Hereditas(Beijing) ›› 2025, Vol. 47 ›› Issue (11): 1197-1213.doi: 10.16288/j.yczz.24-372
• Review • Previous Articles Next Articles
Progress on extracellular vesicles in the nervous system
Jiaqiang Chen1,2( ), Mei Ding1,2(
), Mei Ding1,2( )
)
1. Institute of Genetics and Developmental Biology ,Chinese Academy of Sciences Beijing 100101, China 2. University of Chinese Academy of Sciences Beijing 100093, China
-
Received:2025-04-12Revised:2025-06-20Online:2025-07-07Published:2025-07-07 -
Contact:Mei Ding E-mail:jiaqiang.chen@genetics.ac.cn;mding@genetics.ac.cn -
Supported by:National Natural Science Foundation of China(31921002)
Cite this article
Jiaqiang Chen, Mei Ding. Progress on extracellular vesicles in the nervous system[J]. Hereditas(Beijing), 2025, 47(11): 1197-1213.
share this article
| [1] |
Agnati LF, Guidolin D, Guescini M, Genedani S, Fuxe K. Understanding wiring and volume transmission. Brain Res Rev, 2010, 64(1): 137-159.
pmid: 20347870 |
| [2] |
Budnik V, Ruiz-Cañada C, Wendler F. Extracellular vesicles round off communication in the nervous system. Nat Rev Neurosci, 2016, 17(3): 160-172.
pmid: 26891626 |
| [3] |
Ahmad S, Srivastava RK, Singh P, Naik UP, Srivastava AK. Role of extracellular vesicles in glia-neuron intercellular communication. Front Mol Neurosci, 2022, 15: 844194.
pmid: 35493327 |
| [4] |
Filannino FM, Panaro MA, Benameur T, Pizzolorusso I, Porro C. Extracellular vesicles in the central nervous system: a novel mechanism of neuronal cell communication. Int J Mol Sci, 2024, 25(3): 1629.
pmid: 38338906 |
| [5] |
Holm MM, Kaiser J, Schwab ME. Extracellular vesicles: multimodal envoys in neural maintenance and repair. Trends Neurosci, 2018, 41(6): 360-372.
pmid: 29605090 |
| [6] |
Gage FH. Adult neurogenesis in mammals. Science, 2019, 364(6443): 827-828.
pmid: 31147506 |
| [7] |
Sangani NB, Gomes AR, Curfs LMG, Reutelingsperger CP. The role of extracellular vesicles during CNS development. Prog Neurobiol, 2021, 205: 102124.
pmid: 34314775 |
| [8] |
Ma YZ, Li CH, Huang YL, Wang Y, Xia XH, Zheng JC. Exosomes released from neural progenitor cells and induced neural progenitor cells regulate neurogenesis through miR-21a. Cell Commun Signal, 2019, 17(1): 96.
pmid: 31419975 |
| [9] |
Sharma P, Mesci P, Carromeu C, McClatchy DR, Schiapparelli L, Yates JR, Muotri AR, Cline HT. Exosomes regulate neurogenesis and circuit assembly. Proc Natl Acad Sci USA, 2019, 116(32): 16086-16094.
pmid: 31320591 |
| [10] |
Upadhya R, Madhu LN, Attaluri S, Gitaí DLG, Pinson MR, Kodali M, Shetty G, Zanirati G, Kumar S, Shuai B, Weintraub ST, Shetty AK. Extracellular vesicles from human iPSC-derived neural stem cells: miRNA and protein signatures, and anti-inflammatory and neurogenic properties. J Extracell vesicles, 2020, 9(1): 1809064.
pmid: 32944193 |
| [11] |
Bassi MS, Iezzi E, Gilio L, Centonze D, Buttari F. Synaptic plasticity shapes brain connectivity: implications for network topology. Int J Mol Sci, 2019, 20(24): 6193.
pmid: 31817968 |
| [12] |
Vilcaes AA, Chanaday NL, Kavalali ET. Interneuronal exchange and functional integration of synaptobrevin via extracellular vesicles. Neuron, 2021, 109(6): 971-983.e5.
pmid: 33513363 |
| [13] |
Lee SH, Shin SM, Zhong P, Kim HT, Kim DI, Kim JM, Do Heo W, Kim DW, Yeo CY, Kim CH, Liu QS. Reciprocal control of excitatory synapse numbers by Wnt and Wnt inhibitor PRR7 secreted on exosomes. Nat Commun, 2018, 9(1): 3434.
pmid: 30143647 |
| [14] |
Korkut C, Ataman B, Ramachandran P, Ashley J, Barria R, Gherbesi N, Budnik V. Trans-synaptic transmission of vesicular Wnt signals through Evi/Wntless. Cell, 2009, 139(2): 393-404.
pmid: 19837038 |
| [15] |
Korkut C, Li YH, Koles K, Brewer C, Ashley J, Yoshihara M, Budnik V. Regulation of postsynaptic retrograde signaling by presynaptic exosome release. Neuron, 2013, 77(6): 1039-1046.
pmid: 23522040 |
| [16] |
Goldie BJ, Dun MD, Lin MJ, Smith ND, Verrills NM, Dayas CV, Cairns MJ. Activity-associated miRNA are packaged in Map1b-enriched exosomes released from depolarized neurons. Nucleic Acids Res, 2014, 42(14): 9195-9208.
pmid: 25053844 |
| [17] |
Pastuzyn ED, Day CE, Kearns RB, Kyrke-Smith M, Taibi AV, McCormick J, Yoder N, Belnap DM, Erlendsson S, Morado DR, Briggs JAG, Feschotte C, Shepherd JD. The neuronal gene Arc encodes a repurposed retrotransposon Gag protein that mediates intercellular RNA transfer. Cell, 2018, 172(1-2): 275-288.e18.
pmid: 29328916 |
| [18] |
Shepherd JD, Bear MF. New views of Arc, a master regulator of synaptic plasticity. Nat Neurosci, 2011, 14(3): 279-284.
pmid: 21278731 |
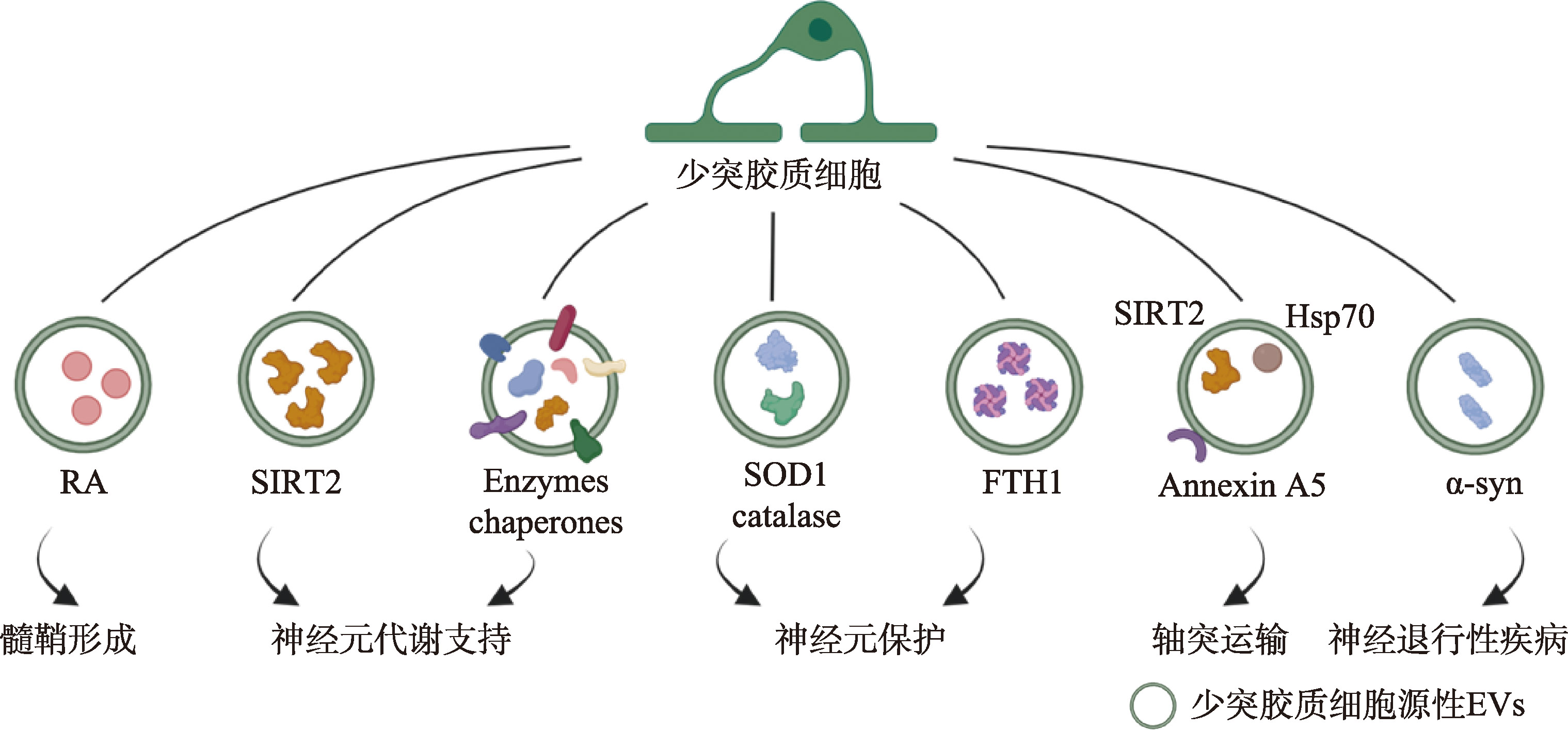
| [19] |
Ashley J, Cordy B, Lucia D, Fradkin LG, Budnik V, Thomson T. Retrovirus-like Gag protein Arc1 binds RNA and traffics across synaptic boutons. Cell, 2018, 172(1-2): 262-274.e11.
pmid: 29328915 |
| [20] |
Morel L, Regan M, Higashimori H, Ng SK, Esau C, Vidensky S, Rothstein J, Yang YJ. Neuronal exosomal miRNA-dependent translational regulation of astroglial glutamate transporter GLT1. J Biol Chem, 2013, 288(10): 7105-7116.
pmid: 23364798 |
| [21] |
Bahrini I, Song JH, Diez D, Hanayama R. Neuronal exosomes facilitate synaptic pruning by up-regulating complement factors in microglia. Sci Rep, 2015, 5(1): 7989.
pmid: 25612542 |
| [22] |
Peng H, Harvey BT, Richards CI, Nixon K. Neuron- derived extracellular vesicles modulate microglia activation and function. Biology (Basel), 2021, 10(10): 948.
pmid: 34681047 |
| [23] | Kaya Z, Belder N, Sever-Bahcekapili M, Donmez-Demir B, Erdener ŞE, Bozbeyoglu N, Bagci C, Eren-Kocak E, Yemisci M, Karatas H, Erdemli E, Gursel I, Dalkara T. Vesicular HMGB1 release from neurons stressed with spreading depolarization enables confined inflammatory signaling to astrocytes. J Neuroinflammation, 2023, 20(1): 295. [DOI] |
| [24] |
Xin DQ, Li TT, Zhao YJ, Guo XF, Gai CC, Jiang ZG, Yu SW, Cheng J, Song Y, Cheng YH, Luo Q, Gu B, Liu DX, Wang Z. MiR-100-5p-rich small extracellular vesicles from activated neuron to aggravate microglial activation and neuronal activity after stroke. J Nanobiotechnology, 2024, 22(1): 534.
pmid: 39227960 |
| [25] |
Cossetti C, Iraci N, Mercer TR, Leonardi T, Alpi E, Drago D, Alfaro-Cervello C, Saini HK, Davis MP, Schaeffer J, Vega B, Stefanini M, Zhao CJ, Muller W, Garcia-Verdugo JM, Mathivanan S, Bachi A, Enright AJ, Mattick JS, Pluchino S. Extracellular vesicles from neural stem cells transfer IFN-γ via Ifngr1 to activate Stat1 signaling in target cells. Mol Cell, 2014, 56(2): 193-204.
pmid: 25242146 |
| [26] |
Simeoli R, Montague K, Jones HR, Castaldi L, Chambers D, Kelleher JH, Vacca V, Pitcher T, Grist J, Al-Ahdal H, Wong LF, Perretti M, Lai J, Mouritzen P, Heppenstall P, Malcangio M. Exosomal cargo including microRNA regulates sensory neuron to macrophage communication after nerve trauma. Nat Commun, 2017, 8(1): 1778.
pmid: 29176651 |
| [27] |
Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron, 2008, 57(2): 178-201.
pmid: 18215617 |
| [28] |
Obermeier B, Daneman R, Ransohoff RM. Development, maintenance and disruption of the blood-brain barrier. Nat Med, 2013, 19(12): 1584-1596.
pmid: 24309662 |
| [29] |
Tam SJ, Watts RJ. Connecting vascular and nervous system development: angiogenesis and the blood-brain barrier. Annu Rev Neurosci, 2010, 33(1): 379-408.
pmid: 20367445 |
| [30] |
Xu B, Zhang Y, Du XF, Li J, Zi HX, Bu JW, Yan Y, Han H, Du JL. Neurons secrete miR-132-containing exosomes to regulate brain vascular integrity. Cell Res, 2017, 27(7): 882-897.
pmid: 28429770 |
| [31] |
Li Y, Jiang JW, Li JM, Liu SL, Wang C, Yu ZT, Xia Y. Exosome-derived CDC42 from hypoxia-pretreated neural stem cells inhibits ACSL4-related ferroptosis to alleviate vascular injury in Parkinson’s disease mice models. J neurochem, 2025, 169(3): e70027.
pmid: 40035385 |
| [32] |
Dong XH, Jiang DY, Wang L, Zhao J, Yu LL, Huang Y, Wu XH, Zhu YQ, Zhao YM, Zhao QS, Zhang GM, Li XY. VPS28 regulates brain vasculature by controlling neuronal VEGF trafficking through extracellular vesicle secretion. iScience, 2022, 25(4): 104042.
pmid: 35330682 |
| [33] |
Wang JW, Xie XF, Wu YG, Zhou Y, Li QF, Li Y, Xu X, Wang M, Murdiyarso L, Houck K, Hilton T, Chung D, Dong JF, Li M, Zhang JN. Brain-derived extracellular vesicles induce vasoconstriction and reduce cerebral blood flow in mice. J Neurotrauma, 2022, 39(11-12): 879-890.
pmid: 35316073 |
| [34] |
Yuyama K, Sun H, Sakai S, Mitsutake S, Okada M, Tahara H, Furukawa JI, Fujitani N, Shinohara Y, Igarashi Y. Decreased amyloid-β pathologies by intracerebral loading of glycosphingolipid-enriched exosomes in Alzheimer model mice. J Biol Chem, 2014, 289(35): 24488-24498.
pmid: 25037226 |
| [35] |
Yuyama K, Sun H, Usuki S, Sakai S, Hanamatsu H, Mioka T, Kimura N, Okada M, Tahara H, Furukawa JI, Fujitani N, Shinohara Y, Igarashi Y. A potential function for neuronal exosomes: sequestering intracerebral amyloid-β peptide. FEBS Lett, 2015, 589(1): 84-88.
pmid: 25436414 |
| [36] |
Melentijevic I, Toth ML, Arnold ML, Guasp RJ, Harinath G, Nguyen KC, Taub D, Parker JA, Neri C, Gabel CV, Hall DH, Driscoll M. C. elegans neurons jettison protein aggregates and mitochondria under neurotoxic stress. Nature, 2017, 542(7641): 367-371.
pmid: 28178240 |
| [37] |
Takeuchi T, Suzuki M, Fujikake N, Popiel HA, Kikuchi H, Futaki S, Wada K, Nagai Y. Intercellular chaperone transmission via exosomes contributes to maintenance of protein homeostasis at the organismal level. Proc Natl Acad Sci USA, 2015, 112(19): E2497-E2506.
pmid: 25918398 |
| [38] | Shang XK, Zhang SM, Ni JJ. Research progress of cathepsin B in brain aging and Alzheimer’s diseases. Hereditas(Beijing), 2023, 45(3): 212-220. |
| 商晓康, 张思萌, 倪军军. 组织蛋白酶B参与脑衰老及阿尔兹海默症发生发展研究进展. 遗传, 2023, 45(3): 212-220. | |
| [39] |
Ruan Z, Pathak D, Venkatesan Kalavai S, Yoshii- Kitahara A, Muraoka S, Bhatt N, Takamatsu-Yukawa K, Hu JQ, Wang YZ, Hersh S, Ericsson M, Gorantla S, Gendelman HE, Kayed R, Ikezu S, Luebke JI, Ikezu T. Alzheimer’s disease brain-derived extracellular vesicles spread tau pathology in interneurons. Brain, 2021, 144(1): 288-309.
pmid: 33246331 |
| [40] |
Sinha MS, Ansell-Schultz A, Civitelli L, Hildesjö C, Larsson M, Lannfelt L, Ingelsson M, Hallbeck M. Alzheimer’s disease pathology propagation by exosomes containing toxic amyloid-beta oligomers. Acta Neuropathol, 2018, 136(1): 41-56.
pmid: 29934873 |
| [41] |
Ding L, Yang XY, Xia XH, Li YX, Wang Y, Li CH, Sun YY, Gao G, Zhao S, Sheng SY, Liu JH, Zheng JC. Exosomes mediate APP dysregulation via APP-miR-185-5p axis. Front Cell Dev Biol, 2022, 10: 793388.
pmid: 35223832 |
| [42] |
Lizarraga-Valderrama LR, Sheridan GK. Extracellular vesicles and intercellular communication in the central nervous system. FEBS Lett, 2021, 595(10): 1391-1410.
pmid: 33728650 |
| [43] |
Freeman MR, Rowitch DH. Evolving concepts of gliogenesis: a look way back and ahead to the next 25 years. Neuron, 2013, 80(3): 613-623.
pmid: 24183014 |
| [44] |
Luarte A, Henzi R, Fernández A, Gaete D, Cisternas P, Pizarro M, Batiz LF, Villalobos I, Masalleras M, Vergara R, Varas-Godoy M, Abarzua-Catalan L, Herrera-Molina R, Lafourcade C, Wyneken U. Astrocyte-derived small extracellular vesicles regulate dendritic complexity through miR-26a-5p activity. Cells, 2020, 9(4): 930.
pmid: 32290095 |
| [45] |
You Y, Borgmann K, Edara VV, Stacy S, Ghorpade A, Ikezu T. Activated human astrocyte-derived extracellular vesicles modulate neuronal uptake, differentiation and firing. J Extracell Vesicles, 2019, 9(1): 1706801.
pmid: 32002171 |
| [46] |
Pascua-Maestro R, González E, Lillo C, Ganfornina MD, Falcón-Pérez JM, Sanchez D. Extracellular vesicles secreted by astroglial cells transport Apolipoprotein D to neurons and mediate neuronal survival upon oxidative stress. Front Cell Neurosci, 2019, 12: 526.
pmid: 30687015 |
| [47] |
Guitart K, Loers G, Buck F, Bork U, Schachner M, Kleene R. Improvement of neuronal cell survival by astrocyte-derived exosomes under hypoxic and ischemic conditions depends on prion protein. Glia, 2016, 64(6): 896-910.
pmid: 26992135 |
| [48] |
Bianco F, Perrotta C, Novellino L, Francolini M, Riganti L, Menna E, Saglietti L, Schuchman EH, Furlan R, Clementi E, Matteoli M, Verderio C. Acid sphingomyelinase activity triggers microparticle release from glial cells. EMBO J, 2009, 28(8): 1043-1054.
pmid: 19300439 |
| [49] |
Dickens AM, Tovar-Y-Romo LB, Yoo SW, Trout AL, Bae M, Kanmogne M, Megra B, Williams DW, Witwer KW, Gacias M, Tabatadze N, Cole RN, Casaccia P, Berman JW, Anthony DC, Haughey NJ. Astrocyte-shed extracellular vesicles regulate the peripheral leukocyte response to inflammatory brain lesions. Sci Signal, 2017, 10(473): eaai7696.
pmid: 28377412 |
| [50] |
Ibáñez F, Montesinos J, Ureña-Peralta JR, Guerri C, Pascual M. TLR4 participates in the transmission of ethanol-induced neuroinflammation via astrocyte- derived extracellular vesicles. J Neuroinflammation, 2019, 16(1): 136.
pmid: 31272469 |
| [51] |
Willis CM, Nicaise AM, Bongarzone ER, Givogri M, Reiter CR, Heintz O, Jellison ER, Sutter PA, TeHennepe G, Ananda G, Vella AT, Crocker SJ. Astrocyte support for oligodendrocyte differentiation can be conveyed via extracellular vesicles but diminishes with age. Sci Rep, 2020, 10(1): 828.
pmid: 31964978 |
| [52] |
Varcianna A, Myszczynska MA, Castelli LM, O'Neill B, Kim Y, Talbot J, Nyberg S, Nyamali I, Heath PR, Stopford MJ, Hautbergue GM, Ferraiuolo L. Micro- RNAs secreted through astrocyte-derived extracellular vesicles cause neuronal network degeneration in C9orf72 ALS. EBioMedicine, 2019, 40: 626-635.
pmid: 30711519 |
| [53] |
Basso M, Pozzi S, Tortarolo M, Fiordaliso F, Bisighini C, Pasetto L, Spaltro G, Lidonnici D, Gensano F, Battaglia E, Bendotti C, Bonetto V. Mutant copper-zinc superoxide dismutase (SOD1) induces protein secretion pathway alterations and exosome release in astrocytes: implications for disease spreading and motor neuron pathology in amyotrophic lateral sclerosis. J Biol Chem, 2013, 288(22): 15699-15711.
pmid: 23592792 |
| [54] |
Hickman S, Izzy S, Sen P, Morsett L, El Khoury J. Microglia in neurodegeneration. Nat Neurosci, 2018, 21(10): 1359-1369.
pmid: 30258234 |
| [55] |
Paolicelli RC, Bergamini G, Rajendran L. Cell-to-cell communication by extracellular vesicles: focus on microglia. Neuroscience, 2019, 405: 148-157.
pmid: 29660443 |
| [56] |
Antonucci F, Turola E, Riganti L, Caleo M, Gabrielli M, Perrotta C, Novellino L, Clementi E, Giussani P, Viani P, Matteoli M, Verderio C. Microvesicles released from microglia stimulate synaptic activity via enhanced sphingolipid metabolism. EMBO J, 2012, 31(5): 1231-1240.
pmid: 22246184 |
| [57] |
Gabrielli M, Battista N, Riganti L, Prada I, Antonucci F, Cantone L, Matteoli M, Maccarrone M, Verderio C. Active endocannabinoids are secreted on extracellular membrane vesicles. EMBO Rep, 2015, 16(2): 213-220.
pmid: 25568329 |
| [58] |
Prada I, Gabrielli M, Turola E, Iorio A, D’Arrigo G, Parolisi R, De Luca M, Pacifici M, Bastoni M, Lombardi M, Legname G, Cojoc D, Buffo A, Furlan R, Peruzzi F, Verderio C. Glia-to-neuron transfer of miRNAs via extracellular vesicles: a new mechanism underlying inflammation-induced synaptic alterations. Acta Neuropathol, 2018, 135(4): 529-550.
pmid: 29302779 |
| [59] |
Bianco F, Pravettoni E, Colombo A, Schenk U, Möller T, Matteoli M, Verderio C. Astrocyte-derived ATP induces vesicle shedding and IL-1β release from microglia. J Immunol, 2005, 174(11): 7268-7277.
pmid: 15905573 |
| [60] |
Yang YY, Boza-Serrano A, Dunning CJR, Clausen BH, Lambertsen KL, Deierborg T. Inflammation leads to distinct populations of extracellular vesicles from microglia. J Neuroinflammation, 2018, 15(1): 168.
pmid: 29807527 |
| [61] |
Kumar A, Stoica BA, Loane DJ, Yang M, Abulwerdi G, Khan N, Kumar A, Thom SR, Faden AI. Microglial- derived microparticles mediate neuroinflammation after traumatic brain injury. J Neuroinflammation, 2017, 14(1): 47.
pmid: 28292310 |
| [62] |
Huang S, Ge XT, Yu JW, Han ZL, Yin ZY, Li Y, Chen FL, Wang HC, Zhang JN, Lei P. Increased miR-124-3p in microglial exosomes following traumatic brain injury inhibits neuronal inflammation and contributes to neurite outgrowth via their transfer into neurons. FASEB J, 2018, 32(1): 512-528.
pmid: 28935818 |
| [63] |
Lombardi M, Parolisi R, Scaroni F, Bonfanti E, Gualerzi A, Gabrielli M, de Rosbo NK, Uccelli A, Giussani P, Viani P, Garlanda C, Abbracchio MP, Chaabane L, Buffo A, Fumagalli M, Verderio C. Detrimental and protective action of microglial extracellular vesicles on myelin lesions: astrocyte involvement in remyelination failure. Acta Neuropathol, 2019, 138(6): 987-1012.
pmid: 31363836 |
| [64] |
Aires ID, Ribeiro-Rodrigues T, Boia R, Ferreira- Rodrigues M, Girão H, Ambrósio AF, Santiago AR. Microglial extracellular vesicles as vehicles for neurodegeneration spreading. Biomolecules, 2021, 11(6): 770.
pmid: 34063832 |
| [65] |
Li J, Li XN, Jiang X, Yang M, Yang R, Burnstock G, Xiang ZH, Yuan HB. Microvesicles shed from microglia activated by the P2X7-p38 pathway are involved in neuropathic pain induced by spinal nerve ligation in rats. Purinergic Signal, 2017, 13(1): 13-26.
pmid: 27683228 |
| [66] |
Guo M, Wang J, Zhao YX, Feng YW, Han SD, Dong Q, Cui M, Tieu K. Microglial exosomes facilitate α-synuclein transmission in Parkinson’s disease. Brain, 2020, 143(5): 1476-1497.
pmid: 32355963 |
| [67] |
Joshi P, Turola E, Ruiz A, Bergami A, Libera DD, Benussi L, Giussani P, Magnani G, Comi G, Legname G, Ghidoni R, Furlan R, Matteoli M, Verderio C. Microglia convert aggregated amyloid-β into neurotoxic forms through the shedding of microvesicles. Cell Death Differ, 2014, 21(4): 582-593.
pmid: 24336048 |
| [68] |
Chen JF, Wang F, Huang NX, Xiao L, Mei F. Oligodendrocytes and myelin: active players in neurodegeneartive brains? Dev Neurobiol, 2022, 82(2): 160-174.
pmid: 35081276 |
| [69] |
Kuhn S, Gritti L, Crooks D, Dombrowski Y. Oligodendrocytes in development, myelin generation and beyond. Cells, 2019, 8(11): 1424.
pmid: 31726662 |
| [70] |
Yu Q, Guan T, Guo Y, Kong JM. The initial myelination in the central nervous system. ASN Neuro, 2023, 15: 17590914231163039.
pmid: 36974372 |
| [71] |
Bakhti M, Winter C, Simons M. Inhibition of myelin membrane sheath formation by oligodendrocyte-derived exosome-like vesicles. J Biol Chem, 2011, 286(1): 787-796.
pmid: 20978131 |
| [72] |
Goncalves MB, Wu Y, Clarke E, Grist J, Hobbs C, Trigo D, Jack J, Corcoran JPT. Regulation of myelination by exosome associated retinoic acid release from NG2-positive cells. J Neurosci, 2019, 39(16): 3013-3027.
pmid: 30760627 |
| [73] |
Chamberlain KA, Huang N, Xie YX, LiCausi F, Li SN, Li Y, Sheng ZH. Oligodendrocytes enhance axonal energy metabolism by deacetylation of mitochondrial proteins through transcellular delivery of SIRT2. Neuron, 2021, 109(21): 3456-3472.e8.
pmid: 34506725 |
| [74] |
Frühbeis C, Fröhlich D, Kuo WP, Amphornrat J, Thilemann S, Saab AS, Kirchhoff F, Möbius W, Goebbels S, Nave KA, Schneider A, Simons M, Klugmann M, Trotter J, Krämer-Albers EM. Neurotransmitter-triggered transfer of exosomes mediates oligodendrocyte-neuron communication. PLoS Biol, 2013, 11(7): e1001604.
pmid: 23874151 |
| [75] |
Fröhlich D, Kuo WP, Frühbeis C, Sun JJ, Zehendner CM, Luhmann HJ, Pinto S, Toedling J, Trotter J, Krämer- Albers EM. Multifaceted effects of oligodendroglial exosomes on neurons: impact on neuronal firing rate, signal transduction and gene regulation. Philos Trans R Soc Lond B Biol Sci, 2014, 369(1652): 20130510.
pmid: 25135971 |
| [76] |
Mukherjee C, Kling T, Russo B, Miebach K, Kess E, Schifferer M, Pedro LD, Weikert U, Fard MK, Kannaiyan N, Rossner M, Aicher ML, Goebbels S, Nave KA, Krämer-Albers EM, Schneider A, Simons M. Oligodendrocytes provide antioxidant defense function for neurons by secreting ferritin heavy chain. Cell Metab, 2020, 32(2): 259-272.e10.
pmid: 32531201 |
| [77] |
Frühbeis C, Kuo-Elsner WP, Müller C, Barth K, Peris L, Tenzer S, Möbius W, Werner HB, Nave KA, Fröhlich D, Krämer-Albers EM. Oligodendrocytes support axonal transport and maintenance via exosome secretion. PLoS Biol, 2020, 18(12): e3000621.
pmid: 33351792 |
| [78] |
Dutta S, Hornung S, Kruayatidee A, Maina KN, del Rosario I, Paul KC, Wong DY, Duarte Folle A, Markovic D, Palma JA, Serrano GE, Adler CH, Perlman SL, Poon WW, Kang UJ, Alcalay RN, Sklerov M, Gylys KH, Kaufmann H, Fogel BL, Bronstein JM, Ritz B, Bitan G. α-synuclein in blood exosomes immunoprecipitated using neuronal and oligodendroglial markers distinguishes Parkinson’s disease from multiple system atrophy. Acta Neuropathol, 2021, 142(3): 495-511.
pmid: 33991233 |
| [79] |
Kratzer I, Ek J, Stolp H. The molecular anatomy and functions of the choroid plexus in healthy and diseased brain. Biochim Biophys Acta Biomembr, 2020, 1862(11): 183430.
pmid: 32750317 |
| [80] |
Balusu S, Van Wonterghem E, De Rycke R, Raemdonck K, Stremersch S, Gevaert K, Brkic M, Demeestere D, Vanhooren V, Hendrix A, Libert C, Vandenbroucke RE. Identification of a novel mechanism of blood-brain communication during peripheral inflammation via choroid plexus-derived extracellular vesicles. EMBO Mol Med, 2016, 8(10): 1162-1183.
pmid: 27596437 |
| [81] |
Yang Z, Shi XF, Gao ZD, Chu L. miR-155-5p in extracellular vesicles derived from choroid plexus epithelial cells promotes autophagy and inflammation to aggravate ischemic brain injury in mice. Oxid Med Cell Longev, 2022, 2022: 8603427.
pmid: 35222806 |
| [82] |
Vandendriessche C, Balusu S, Van Cauwenberghe C, Brkic M, Pauwels M, Plehiers N, Bruggeman A, Dujardin P, Van Imschoot G, Van Wonterghem E, Hendrix A, Baeke F, De Rycke R, Gevaert K, Vandenbroucke RE. Importance of extracellular vesicle secretion at the blood-cerebrospinal fluid interface in the pathogenesis of Alzheimer’s disease. Acta Neuropathol Commun, 2021, 9(1): 143.
pmid: 34425919 |
| [83] |
O’Hara BA, Morris-Love J, Gee GV, Haley SA, Atwood WJ. JC virus infected choroid plexus epithelial cells produce extracellular vesicles that infect glial cells independently of the virus attachment receptor. PLoS Pathog, 2020, 16(3): e1008371.
pmid: 32130281 |
| [84] |
Grapp M, Wrede A, Schweizer M, Hüwel S, Galla HJ, Snaidero N, Simons M, Bückers J, Low PS, Urlaub H, Gärtner J, Steinfeld R. Choroid plexus transcytosis and exosome shuttling deliver folate into brain parenchyma. Nat Commun, 2013, 4(1): 2123.
pmid: 23828504 |
| [85] |
Ditte Z, Silbern I, Ditte P, Urlaub H, Eichele G. Extracellular vesicles derived from the choroid plexus trigger the differentiation of neural stem cells. J Extracell Vesicles, 2022, 11(11): e12276.
pmid: 36325603 |
| [86] |
Liu LL, Shannahan J, Zheng W. Choroid plexus modulates subventricular zone adult neurogenesis and olfaction through secretion of small extracellular vesicles. bioRxiv, 2023, doi: 10.1101/2023.03.16.532966.
pmid: 36993578 |
| [87] |
Kastriti ME, Adameyko I. Specification, plasticity and evolutionary origin of peripheral glial cells. Curr Opin Neurobiol, 2017, 47: 196-202.
pmid: 29161639 |
| [88] |
Taveggia C, Feltri ML. Beyond wrapping: canonical and noncanonical functions of Schwann cells. Annu Rev Neurosci, 2022, 45(1): 561-580.
pmid: 35440141 |
| [89] |
Wong FC, Ye LH, Demir IE, Kahlert C. Schwann cell-derived exosomes: janus-faced mediators of regeneration and disease. Glia, 2022, 70(1): 20-34.
pmid: 34519370 |
| [90] |
Lopez-Verrilli MA, Picou F, Court FA. Schwann cell- derived exosomes enhance axonal regeneration in the peripheral nervous system. Glia, 2013, 61(11): 1795-1806.
pmid: 24038411 |
| [91] |
López-Leal R, Díaz-Viraqué F, Catalán RJ, Saquel C, Enright A, Iraola G, Court FA. Schwann cell reprogramming into repair cells increases miRNA-21 expression in exosomes promoting axonal growth. J Cell Sci, 2020, 133(12): jcs239004.
pmid: 32409566 |
| [92] |
Zhou M, Hu M, He SM, Li BS, Liu C, Min J, Hong L. Effects of RSC96 Schwann cell-derived exosomes on proliferation, senescence, and apoptosis of dorsal root ganglion cells in vitro. Med Sci Monit, 2018, 24: 7841-7849.
pmid: 30387453 |
| [93] | Hyung S, Kim JY, Yu CJ, Jung HS, Hong JW. Neuroprotective effect of glial cell-derived exosomes on neurons. Immunother (Los Angel), 2019, 5(1): 156. |
| [94] |
Wu ZG, Pu PJ, Su Z, Zhang XC, Nie LY, Chang YM. Schwann cell-derived exosomes promote bone regeneration and repair by enhancing the biological activity of porous Ti6Al4V scaffolds. Biochem Biophys Res Commun, 2020, 531(4): 559-565.
pmid: 32811642 |
| [95] |
Jia LF, Chopp M, Wang L, Lu XR, Szalad A, Zhang ZG. Exosomes derived from high-glucose-stimulated Schwann cells promote development of diabetic peripheral neuropathy. FASEB J, 2018, 32(12): fj201800597R.
pmid: 29932869 |
| [96] |
Wang L, Chopp M, Szalad A, Lu XR, Zhang Y, Wang XL, Cepparulo P, Lu M, Li C, Zhang ZG. Exosomes derived from Schwann cells ameliorate peripheral neuropathy in type 2 diabetic mice. Diabetes, 2020, 69(4): 749-759.
pmid: 31915154 |
| [97] |
Chignon-Sicard B, Hofman V, Chevallier D, Cucchi JM, Ilié M, Dadone-Montaudié B, Paul F, Carpentier X, Quintens H, Bence-Gauchiez C, Caselles D, Rossant J, Durand M, Bertolotti R. Age-related schwannomatosis with potential exosome-mediated contribution to prostate hyperplasia: a case report and mini-review. Ther Adv Urol, 2019, 11: 1756287219875578.
pmid: 31632463 |
| [98] |
Milosavljević A, Jančić J, Mirčić A, Dožić A, Boljanović J, Milisavljević M, Ćetković M. Morphological and functional characteristics of satellite glial cells in the peripheral nervous system. Folia Morphol (Warsz), 2021, 80(4): 745-755.
pmid: 33330971 |
| [99] |
Vinterhøj HSH, Stensballe A, Duroux M, Gazerani P. Characterization of rat primary trigeminal satellite glial cells and associated extracellular vesicles under normal and inflammatory conditions. J Proteomics, 2019, 190: 27-34.
pmid: 29581063 |
| [100] |
Zhao LP, Liu SJ, Zhang XB, Yang J, Mao M, Zhang SS, Xu SQ, Feng SW, Wang X. Satellite glial cell-secreted exosomes after in-vitro oxaliplatin treatment presents a pro-nociceptive effect for dorsal root ganglion neurons and induce mechanical hypersensitivity in naïve mice. Mol Cell Neurosci, 2023, 126: 103881.
pmid: 37467904 |
| [101] |
Xin HQ, Li Y, Buller B, Katakowski M, Zhang Y, Wang XL, Shang X, Zhang ZG, Chopp M. Exosome-mediated transfer of miR-133b from multipotent mesenchymal stromal cells to neural cells contributes to neurite outgrowth. Stem Cells, 2012, 30(7): 1556-1564.
pmid: 22605481 |
| [102] |
Ridder K, Keller S, Dams M, Rupp AK, Schlaudraff J, Del Turco D, Starmann J, Macas J, Karpova D, Devraj K, Depboylu C, Landfried B, Arnold B, Plate KH, Höglinger G, Sültmann H, Altevogt P, Momma S. Extracellular vesicle-mediated transfer of genetic information between the hematopoietic system and the brain in response to inflammation. PLoS Biol, 2014, 12(6): e1001874.
pmid: 24893313 |
| [103] |
Picco F, Zeboudj L, Oggero S, Prato V, Burgoyne T, Gamper N, Malcangio M. Macrophage to neuron communication via extracellular vesicles in neuropathic pain conditions. Heliyon, 2024, 11(1): e41268.
pmid: 39811367 |
| [104] |
Sugihara Y, Onoue S, Tashiro K, Sato M, Hasegawa T, Katakura Y. Carnosine induces intestinal cells to secrete exosomes that activate neuronal cells. PLoS One, 2019, 14(5): e0217394.
pmid: 31136600 |
| [105] |
Verdi V, Bécot A, van Niel G, Verweij FJ. In vivo imaging of EVs in zebrafish: new perspectives from “the waterside”. FASEB BioAdv, 2021, 3(11): 918-929.
pmid: 34761174 |
| [106] |
Bécot A, Corona ML, van Niel G. In vivo imaging: an essential tool to better understand the biology of extracellular vesicles. Med Sci (Paris), 2021, 37(12): 1108-1115.
pmid: 34928213 |
| [107] |
Zhang RL, Mao WB, Niu LM, Bao WD, Wang YQ, Wang Y, Zhu YS, Yang ZH, Chen JC, Dong JW, Cai M, Yuan ZL, Song HK, Li GQ, Zhang M, Xiong NX, Wei J, Dong ZQ. NSC-derived exosomes enhance therapeutic effects of NSC transplantation on cerebral ischemia in mice. eLife, 2023, 12: e84493.
pmid: 37104115 |
| [108] |
Lu WC, Yan JF, Wang CY, Qin WP, Han XX, Qin ZX, Wei Y, Xu HQ, Gao JL, Gao CH, Ye T, Tay FR, Niu LN, Jiao K. Interorgan communication in neurogenic heterotopic ossification: the role of brain-derived extracellular vesicles. Bone Res, 2024, 12(1): 11.
pmid: 38383487 |
| [109] |
Arifin DR, Witwer KW, Bulte JWM. Non-invasive imaging of extracellular vesicles: quo vaditis in vivo? J Extracell vesicles, 2022, 11(7): e12241.
pmid: 35844061 |
| [1] | Jiaqiang Chen, Mei Ding. Progress on extracellular vesicles [J]. Hereditas(Beijing), 2025, 47(10): 1078-1098. |
| [2] | Xiaokang Shang, Simeng Zhang, Junjun Ni. Research progress of cathepsin B in brain aging and Alzheimer’s diseases [J]. Hereditas(Beijing), 2023, 45(3): 212-220. |
| [3] | Pengfei Zheng, Haibo Xie, Panpan Zhu, Chengtian Zhao. Distribution pattern of floor plate neurons in zebrafish [J]. Hereditas(Beijing), 2022, 44(6): 510-520. |
| [4] | Fang Li,Qingyun Huang,Sijia Liu,Zhongxin Guo,Xinxin Xiong,Lin Gui,Huijuan Shu,Shaoming Huang,Guohe Tan,Yuanyuan Liu. The role of Bmal1 in neuronal radial migration and axonal projection of the embryonic mouse cerebral cortex [J]. Hereditas(Beijing), 2019, 41(6): 524-533. |
| [5] | Xiulian Shen, Yichao Lu, Zhilian Jia, Qiang Wu. N-WASP regulates cortical neuron migration through its polyPro and VCA domains [J]. Hereditas(Beijing), 2018, 40(5): 390-401. |
| [6] | Xiumei Zhang, Jie Gao, Chunhong Chen, Haijun Tu. Progress in the mechanisms of neural modulation of innate immunity in Caenorhabditis elegans [J]. Hereditas(Beijing), 2018, 40(12): 1066-1074. |
| [7] | Xiaomei Bao, Qing He, Ying Wang, Zhihui Huang, Zengqiang Yuan, . The roles and mechanisms of the Hippo/YAP signaling pathway in the nervous system [J]. Hereditas(Beijing), 2017, 39(7): 630-641. |
| [8] | Wanjin Chen, Qijie Zhang, Jin He, Xiang Lin, Ning Wang. The construction of urine-derived cell lines from patients with spinal muscular atrophy [J]. HEREDITAS(Beijing), 2014, 36(11): 1168-1172. |
| [9] | LAI Ping, WANG Ping-Qing, ZHANG Bao-Yun, CHU Ming-Xing, LIU Chong-Xu, TAN Ying, FAN Qi. The neuroendocrine regulatory mechanisms of mammalian seasonal reproduction [J]. HEREDITAS, 2012, 34(3): 281-288. |
| [10] | WANG Zhen-Dong, XUE Yuan, SHAN Zhi-Yan, ZHENG Zhong, LI Xue, WU Yan-Shuang, SUN Rui-Zhen, SHI Jian, LEI Lei. Generation of mouse parthenogenetic embryonic stem cells and preliminary study of the differentiation ability to motor neurons [J]. HEREDITAS, 2011, 33(11): 1231-1238. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||