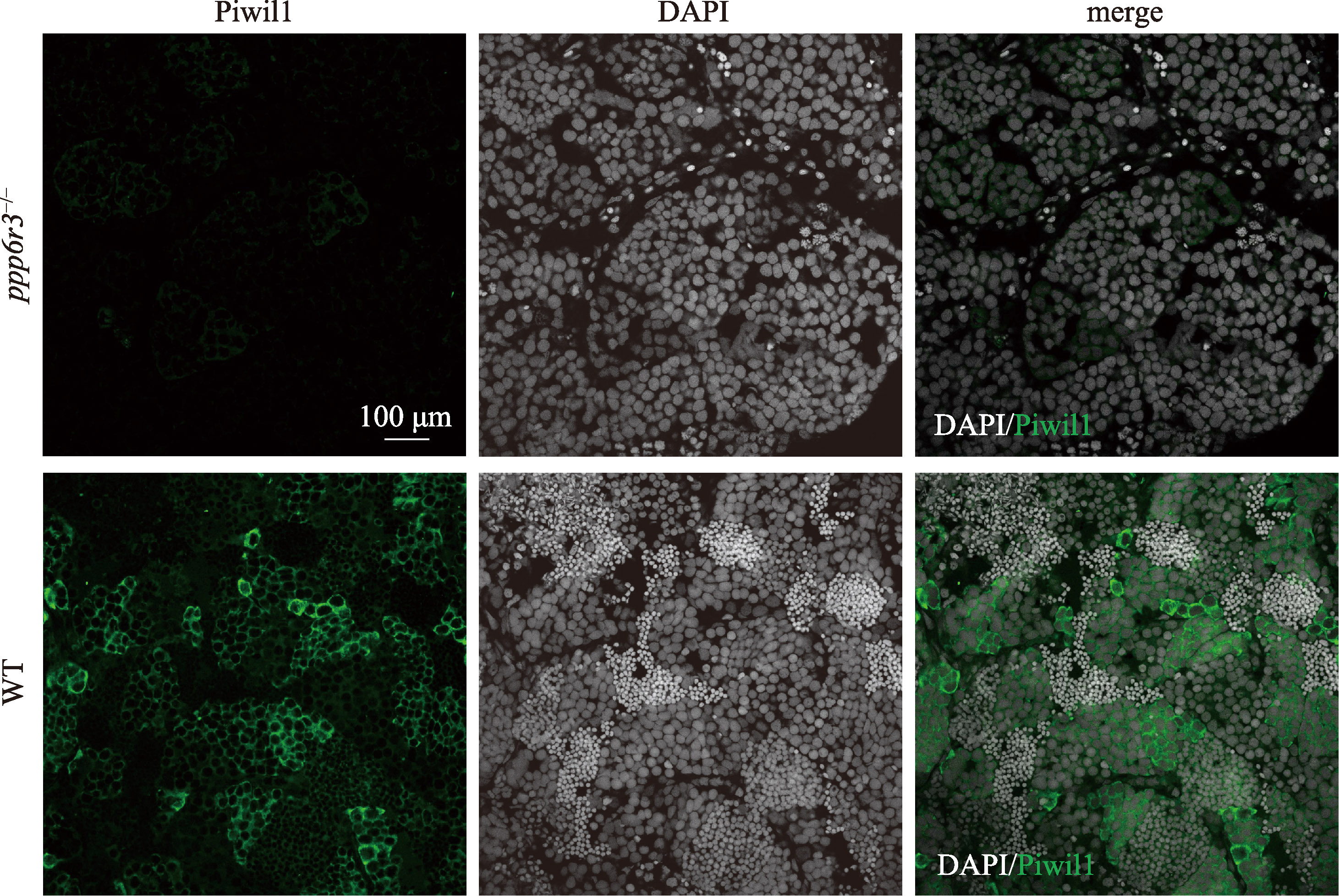
Hereditas(Beijing) ›› 2025, Vol. 47 ›› Issue (9): 1023-1031.doi: 10.16288/j.yczz.25-093
• Research Article • Previous Articles Next Articles
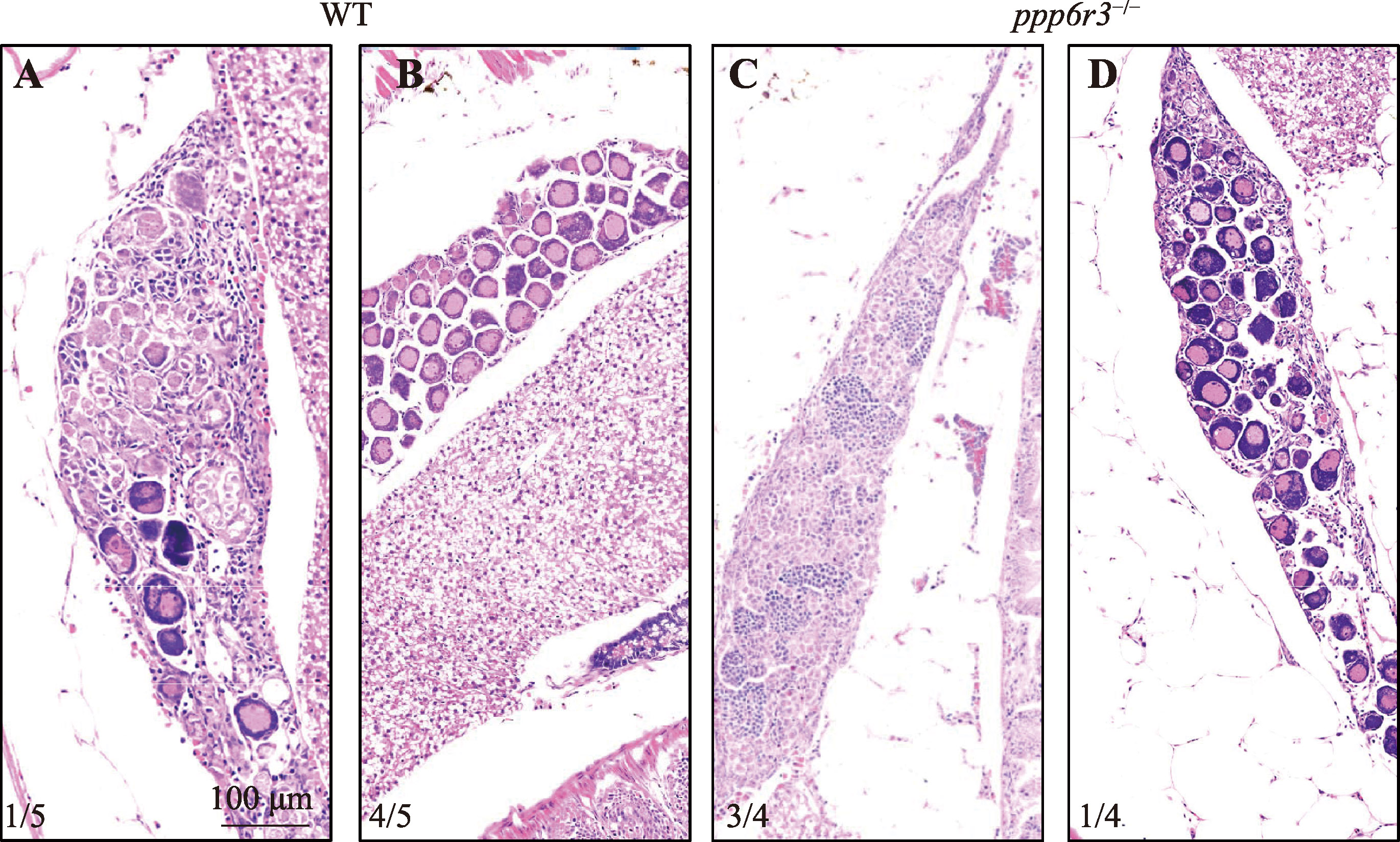
Role of ppp6r3 in zebrafish gonadal differentiation and gametogenesis
Yunhai Gao1,2( ), Jiajie Deng2, Xiao Xiao2, Luyuan Pan3, Mudan He2(
), Jiajie Deng2, Xiao Xiao2, Luyuan Pan3, Mudan He2( ), Yunbin Zhang1(
), Yunbin Zhang1( )
)
- 1. Center for Excellence in Molecular Cell Science, Chinese Academy of Sciences, Shanghai 200031, China
2. Institute of Hydrobiology, Chinese Academy of Science, Wuhan 430070, China
3. China Zebrafish Resource Center, National Aquatic Biological Resource Center, Institute of Hydrobiology, Chinese Academy of Science, Wuhan 430070, China
-
Received:2025-04-02Revised:2025-04-24Online:2025-05-13Published:2025-05-13 -
Contact:Mudan He, Yunbin Zhang E-mail:gaoyunhai2022@sibcb.ac.cn;hemudan@ihb.ac.cn;zhangyb@sibcb.ac.cn -
Supported by:National Key R&D Program of China(2022YFF0710500);National Key R&D Program of China(2024YFD2402300);STI 2030-Major Projects(2023ZD04069)
Cite this article
Yunhai Gao, Jiajie Deng, Xiao Xiao, Luyuan Pan, Mudan He, Yunbin Zhang. Role of ppp6r3 in zebrafish gonadal differentiation and gametogenesis[J]. Hereditas(Beijing), 2025, 47(9): 1023-1031.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
Table 1
Primers used in this study"
| 引物 | 序列(5′→3′) | 用途 |
|---|---|---|
| ppp6r3-gRNA-F | TGTAATACGACTCACTATAACGGATGGTTCATTCCAGGGGTTTTA GAGCTAGAAATAGC | gRNA扩增 |
| gRNA-RP | AAAAAAAGCACCGACTCGGTGCCAC | |
| ppp6r3-cas9-F | TGGAGGGATTAAGGTTGAGCT | 靶点扩增和突变体筛选 |
| ppp6r3-cas9-R | GTCCAAGACTCTGCCAGGTT | |
| Ppp6r3-P1 | CCTCGTAAATCCTCATCAAAAGCTGTAGAGTTTCATCAGTAAACT | 单分子荧光检测 |
| Ppp6r3-P2 | GGTTGAGCGGAGGCTCGTTCTGTAAAAATCATCCAGTAAACCGCC | |
| Ppp6r3-P3 | CCTCGTAAATCCTCATCAAATCAAGACTTTGCTGAAGAAACTGGC | |
| Ppp6r3-P4 | GCTCTGGTTTTCTCCCAATAAGTATAAATCATCCAGTAAACCGCC | |
| Ppp6r3-P5 | CCTCGTAAATCCTCATCAAAAGTCTTCCCTCTTCCTGAGGAACTC | |
| Ppp6r3-P6 | CTATGTGTTTGATCATCAGATCCACAAATCATCCAGTAAACCGCC | |
| Ppp6r3-P7 | CCTCGTAAATCCTCATCAAATCCTGAGCAGGAGGTCCATAATAGC | |
| Ppp6r3-P8 | GCTGCTGTGGCTCAATGCAGGTGAGAAATCATCCAGTAAACCGCC | |
| Ppp6r3-P9 | CCTCGTAAATCCTCATCAAACTTCATTTAGCCAATTTAATACATC | |
| Ppp6r3-P10 | TATCAACCAGCCGCTGTATAACTTTAAATCATCCAGTAAACCGCC |
| [1] |
Ye D, Zhu L, Zhang QF, Xiong F, Wang HP, Wang XS, He MD, Zhu ZY, Sun YH. Abundance of early embryonic primordial germ cells promotes zebrafish female differentiation as revealed by lifetime labeling of germline. Mar Biotechnol (NY), 2019, 21(2): 217-228.
pmid: 30671659 |
| [2] |
Uchida D, Yamashita M, Kitano T, Iguchi T. Oocyte apoptosis during the transition from ovary-like tissue to testes during sex differentiation of juvenile zebrafish. J Exp Biol, 2002, 205(Pt 6): 711-718.
pmid: 11914381 |
| [3] |
Zhang R, Tu YX, Ye D, Gu ZL, Chen ZX, Sun YH. A germline-specific regulator of mitochondrial fusion is required for maintenance and differentiation of germline stem and progenitor cells. Adv Sci (Weinh), 2022, 9(36): e2203631.
pmid: 36257818 |
| [4] |
Wang YQ, Ye D, Zhang FH, Zhang R, Zhu JW, Wang HP, He MD, Sun YH. Cyp11a2 is essential for oocyte development and spermatogonial stem cell differentiation in zebrafish. Endocrinology, 2022, 163(2): bqab258.
pmid: 34932120 |
| [5] |
Ren ZQ, Ye D, Su NK, Wang CF, He LJ, Wang HP, He MD, Sun YH. Foxl2l is a germ cell-intrinsic gatekeeper of oogenesis in zebrafish. Zool Res, 2024, 45(5): 1116-1130.
pmid: 39257375 |
| [6] |
Ohama T. The multiple functions of protein phosphatase 6. Biochim Biophys Acta Mol Cell Res, 2019, 1866(1): 74-82.
pmid: 30036567 |
| [7] |
Zolnierowicz S. Type 2A protein phosphatase, the complex regulator of numerous signaling pathways. Biochem Pharmacol, 2000, 60(8): 1225-1235.
pmid: 11007961 |
| [8] |
Hu MW, Wang ZB, Teng Y, Jiang ZZ, Ma XS, Hou N, Cheng X, Schatten H, Xu XZ, Yang X, Sun QY. Loss of protein phosphatase 6 in oocytes causes failure of meiosis II exit and impaired female fertility. J Cell Sci, 2015, 128(20): 3769-3780.
pmid: 26349807 |
| [9] |
Hu MW, Meng TG, Jiang ZZ, Dong MZ, Schatten H, Xu XZ, Wang ZB, Sun QY. Protein phosphatase 6 protects prophase I-arrested oocytes by safeguarding genomic integrity. PLoS Genet, 2016, 12(12): e1006513.
pmid: 27930667 |
| [10] |
Lei WL, Han F, Hu MW, Liang QX, Meng TG, Zhou Q, Ouyang YC, Hou Y, Schatten H, Wang ZB, Sun QY. Protein phosphatase 6 is a key factor regulating spermatogenesis. Cell Death Differ, 2020, 27(6): 1952-1964.
pmid: 31819157 |
| [11] |
Lei WL, Li YY, Meng TG, Ning Y, Sun SM, Zhang CH, Gui YT, Wang ZB, Qian WP, Sun QY. Specific deletion of protein phosphatase 6 catalytic subunit in Sertoli cells leads to disruption of spermatogenesis. Cell Death Dis, 2021, 12(10): 883.
pmid: 34580275 |
| [12] |
Sents W, Ivanova E, Lambrecht C, Haesen D, Janssens V. The biogenesis of active protein phosphatase 2A holoenzymes: a tightly regulated process creating phosphatase specificity. FEBS J, 2013, 280(2): 644-661.
pmid: 22443683 |
| [13] |
Douglas P, Ye RQ, Trinkle-Mulcahy L, Neal JA, De Wever V, Morrice NA, Meek K, Lees-Miller SP. Polo-like kinase 1 (PLK1) and protein phosphatase 6 (PP6) regulate DNA- dependent protein kinase catalytic subunit (DNA-PKcs) phosphorylation in mitosis. Biosci Rep, 2014, 34(3): e00113.
pmid: 24844881 |
| [14] |
Stefansson B, Brautigan DL. Protein phosphatase 6 subunit with conserved Sit4-associated protein domain targets IkappaBepsilon. J Biol Chem, 2006, 281(32): 22624-22634.
pmid: 16769727 |
| [15] |
Zhong JN, Liao J, Liu X, Wang P, Liu JP, Hou WY, Zhu BT, Yao L, Wang JS, Li J, Stark JM, Xie YT, Xu XZ. Protein phosphatase PP6 is required for homology- directed repair of DNA double-strand breaks. Cell Cycle, 2011, 10(9): 1411-1419.
pmid: 21451261 |
| [16] |
Houwing S, Kamminga LM, Berezikov E, Cronembold D, Girard A, van den Elst H, Filippov DV, Blaser H, Raz E, Moens CB, Plasterk RHA, Hannon GJ, Draper BW, Ketting RF. A role for Piwi and piRNAs in germ cell maintenance and transposon silencing in zebrafish. Cell, 2007, 129(1): 69-82.
pmid: 17418787 |
| [17] |
Luke MM, Dellaseta F, Di Como CJ, Sugimoto H, Kobayashi R, Arndt KT. The SAP, a new family of proteins, associate and function positively with the SIT4 phosphatase. Mol Cell Biol, 1996, 16(6): 2744-2755.
pmid: 8649382 |
| [18] |
Stefansson B, Ohama T, Daugherty AE, Brautigan DL. Protein phosphatase 6 regulatory subunits composed of ankyrin repeat domains. Biochemistry, 2008, 47(5): 1442-1451.
pmid: 18186651 |
| [19] |
Guergnon J, Derewenda U, Edelson JR, Brautigan DL. Mapping of protein phosphatase-6 association with its SAPS domain regulatory subunit using a model of helical repeats. BMC Biochem, 2009, 10: 24.
pmid: 19835610 |
| [1] | Jiehao Lin, Tongshu Yang, Wenqing Zhang, Wei Liu. Role of different Lyl1 transcripts in zebrafish primitive hematopoiesis [J]. Hereditas(Beijing), 2025, 47(5): 573-588. |
| [2] | Liu Jixiang, Lai Siting, Bai Jing, Xu Jin. Il34 rescues metronidazole-induced impairment of spinal cord regeneration in zebrafish central nervous system [J]. Hereditas(Beijing), 2024, 46(6): 478-489. |
| [3] | Jiaxin Hong, Song’en Xu, Wenqing Zhang, Wei Liu. The interaction of Pu.1 and cMyb in zebrafish neutrophil development [J]. Hereditas(Beijing), 2024, 46(4): 319-332. |
| [4] | Piao Sun, Ying Li, Fan Liu, Lu Wang. Generation and analysis of TPI deficiency zebrafish model [J]. Hereditas(Beijing), 2024, 46(3): 232-241. |
| [5] | Xiaojun Yang, Zhenhan Huang, Wei Liu, Wenqing Zhang, Zhibin Huang. Identification and functional characterization of CD209 homologous genes in zebrafish [J]. Hereditas(Beijing), 2024, 46(11): 947-957. |
| [6] | Kailun Li, Jingao Lu, Xiaohui Chen, Wenqing Zhang, Wei Liu. The role of the allantoin in promoting fracture healing in osteoclast-deficient zebrafish [J]. Hereditas(Beijing), 2023, 45(4): 341-353. |
| [7] | Jing’ao Lu, Chunyan Huang, Zhiyin Lin, Zheng Tang, Ning Ma, Zhibin Huang. The role of the cd99l2 gene on leukocyte interstitial migration in zebrafish [J]. Hereditas(Beijing), 2022, 44(9): 798-809. |
| [8] | Pengfei Zheng, Haibo Xie, Panpan Zhu, Chengtian Zhao. Distribution pattern of floor plate neurons in zebrafish [J]. Hereditas(Beijing), 2022, 44(6): 510-520. |
| [9] | Tingting Zhang, Feng Liu. Study on a detection method of protein tyrosine sulfation modification in zebrafish [J]. Hereditas(Beijing), 2022, 44(2): 178-186. |
| [10] | Tingting Jia, Lei Lei, Xinyuan Wu, Shunyou Cai, Yixuan Chen, Yu Xue. Study on the mechanism of metformin on zebrafish skeletal development and damage repair [J]. Hereditas(Beijing), 2022, 44(1): 68-79. |
| [11] | Jiani Guo, Fan Liu, Lu Wang. Zebrafish blood disease models and applications [J]. Hereditas(Beijing), 2020, 42(8): 725-738. |
| [12] | Feng Xiong,Xunwei Xie,Luyuan Pan,Kuoyu Li,Liyue Liu,Yun Zhang,Linglu Li,Yonghua Sun. Development of resources, technologies and services at the China Zebrafish Resource Center [J]. Hereditas(Beijing), 2018, 40(8): 683-692. |
| [13] | Jingjin Xu, Wenjuan Zhang, Jingyi Wang, Liyun Yao, Yutian Pan, Yixin Ou, Yu Xue. The active component screening of Anoectochilus roxburghii and the functional study on inhibition of melanogenesis in zebrafish [J]. Hereditas(Beijing), 2017, 39(12): 1178-1187. |
| [14] | Shanshan Liu, Cuizhen Zhang, Gang Peng. Effects of starvation on the expression of feeding related neuropeptides in the larval zebrafish hypothalamus [J]. Hereditas(Beijing), 2016, 38(9): 821-830. |
| [15] | Fenghua Zhang, Houpeng Wang, Siyu Huang, Feng Xiong, Zuoyan Zhu, Yonghua Sun. A comparison of the knockout efficiencies of two codon-optimized Cas9 coding sequences in zebrafish embryos [J]. HEREDITAS(Beijing), 2016, 38(2): 144-154. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||