Hereditas(Beijing) ›› 2021, Vol. 43 ›› Issue (7): 642-653.doi: 10.16288/j.yczz.20-421
• Review • Previous Articles Next Articles
Roles of NEK family in cell cycle regulation
Yuanyuan Li1( ), Lei Guo2, Zhiming Han1,3(
), Lei Guo2, Zhiming Han1,3( )
)
- 1. State Key Laboratory of Stem Cell and Reproductive Biology, Institute of Zoology, Chinese Academy of Sciences, Beijing 100101, China
2. Fertility Preservation Lab, Reproductive Medicine Center, Guangdong Second Provincial General Hospital, Guangzhou 510317, China
3. Beijing Institute for Stem Cell and Regenerative Medicine, Beijing 100101, China
-
Received:2021-03-27Revised:2021-05-12Online:2021-07-20Published:2021-06-25 -
Contact:Han Zhiming E-mail:liyuanyuan891116@163.com;hanzm@ioz.ac.cn -
Supported by:Supported by the National Key R&D Program of China Nos(2018YFC1004000);Supported by the National Key R&D Program of China Nos(2019YFA0109900);the National Natural Science Foundation of China No(31970509)
Cite this article
Yuanyuan Li, Lei Guo, Zhiming Han. Roles of NEK family in cell cycle regulation[J]. Hereditas(Beijing), 2021, 43(7): 642-653.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
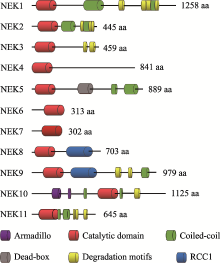
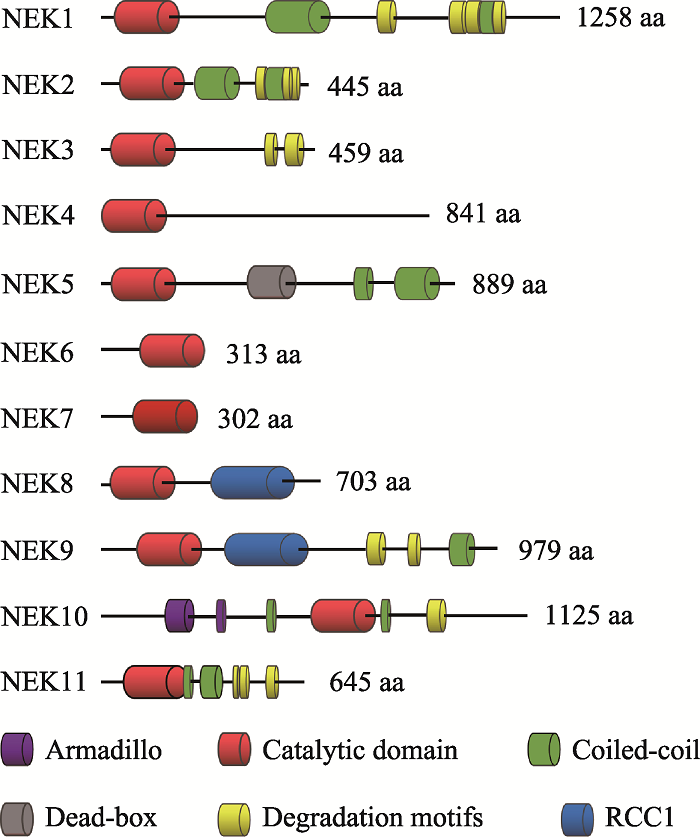
Table 1
Subcellular localization and functions of human and mammalian NEK family"
| NEK | 亚细胞定位 | 功能 |
|---|---|---|
| 1 | 细胞质、中心体、纤毛、DNA损伤部位 | 纤毛发生[ |
| 2 | 中心体 | 有丝分裂[ |
| 3 | 细胞质 | 催乳素依赖性信号传导[ |
| 4 | 纤毛基体、纤毛小根 | DNA损伤反应[ |
| 5 | 细胞质、中心体、纺锤体、线粒体 | 中心体分离[ |
| 6 | 细胞质、纺锤体、中心体 | 有丝分裂[ |
| 7 | 纺锤体两极 | 中心体分离[ |
| 8 | 中心体、纤毛 | 纤毛发生[ |
| 9 | 中心体, 纺锤体两极 | 纺锤体形成[ |
| 10 | 线粒体 | 细胞周期[ |
| 11 | 细胞核、核仁、纺锤体 | 细胞周期[ |
| [1] |
Morris NR. Mitotic mutants of Aspergillus nidulans. Genet Res , 1976, 26(3):237-254.
doi: 10.1017/S0016672300016049 |
| [2] |
Oakley BR, Morris NR. A mutation inAspergillus nidulansthat blocks the transition from interphase to prophase. J Cell Biol , 1983, 96(4):1155-1158.
pmid: 6339527 |
| [3] |
Osmani SA, May GS, Morris NR. Regulation of the mRNA Levels of nimA, a Gene Required for the G2-M Transition inAspergillus nidulans. J Cell Biol , 1987, 104(6):1495-1504.
pmid: 3294854 |
| [4] |
Osmani SA, Pu RT, Morris NR. Mitotic induction and maintenance by overexpression of a G2-specific gene that encodes a potential protein kinase. Cell, 1988, 53(2):237-244.
pmid: 3359487 |
| [5] |
Osmani AH, McGuire SL, Osmani SA. Parallel activation of the NIMA and p34cdc2 cell cycle-regulated protein kinases is required to initiate mitosis in A. nidulans. Cell , 1991, 67(2):283-291.
pmid: 1913824 |
| [6] |
Pu RT, Osmani SA. Mitotic destruction of the cell cycle regulated NIMA protein kinase of Aspergillus nidulans is required for mitotic exit. EMBO J, 1995, 14(5):995-1003.
pmid: 7889945 |
| [7] |
Krien MJ, Bugg SJ, Palatsides M, Asouline G, Morimyo M, O'Connell MJ. A NIMA homologue promotes chromatin condensation in fission yeast. J Cell Sci, 1998, 111(Pt7):967-976.
doi: 10.1242/jcs.111.7.967 |
| [8] |
Wu L, Osmani SA, Mirabito PM. A role for NIMA in the nuclear localization of cyclin B inAspergillus nidulans. J Cell Biol , 1998, 141(7):1575-1587.
pmid: 9647650 |
| [9] |
De Souza CP, Osmani AH, Wu LP, Spotts JL, Osmani SA. Mitotic histone H3 phosphorylation by the NIMA kinase in Aspergillus nidulans. Cell , 2000, 102(3):293-302.
pmid: 10975520 |
| [10] |
Grallert A, Hagan IM. Schizosaccharomyces pombe NIMA-related kinase, Fin1, regulates spindle formation and an affinity of Polo for the SPB. EMBO J , 2002, 21(12):3096-3107.
pmid: 12065422 |
| [11] |
Grallert A, Krapp A, Bagley S, Simanis V, Hagan IM. Recruitment of NIMA kinase shows that maturation of theS. pombe spindle-pole body occurs over consecutive cell cycles and reveals a role for NIMA in modulating SIN activity. Genes Dev , 2004, 18(9):1007-1021.
doi: 10.1101/gad.296204 |
| [12] |
Letwin K, Mizzen L, Motro B, Ben-David Y, Bernstein A, Pawson T. A mammalian dual specificity protein kinase, Nek1, is related to the NIMA cell cycle regulator and highly expressed in meiotic germ cells. EMBO J, 1992, 11(10):3521-3531.
pmid: 1382974 |
| [13] |
Sonn S, Khang I, Kim K, Rhee K. Suppression of Nek2A in mouse early embryos confirms its requirement for chromosome segregation. J Cell Sci, 2004, 117(Pt 23):5557-5566.
doi: 10.1242/jcs.01476 |
| [14] |
Bowers AJ, Boylan JF. Nek8, a NIMA family kinase member, is overexpressed in primary human breast tumors. Gene, 2004, 328:135-142.
pmid: 15019993 |
| [15] | Bradley BA, Wagner JJ, Quarmby LM. Identification and sequence analysis of six new members of the NIMA-related kinase family in Chlamydomonas. J Eukaryot Microbioly , 2004, 51(1):66-72. |
| [16] |
Dorin D, Le Roch K, Sallicandro P, Alano P, Parzy D, Poullet P, Meijer L, Doerig C. Pfnek-1, a NIMA-related kinase from the human malaria parasite Plasmodium falciparum Biochemical properties and possible involvement in MAPK regulation. Eur J Biochem, 2001, 268(9):2600-2608.
pmid: 11322879 |
| [17] |
Prigent C, Glover DM, Giet R. Drosophila Nek2 protein kinase knockdown leads to centrosome maturation defects while overexpression causes centrosome fragmentation and cytokinesis failure. Exp Cell Res, 2005, 303(1):1-13.
pmid: 15572022 |
| [18] | Uto K, Nakajo N, Sagata N. Two structural variants of Nek2 kinase, termed Nek2A and Nek2B, are differentially expressed in Xenopus tissues and development. Dev Biol , 1999, 208(2):456-464. |
| [19] | Fry AM, O'Regan L, Sabir SR, Bayliss R. Cell cycle regulation by the NEK family of protein kinases. J Cell Sci, 2012, 125(Pt 19):4423-4433. |
| [20] |
Moniz L, Dutt P, Haider N, Stambolic V. Nek family of kinases in cell cycle, checkpoint control and cancer. Cell Div, 2011, 6(1):18.
doi: 10.1186/1747-1028-6-18 |
| [21] |
Fry AM. The Nek2 protein kinase: a novel regulator of centrosome structure. Oncogene, 2002, 21(40):6184-6194.
doi: 10.1038/sj.onc.1205711 |
| [22] |
Kandli M, Feige E, Chen A, Kilfin G, Motro B. Isolation and characterization of two evolutionarily conserved murine kinases (Nek6 and Nek7) related to the fungal mitotic regulator, NIMA. Genomics, 2000, 68(2):187-196.
pmid: 10964517 |
| [23] |
Bertran MT, Sdelci S, Regué L, Avruch J, Caelles C, Roig J. Nek9 is a Plk1-activated kinase that controls early centrosome separation through Nek6/7 and Eg5. EMBO J, 2011, 30(13):2634-2647.
doi: 10.1038/emboj.2011.179 |
| [24] |
Belham C, Roig J, Caldwell JA, Aoyama Y, Kemp BE, Comb M, Avruch J. A mitotic cascade of NIMA family kinases. Nercc1/Nek9 activates the Nek6 and Nek7 kinases. J Biol Chem, 2003, 278(37):34897-34909.
doi: 10.1074/jbc.M303663200 |
| [25] |
Roig J, Mikhailov A, Belham C, Avruch J. Nercc1, a mammalian NIMA-family kinase, binds the Ran GTPase and regulates mitotic progression. Genes Dev, 2002, 16(13):1640-1658.
doi: 10.1101/gad.972202 |
| [26] |
Rellos P, Ivins FJ, Baxter JE, Pike A, Nott TJ, Parkinson DM, Das S, Howell S, Fedorov O, Shen QY, Fry AM, Knapp S, Smerdon SJ. Structure and regulation of the human Nek2 centrosomal kinase. J Biol Chem, 2007, 282(9):6833-6842.
pmid: 17197699 |
| [27] |
Lu KP, Kemp BE, Means AR. Identification of substrate specificity determinants for the cell cycle-regulated NIMA protein kinase. J Biol Chem, 1994, 269(9):6603-6607.
pmid: 8120013 |
| [28] | Alexander J, Lim D, Joughin BA, Hegemann B, Hutchins JR, Ehrenberger T, Ivins F, Sessa F, Hudecz O, Nigg EA, Fry AM, Musacchio A, Stukenberg PT, Mechtler K, Peters JM, Smerdon SJ, Yaffe MB. Spatial exclusivity combined with positive and negative selection of phosphorylation motifs is the basis for context-dependent mitotic signaling.Sci Signal , 2011, 4(179): ra42. |
| [29] |
Lizcano JM, Deak M, Morrice N, Kieloch A, Hastie CJ, Dong L, Schutkowski M, Reimer U, Alessi DR. Molecular basis for the substrate specificity of NIMA-related kinase-6 (NEK6). Evidence that NEK6 does not phosphorylate the hydrophobic motif of ribosomal S6 protein kinase and serum- and glucocorticoid-induced protein kinase in vivo. J Biol Chem, 2002, 277(31):27839-27849.
pmid: 12023960 |
| [30] |
Zalli D, Bayliss R, Fry AM. The Nek8 protein kinase, mutated in the human cystic kidney disease nephronophthisis, is both activated and degraded during ciliogenesis. Hum Mol Genet, 2012, 21(5):1155-1171.
doi: 10.1093/hmg/ddr544 |
| [31] |
Hames RS, Wattam SL, Yamano H, Bacchieri R, Fry AM. APC/C-mediated destruction of the centrosomal kinase Nek2A occurs in early mitosis and depends upon a cyclin A-type D-box. EMBO J, 2001, 20(24):7117-7127.
pmid: 11742988 |
| [32] |
Hayes MJ, Kimata Y, Wattam SL, Lindon C, Mao G, Yamano H, Fry AM. Early mitotic degradation of Nek2A depends on Cdc20-independent interaction with the APC/C. Nat Cell Biol, 2006, 8(6):607-614.
doi: 10.1038/ncb1410 |
| [33] | 李梅章, 褚嘉祐, 杨昭庆, 余龙. 一个 NEK基因家族新成员的克隆和鉴定. 遗传 , 2001, 23(2):97-102. |
| Li MZ, Zhu JY, Yang ZQ, YL. Isolating and identifying a novel member belonging to NEK gene family. Hereditas (Beijing) , 2001, 23(2):97-102. | |
| [34] |
Kimura M, Okano Y. Identification and assignment of the human NIMA-related protein kinase 7 gene (NEK7) to human chromosome 1q31.3. Cytogenet Cell Genet, 2001, 94(1-2):33-38.
pmid: 11701951 |
| [35] |
Vaz Meirelles G, Ferreira Lanza DC, da Silva JC, Santana Bernachi J, Paes Leme AF, Kobarg J. Characterization of hNek6 interactome reveals an important role for its short N-terminal domain and colocalization with proteins at the centrosome. J Proteome Res, 2010, 9(12):6298-6316.
doi: 10.1021/pr100562w pmid: 20873783 |
| [36] |
Upadhya P, Birkenmeier EH, Birkenmeier CS, Barker JE. Mutations in a NIMA-related kinase gene, Nek1, cause pleiotropic effects including a progressive polycystic kidney disease in mice. Proc Natl Acad Sci USA, 2000, 97(1):217-221.
doi: 10.1073/pnas.97.1.217 |
| [37] |
Thiel C, Kessler K, Giessl A, Dimmler A, Shalev SA, von der Haar S, Zenker M, Zahnleiter D, Stöss H, Beinder E, Abou Jamra R, Ekici AB, Schröder-Kress N, Aigner T, Kirchner T, Reis A, Brandstätter JH, Rauch A. NEK1 mutations cause short-rib polydactyly syndrome type majewski. Am J Hum Genet, 2011, 88(1):106-114.
doi: 10.1016/j.ajhg.2010.12.004 |
| [38] |
Melo-Hanchuk TD, Slepicka PF, Meirelles GV, Basei FL, Lovato DV, Granato DC, Pauletti BA, Domingues RR, Leme AFP, Pelegrini AL, Lenz G, Knapp S, Elkins JM, Kobarg J. NEK1 kinase domain structure and its dynamic protein interactome after exposure to Cisplatin. Sci Rep, 2017, 7(1):5445.
doi: 10.1038/s41598-017-05325-w pmid: 28710492 |
| [39] |
Singh V, Khalil MI, De Benedetti A. The TLK1/Nek1 axis contributes to mitochondrial integrity and apoptosis prevention via phosphorylation of VDAC1. Cell Cycle, 2020, 19(3):363-375.
doi: 10.1080/15384101.2019.1711317 |
| [40] |
Brieño-Enríquez MA, Moak SL, Holloway JK, Cohen PE. NIMA-related kinase 1 (NEK1) regulates meiosis I spindle assembly by altering the balance between α-Adducin and Myosin X. PLoS One, 2017, 12(10):e0185780.
doi: 10.1371/journal.pone.0185780 |
| [41] |
Fry AM, Meraldi P, Nigg EA. A centrosomal function for the human Nek2 protein kinase, a member of the NIMA family of cell cycle regulators. EMBO J, 1998, 17(2):470-481.
pmid: 9430639 |
| [42] |
Sonn S, Oh GT, Rhee K. Nek2 and its substrate, centrobin/Nip2, are required for proper meiotic spindle formation of the mouse oocytes. Zygote, 2011, 19(1):15-20.
doi: 10.1017/S0967199410000183 |
| [43] |
Endicott SJ, Basu B, Khokha M, Brueckner M. The NIMA-like kinase Nek2 is a key switch balancing cilia biogenesis and resorption in the development of left- right asymmetry. Development, 2015, 142(23):4068-4079.
doi: 10.1242/dev.126953 pmid: 26493400 |
| [44] |
Viol L, Hata S, Pastor-Peidro A, Neuner A, Murke F, Wuchter P, Ho AD, Giebel B, Pereira G. Nek2 kinase displaces distal appendages from the mother centriole prior to mitosis. J Cell Biol, 2020, 219(3):e201907136.
doi: 10.1083/jcb.201907136 |
| [45] |
Miller SL, DeMaria JE, Freier DO, Riegel AM, Clevenger CV. Novel association of Vav2 and Nek3 modulates signaling through the human prolactin receptor. Mol Endocrinol, 2005, 19(4):939-949.
doi: 10.1210/me.2004-0443 |
| [46] |
Nguyen CL, Possemato R, Bauerlein EL, Xie A, Scully R, Hahn WC. Nek4 regulates entry into replicative senescence and the response to DNA damage in human fibroblasts. Mol Cell Biol, 2012, 32(19):3963-3977.
doi: 10.1128/MCB.00436-12 |
| [47] |
Doles J, Hemann MT. Nek4 status differentially alters sensitivity to distinct microtubule poisons. Cancer Res, 2010, 70(3):1033-1041.
doi: 10.1158/0008-5472.CAN-09-2113 |
| [48] |
Basei FL, Meirelles GV, Righetto GL, Dos Santos Migueleti DL, Smetana JH, Kobarg J. New interaction partners for Nek4.1 and Nek4.2 isoforms: from the DNA damage response to RNA splicing. Proteome Sci, 2015, 13:11.
doi: 10.1186/s12953-015-0065-6 |
| [49] |
Coene KL, Mans DA, Boldt K, Gloeckner CJ, van Reeuwijk J, Bolat E, Roosing S, Letteboer SJ, Peters TA, Cremers FP, Ueffing M, Roepman R. The ciliopathy- associated protein homologs RPGRIP1 and RPGRIP1L are linked to cilium integrity through interaction with Nek4 serine/threonine kinase. Hum Mol Genet, 2011, 20(18):3592-3605.
doi: 10.1093/hmg/ddr280 |
| [50] |
Prosser SL, Sahota NK, Pelletier L, Morrison CG, Fry AM. Nek5 promotes centrosome integrity in interphase and loss of centrosome cohesion in mitosis. J Cell Biol, 2015, 209(3):339-348.
doi: 10.1083/jcb.201412099 |
| [51] |
Li YY, Guo L, Li H, Li J, Dong F, Yi ZY, Ouyang YC, Hou Y, Wang ZB, Sun QY, Lu SS, Han ZM. NEK5 regulates cell cycle progression during mouse oocyte maturation and preimplantation embryonic development. Mol Reprod Dev, 2019, 86(9):1189-1198.
doi: 10.1002/mrd.v86.9 |
| [52] |
Melo-Hanchuk TD, Slepicka PF, Pelegrini AL, Menck CFM, Kobarg J. NEK5 interacts with topoisomerase IIβ and is involved in the DNA damage response induced by etoposide. J Cell Biochem, 2019, 120(10):16853-16866.
doi: 10.1002/jcb.28943 pmid: 31090963 |
| [53] |
Shimizu K, Sawasaki T. Nek5, a novel substrate for caspase-3, promotes skeletal muscle differentiation by up-regulating caspase activity. FEBS Lett, 2013, 587(14):2219-2225.
doi: 10.1016/j.febslet.2013.05.049 pmid: 23727203 |
| [54] |
Ferezin CC, Basei FL, Melo-Hanchuk TD, de Oliveira AL, Peres de Oliveira A, Mori MP, de Souza-Pinto NC, Kobarg J. NEK5 interacts with LonP1 and its kinase activity is essential for the regulation of mitochondrial functions and mtDNA maintenance. FEBS Open Bio, 2021, 11(3):546-563.
doi: 10.1002/feb4.v11.3 |
| [55] |
Melo Hanchuk TD, Papa PF, La Guardia PG, Vercesi AE, Kobarg J. Nek5 interacts with mitochondrial proteins and interferes negatively in mitochondrial mediated cell death and respiration. Cell Signal, 2015, 27(6):1168-1177.
doi: 10.1016/j.cellsig.2015.02.021 pmid: 25725288 |
| [56] |
O'Regan L, Fry AM. The Nek6 and Nek7 protein kinases are required for robust mitotic spindle formation and cytokinesis. Mol Biol Cell, 2009, 29(14):3975-3990.
doi: 10.1128/MCB.01867-08 |
| [57] |
Yin MJ, Shao L, Voehringer D, Smeal T, Jallal B. The serine/threonine kinase Nek6 is required for cell cycle progression through mitosis. J Biol Chem, 2003, 278(52):52454-52460.
doi: 10.1074/jbc.M308080200 |
| [58] |
Lee MY, Kim HJ, Kim MA, Jee HJ, Kim AJ, Bae YS, Park JI, Chung JH, Yun J. Nek6 is involved in G2/M phase cell cycle arrest through DNA damage-induced phosphorylation. Cell Cycle, 2008, 7(17):2705-2709.
doi: 10.4161/cc.7.17.6551 |
| [59] |
Gerçeker E, Boyacioglu SO, Kasap E, Baykan A, Yuceyar H, Yildirim H, Ayhan S, Ellidokuz E, Korkmaz M. Never in mitosis gene A-related kinase 6 and aurora kinase A: New gene biomarkers in the conversion from ulcerative colitis to colorectal cancer. Oncol Rep, 2015, 34(4):1905-1914.
doi: 10.3892/or.2015.4187 pmid: 26259750 |
| [60] |
Sdelci S, Bertran MT, Roig J. Nek9, Nek6, Nek7 and the separation of centrosomes. Cell Cycle, 2011, 10(22):3816-3817.
doi: 10.4161/cc.10.22.18226 pmid: 22064517 |
| [61] |
Gross O, Thomas CJ, Guarda G, Tschopp J. The inflammasome: an integrated view. Immunol Rev, 2011, 243(1):136-151.
doi: 10.1111/j.1600-065X.2011.01046.x pmid: 21884173 |
| [62] |
Zhao N, Li CC, Di B, Xu LL. Recent advances in the NEK7-licensed NLRP3 inflammasome activation: Mechanisms, role in diseases and related inhibitors. J Autoimmun, 2020, 113:102515.
doi: S0896-8411(20)30137-2 pmid: 32703754 |
| [63] |
Sun ZZ, Gong W, Zhang Y, Jia ZJ. Physiological and pathological roles of mammalian NEK7. Front Physiol, 2020, 11:606996.
doi: 10.3389/fphys.2020.606996 |
| [64] |
de Souza EE, Meirelles GV, Godoy BB, Perez AM, Smetana JH, Doxsey SJ, McComb ME, Costello CE, Whelan SA, Kobarg J. Characterization of the human NEK7 interactome suggests catalytic and regulatory properties distinct from those of NEK6. J Proteome Res, 2014, 13(9):4074-4090.
doi: 10.1021/pr500437x |
| [65] | Tan R, Nakajima S, Wang Q, Sun H, Xue J, Wu J, Hellwig S, Zeng X, Yates NA, Smithgall TE, Lei M, Jiang Y, Levine AS, Su B, Lan L. Nek7 protects telomeres from oxidative DNA damage by phosphorylation and stabilization of TRF1. Mol Cell , 2017, 65(5): 818- 831.e5. |
| [66] |
Kim S, Lee K, Rhee K. NEK7 is a centrosomal kinase critical for microtubule nucleation. Biochem Biophys Res Commun, 2007, 360(1):56-62.
doi: 10.1016/j.bbrc.2007.05.206 |
| [67] |
de Souza EE, Hehnly H, Perez AM, Meirelles GV, Smetana JH, Doxsey S, Kobarg J. Human Nek7- interactor RGS2 is required for mitotic spindle organization. Cell Cycle, 2015, 14(4):656-667.
doi: 10.4161/15384101.2014.994988 |
| [68] |
Sohara E, Luo Y, Zhang J, Manning DK, Beier DR, Zhou J. Nek8 regulates the expression and localization of polycystin-1 and polycystin-2. J Am Soc Nephrol, 2008, 19(3):469-476.
doi: 10.1681/ASN.2006090985 |
| [69] |
Zalli D, Bayliss R, Fry AM. The Nek8 protein kinase, mutated in the human cystic kidney disease nephronophthisis, is both activated and degraded during ciliogenesis. Hum Mol Genet, 2012, 21(5):1155-1171.
doi: 10.1093/hmg/ddr544 |
| [70] |
Choi HJ, Lin JR, Vannier JB, Slaats GG, Kile AC, Paulsen RD, Manning DK, Beier DR, Giles RH, Boulton SJ, Cimprich KA. NEK8 links the ATR- regulated replication stress response and S phase CDK activity to renal ciliopathies. Mol Cell, 2013, 51(4):423-439.
doi: 10.1016/j.molcel.2013.08.006 |
| [71] |
Abeyta A, Castella M, Jacquemont C, Taniguchi T. NEK8 regulates DNA damage-induced RAD51 foci formation and replication fork protection. Cell Cycle, 2017, 16(4):335-347.
doi: 10.1080/15384101.2016.1259038 |
| [72] |
Kaneta Y, Ullrich A. NEK9 depletion induces catastrophic mitosis by impairment of mitotic checkpoint control and spindle dynamics. Biochem Biophys Res Commun, 2013, 442(3-4):139-146.
doi: 10.1016/j.bbrc.2013.04.105 |
| [73] |
Sdelci S, Schutz M, Pinyol R, Bertran MT, Regue L, Caelles C, Vernos I, Roig J. Nek9 phosphorylation of NEDD1/GCP-WD contributes to Plk1 control of gamma-tubulin recruitment to the mitotic centrosome. Curr Biol, 2012, 22(16):1516-1523.
doi: 10.1016/j.cub.2012.06.027 |
| [74] |
Smith SC, Petrova AV, Madden MZ, Wang H, Pan Y, Warren MD, Hardy CW, Liang D, Liu EA, Robinson MH, Rudra S, Wang J, Ehdaivand S, Torres MA, Wang Y, Yu DS. A gemcitabine sensitivity screen identifies a role for NEK9 in the replication stress response. Nucleic Acids Res, 2014, 42(18):11517-11527.
doi: 10.1093/nar/gku840 |
| [75] |
Moniz LS, Stambolic V. Nek10 mediates G2/M cell cycle arrest and MEK autoactivation in response to UV irradiation. Mol Cell Biol, 2011, 31(1):30-42.
doi: 10.1128/MCB.00648-10 |
| [76] |
Peres de Oliveira A, Basei FL, Slepicka PF, de Castro Ferezin C, Melo-Hanchuk TD, de Souza EE, Lima TI, Dos Santos VT, Mendes D, Silveira LR, Menck CFM, Kobarg J. NEK10 interactome and depletion reveal new roles in mitochondria. Proteome Sci, 2020, 18:4.
doi: 10.1186/s12953-020-00160-w |
| [77] |
Porpora M, Sauchella S, Rinaldi L, Delle Donne R, Sepe M, Torres-Quesada O, Intartaglia D, Garbi C, Insabato L, Santoriello M, Bachmann VA, Synofzik M, Lindner HH, Conte I, Stefan E, Feliciello A. Counterregulation of cAMP-directed kinase activities controls ciliogenesis. Nat Commun, 2018, 9(1):1224.
doi: 10.1038/s41467-018-03643-9 pmid: 29581457 |
| [78] |
Noguchi K, Fukazawa H, Murakami Y, Uehara Y. Nucleolar Nek11 is a novel target of Nek2A in G1/S-arrested cells. J Biol Chem, 2004, 279(31):32716-32727.
pmid: 15161910 |
| [79] |
Melixetian M, Klein DK, Sørensen CS, Helin K. NEK11 regulates CDC25A degradation and the IR-induced G2/M checkpoint. Nat Cell Biol, 2009, 11(10):1247-1253.
doi: 10.1038/ncb1969 pmid: 19734889 |
| [80] |
Noguchi K, Fukazawa H, Murakami Y, Uehara Y. Nek11, a new member of the NIMA family of kinases, involved in DNA replication and genotoxic stress responses. J Biol Chem, 2002, 277(42):39655-39665.
pmid: 12154088 |
| [81] |
Guo L, Wang ZB, Wang HH, Zhang T, Qi ST, Ouyang YC, Hou Y, Sun QY. Nek11 regulates asymmetric cell division during mouse oocyte meiotic maturation. Biochem Biophys Res Commun, 2016, 474(4):667-672.
doi: 10.1016/j.bbrc.2016.05.002 |
| [82] |
O'Connell MJ, Norbury C, Nurse P. Premature chromatin condensation upon accumulation of NIMA. EMBO J, 1994, 13(20):4926-4937.
pmid: 7957060 |
| [83] |
Lu KP, Hunter T. Evidence for a NIMA-like mitotic pathway in vertebrate cells. Cell, 1995, 81(3):413-424.
pmid: 7736593 |
| [84] |
Hégarat N, Rata S, Hochegger H. Bistability of mitotic entry and exit switches during open mitosis in mammalian cells. Bioessays, 2016, 38(7):627-643.
doi: 10.1002/bies.201600057 pmid: 27231150 |
| [85] |
Wloga D, Camba A, Rogowski K, Manning G, Jerka- Dziadosz M, Gaertig J. Members of the NIMA-related kinase family promote disassembly of cilia by multiple mechanisms. Mol Biol Cell, 2006, 17(6):2799-2810.
doi: 10.1091/mbc.e05-05-0450 |
| [86] |
Krien MJ, West RR, John UP, Koniaras K, McIntosh JR, O'Connell MJ. The fission yeast NIMA kinase Fin1p is required for spindle function and nuclear envelope integrity. EMBO J, 2002, 21:1713-1722.
doi: 10.1093/emboj/21.7.1713 |
| [87] | Mahjoub MR, Montpetit B, Zhao L, Finst RJ, Goh B, Kim AC, Quarmby LM. The FA2 gene of Chlamydomonas encodes a NIMA family kinase with roles in cell cycle progression and microtubule severing during deflagellation.J Cell Sci , 2002, 115:1759-1768. |
| [88] |
Andersen JS, Wilkinson CJ, Mayor T, Mortensen P, Nigg EA, Mann M. Proteomic characterization of the human centrosome by protein correlation profiling. Nature, 2003, 426(6966):570-574.
doi: 10.1038/nature02166 |
| [89] |
O'regan L, Blot J, Fry AM. Mitotic regulation by NIMA-related kinases. Cell Div, 2007, 2(25):1-12.
doi: 10.1186/1747-1028-2-1 |
| [90] |
Bahe S, Stierhof YD, Wilkinson CJ, Leiss F, Nigg EA. Rootletin forms centriole-associated filaments and functions in centrosome cohesion. J Cell Biol, 2005, 171(1):27-33.
doi: 10.1083/jcb.200504107 |
| [91] |
Faragher AJ, Fry AM. Nek2A kinase stimulates centrosome disjunction and is required for formation of bipolar mitotic spindles. Mol Biol Cell, 2003, 14(7):2876-2889.
pmid: 12857871 |
| [92] |
Fry AM, Mayor T, Meraldi P, Stierhof YD, Tanaka K, Nigg EA. C-Nap1, a novel centrosomal coiled-coil protein and candidate substrate of the cell cycle-regulated protein kinase Nek2. J Cell Biol, 1998, 141(7):1563-1574.
pmid: 9647649 |
| [93] |
Yang J, Adamian M, Li T. Rootletin interacts with C-Nap1 and may function as a physical linker between the pair of centrioles/basal bodies in cells. Mol Biol Cell, 2006, 17(2):1033-1040.
pmid: 16339073 |
| [94] | Fang G, Zhang D, Yin H, Zheng L, Bi X, Yuan L. Centlein mediates an interaction between C-Nap1 and Cep68 to maintain centrosome cohesion. J Cell Sci, 2014, 127(Pt 8):1631-1639. |
| [95] |
Au FKC, Hau BKT, Qi RZ. Nek2-mediated GAS2L1 phosphorylation and centrosome-linker disassembly induce centrosome disjunction. J Cell Biol, 2020, 219(5):e201909094.
doi: 10.1083/jcb.201909094 |
| [96] |
Au FK, Jia Y, Jiang K, Grigoriev I, Hau BK, Shen Y, Du S, Akhmanova A, Qi RZ. GAS2L1 is a centriole- associated protein required for centrosome dynamics and disjunction. Dev Cell, 2017, 40(1):81-94.
doi: 10.1016/j.devcel.2016.11.019 |
| [97] |
Hata S, Pastor Peidro A, Panic M, Liu P, Atorino E, Funaya C, Jäkle U, Pereira G, Schiebel E. The balance between KIFC3 and EG5 tetrameric kinesins controls the onset of mitotic spindle assembly. Nat Cell Biol, 2019, 21(9):1138-1151.
doi: 10.1038/s41556-019-0382-6 |
| [98] |
Meirelles GV, Perez AM, de Souza EE, Basei FL, Papa PF, Melo Hanchuk TD, Cardoso VB, Kobarg J. “Stop Ne(c)king around”: How interactomics contributes to functionally characterize Nek family kinases. World J Biol Chem, 2014, 5(2):141-160.
doi: 10.4331/wjbc.v5.i2.141 pmid: 24921005 |
| [99] |
Loncarek J, Hergert P, Magidson V, Khodjakov A. Control of daughter centriole formation by the pericentriolar material. Nat Cell Biol, 2008, 10(3):322-328.
doi: 10.1038/ncb1694 |
| [100] |
Gupta A, Tsuchiya Y, Ohta M, Shiratsuchi G, Kitagawa D. NEK7 is required for G1 progression and procentriole formation. Mol Biol Cell, 2017, 28(15):2123-2134.
doi: 10.1091/mbc.e16-09-0643 |
| [101] |
Kim S, Kim S, Rhee K. NEK7 is essential for centriole duplication and centrosomal accumulation of pericentriolar material proteins in interphase cells. J Cell Sci, 2011, 124(Pt 22):3760-3770.
doi: 10.1242/jcs.078089 |
| [102] |
Roig J, Groen A, Caldwell J, Avruch J. Active Nercc1 protein kinase concentrates at centrosomes early in mitosis and is necessary for proper spindle assembly. Mol Biol Cell, 2005, 16(10):4827-4840.
doi: 10.1091/mbc.e05-04-0315 |
| [103] |
Goshima G, Kimura A. New look inside the spindle: microtubule-dependent microtubule generation within the spindle. Curr Opin Cell Biol, 2010, 22(1):44-49.
doi: 10.1016/j.ceb.2009.11.012 pmid: 20022736 |
| [104] |
Blangy A, Lane HA, d'Hérin P, Harper M, Kress M, Nigg EA. Phosphorylation by p34cdc2 regulates spindle association of human Eg5, a kinesin-related motor essential for bipolar spindle formation in vivo. Cell, 1995, 83(7):1159-1169.
pmid: 8548803 |
| [105] |
Sawin KE, Mitchison TJ. Mutations in the kinesin-like protein Eg5 disrupting localization to the mitotic spindle. Proc Natl Acad Sci USA, 1995, 92(10):4289-4293.
doi: 10.1073/pnas.92.10.4289 |
| [106] |
Rapley J, Nicolàs M, Groen A, ReguéL, Bertran MT, Caelles C, Avruch J, Roig J. The NIMA-family kinase Nek6 phosphorylates the kinesin Eg5 at a novel site necessary for mitotic spindle formation. J Cell Sci, 2008, 121(Pt 23):3912-3921.
doi: 10.1242/jcs.035360 |
| [107] | Adib R, Montgomery JM, Atherton J, O'Regan L, Richards MW, Straatman KR, Roth D, Straube A, Bayliss R, Moores CA, Fry AM. Mitotic phosphorylation by NEK6 and NEK7 reduces the microtubule affinity of EML4 to promote chromosome congression. Sci Signal , 2019, 12(594): eaaw2939. |
| [108] |
Wu W, Baxter JE, Wattam SL, Hayward DG, Fardilha M, Knebel A, Ford EM, da Cruz e Silva EF, Fry AM. Alternative splicing controls nuclear translocation of the cell cycle-regulated Nek2 kinase. J Biol Chem, 2007, 282(36):26431-26440.
doi: 10.1074/jbc.M704969200 |
| [109] |
Laurell E, Beck K, Krupina K, Theerthagiri G, Bodenmiller B, Horvath P, Aebersold R, Antonin W, Kutay U. Phosphorylation of Nup98 by multiple kinases is crucial for NPC disassembly during mitotic entry. Cell, 2011, 144(4):539-550.
doi: 10.1016/j.cell.2011.01.012 pmid: 21335236 |
| [110] |
Holland PM, Milne A, Garka K, Johnson RS, Willis C, Sims JE, Rauch CT, Bird TA, Virca GD. Purification, cloning, and characterization of Nek8, a novel NIMA-related kinase, and its candidate substrate Bicd2. J Biol Chem, 2002, 277(18):16229-16240.
pmid: 11864968 |
| [111] |
Fletcher L, Cerniglia GJ, Nigg EA, Yend TJ, Muschel RJ. Inhibition of centrosome separation after DNA damage: a role for Nek2. Radiat Res, 2004, 162(2):128-135.
pmid: 15387139 |
| [112] |
Pelegrini AL, Moura DJ, Brenner BL, Ledur PF, Maques GP, Henriques JA, Saffi J, Lenz G. Nek1 silencing slows down DNA repair and blocks DNA damage-induced cell cycle arrest. Mutagenesis, 2010, 25(5):447-454.
doi: 10.1093/mutage/geq026 |
| [113] |
Chen YM, Chen PL, Chen CF, Jiang XZ, Riley DJ. Never-in-mitosis related kinase 1 functions in DNA damage response and checkpoint control. Cell Cycle, 2008, 7(20):3194-3201.
doi: 10.4161/cc.7.20.6815 |
| [114] |
Chen YM, Chen CF, Riley DJ, Chen PL. Nek1 kinase functions in DNA damage response and checkpoint control through a pathway independent of ATM and ATR. Cell Cycle, 2011, 10(4):655-663.
doi: 10.4161/cc.10.4.14814 |
| [115] |
Polci R, Peng AM, Chen PL, Riley DJ, Chen YM. NIMA-related protein kinase 1 is involved early in the ionizing radiation-induced DNA damage response. Cancer Res, 2004, 64(24):8800-8803.
doi: 10.1158/0008-5472.CAN-04-2243 |
| [116] |
Du J, Cai X, Yao J, Ding X, Wu Q, Pei S, Jiang K, Zhang Y, Wang W, Shi Y, Lai Y, Shen J, Teng M, Huang H, Fei Q, Reddy ES, Zhu J, Jin C, Yao X. The mitotic checkpoint kinase NEK2A regulates kinetochore microtubule attachment stability. Oncogene, 2008, 27(29):4107-4114.
doi: 10.1038/onc.2008.34 pmid: 18297113 |
| [117] |
Lou Y, Yao JH, Zereshki A, Dou Z, Ahmed K, Wang HM, Hu JB, Wang YZ, Yao XB. NEK2A interacts with MAD1 and possibly functions as a novel integrator of the spindle checkpoint signaling. J Biol Chem, 2004, 279(19):20049-20057.
doi: 10.1074/jbc.M314205200 |
| [118] |
Wei R, Ngo B, Wu GK, Lee WH. Phosphorylation of the Ndc80 complex protein, HEC1, by Nek2 kinase modulates chromosome alignment and signaling of the spindle assembly checkpoint. Mol Biol Cell, 2011, 22(19):3584-3594.
doi: 10.1091/mbc.e11-01-0012 |
| [119] |
Martin-Lluesma S, Stucke VM, Nigg EA. Role of Hec1 in spindle checkpoint signaling and kinetochore recruitment of Mad1/Mad2. Science, 2002, 297(5590):2267-2270.
pmid: 12351790 |
| [120] |
Fletcher L, Cerniglia GJ, Yen TJ, Muschel RJ. Live cell imaging reveals distinct roles in cell cycle regulation for Nek2A and Nek2B. Biochim Biophys Acta, 2005, 1744(2):89-92.
pmid: 15950749 |
| [121] |
Salem H, Rachmin I, Yissachar N, Cohen S, Amiel A, Haffner R, Lavi L, Motro B. Nek7 kinase targeting leads to early mortality, cytokinesis disturbance and polyploidy. Oncogene, 2010, 29(28):4046-4057.
doi: 10.1038/onc.2010.162 pmid: 20473324 |
| [122] |
Cullati SN, Kabeche L, Kettenbach AN, Gerber SA. A bifurcated signaling cascade of NIMA-related kinases controls distinct kinesins in anaphase. J Cell Biol, 2017, 216(8):2339-2354.
doi: 10.1083/jcb.201512055 |
| [123] |
Chan PC, Hsu RYC, Liu CW, Lai CC, Chen HC. Adducin-1 is essential for mitotic spindle assembly through its interaction with myosin-X. J Cell Biol, 2014, 204(1):19-28.
doi: 10.1083/jcb.201306083 |
| [124] | Hsu WH, Wang WJ, Lin WY, Huang YM, Lai CC, Liao JC, Chen HC. Adducin-1 is essential for spindle pole integrity through its interaction with TPX2. EMBO Rep, 2018, 19(8):e45607. |
| [125] |
Jeong Y, Lee J, Kim K, Yoo JC, Rhee K. Characterization of NIP2/centrobin, a novel substrate of Nek2, and its potential role in microtubule stabilization. J Cell Sci, 2007, 120(Pt 12):2106-2116.
doi: 10.1242/jcs.03458 |
| [126] |
Lee J, Kim S, Jeong Y, Rhee K. Centrobin/Nip2 expression in vivo suggests its involvement in cell proliferation. Mol Cells, 2009, 28(1):31-36.
doi: 10.1007/s10059-009-0097-9 |
| [127] |
Di Agostino S, Fedele M, Chieffi P, Fusco A, Rossi P, Geremia R, Sette C. Phosphorylation of high-mobility group protein A2 by Nek2 kinase during the first meiotic division in mouse spermatocytes. Mol Biol Cell, 2004, 15(3):1224-1232.
pmid: 14668482 |
| [128] |
Yang SW, Gao C, Chen L, Song YL, Zhu JL, Qi ST, Jiang ZZ, Wang ZW, Lin F, Huang H, Xing FQ, Sun QY. Nek9 regulates spindle organization and cell cycle progression during mouse oocyte meiosis and its location in early embryo mitosis. Cell Cycle, 2012, 11(23):4366-4377.
doi: 10.4161/cc.22690 |
| [1] | Xiangjiang Lv, Jing Guo, Ge Lin. Novel mutations in TRIP13 lead to female infertility with oocyte maturation arrest [J]. Hereditas(Beijing), 2023, 45(6): 514-525. |
| [2] | Yuxuan Guo, Shunping Yan, Yingxiang Wang. Recent advances in functional conservation and divergence of recombinase RAD51 and DMC1 [J]. Hereditas(Beijing), 2022, 44(5): 398-413. |
| [3] | Hui Nie, Yiwen Zhang, Jianing Li, Nannan Wang, Lan Xu. Progress on the correlation between the abnormal synaptonemal complex and infertility [J]. Hereditas(Beijing), 2021, 43(12): 1142-1148. |
| [4] | Fan Li, Rongpei Yu, Dan Sun, Jihua Wang, Shenchong Li, Jiwei Ruan, Qinli Shan, Pingli Lu, Guoxian Wang. Molecular mechanisms of meiotic recombination suppression in plants [J]. Hereditas(Beijing), 2019, 41(1): 52-65. |
| [5] | Yaping Liao,Chunjing Wang,Meng Liang,Xiaomei Hu,Qi Wu. Analysis of genetic characteristics and reproductive risks of balanced complex chromosome rearrangement carriers in China [J]. Hereditas(Beijing), 2017, 39(5): 396-412. |
| [6] | Qingxia Hu,Ang Gao,Weijia Zeng,Yanxin Wang,Jintang Dong,Zhengmao Zhu. Structure and biological functions of mammalian LEM-Domain proteins [J]. HEREDITAS(Beijing), 2015, 37(2): 128-139. |
| [7] | Shanshan Yue,Laixin Xia. Identification of C(2)M interacting proteins by yeast two-hybrid screening [J]. HEREDITAS(Beijing), 2015, 37(11): 1160-1166. |
| [8] | ZHANG Bao-Le GAO Dian-Shuai XU Yin-Xue. G protein-coupled receptor 3: a key factor in the regulation of the nervous system and follicle development [J]. HEREDITAS, 2013, 35(5): 578-586. |
| [9] | XIE Wen-Jun, SHI Dian-Yi, CAI Ze-Xi, CHEN Xiao-Yang, JIN Wei-Wei. Organization, function and genetic controlling of synaptonemal complex [J]. HEREDITAS, 2012, 34(2): 167-176. |
| [10] | DUAN Chao, YANG Qiang-Ling, WANG Liu, SHI Qiang-Hua, XU De-Xin. Correlation analysis between meiotic recombination frequencies and age in human spermatocyte [J]. HEREDITAS, 2011, 33(7): 725-730. |
| [11] | CHEN Jun, LUO Wei-Xiong, LI Meng, LUO Qiong. Chromosome recombination in rice meiosis [J]. HEREDITAS, 2011, 33(6): 648-653. |
| [12] | MENG Ya-Nan, MENG Li-Jun, SONG E-Juan, LIU Mei-Ling, ZHANG Xiu-Jun. Small RNA molecules and regulation of spermatogenesis [J]. HEREDITAS, 2011, 33(1): 9-16. |
| [13] | WANG Bin, LIU Zhi-Yu, MIAO Long. Recent advances in the study of spermatogenesis and fer-tilization in Caenorhabditis elegans [J]. HEREDITAS, 2008, 30(6): 677-686. |
| [14] | LONG Wen-Bo, LUAN Li, WANG Xing, LIU Yu-Hua, TU Sheng-Bin, KONG Fan-Lun, HE Tao. Cytogenetical comparison of restorers TP-4 and D minghui63 and maintainer D46B of autotetraploid rice [J]. HEREDITAS, 2007, 29(4): 462-470. |
| [15] | ZHANG Wei, ZHANG Si-Zhong, A Zhou-Cun. Synaptonemal Complex——An Essential Role in Etiology of Idiopathic Azoospermia [J]. HEREDITAS, 2006, 28(2): 231-235. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||