Hereditas(Beijing) ›› 2022, Vol. 44 ›› Issue (12): 1148-1157.doi: 10.16288/j.yczz.22-217
• Genetic Resource • Previous Articles Next Articles
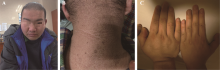
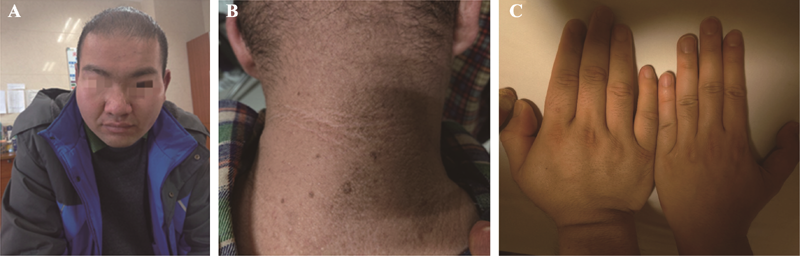
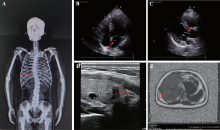
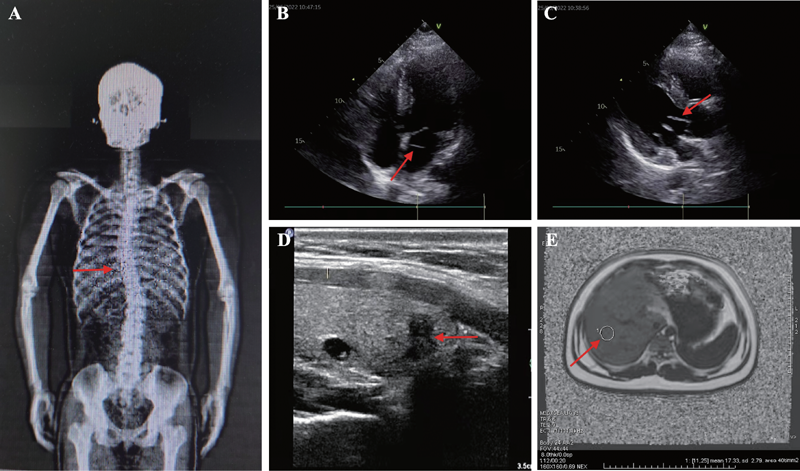
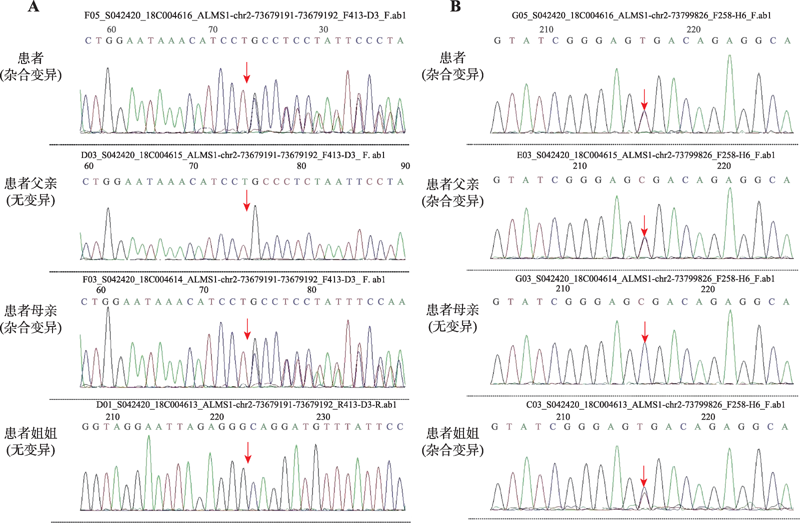
Diagnosis, treatment and genetic analysis of a case of Alstrom syndrome caused by compoud heterozygous mutation of ALMS1
Huijie Yang( ), De Li(
), De Li( ), Huiling Bai, Ming Zhang, Jun Huang, Xiaoqing Yuan(
), Huiling Bai, Ming Zhang, Jun Huang, Xiaoqing Yuan( )
)
- Department of Endocrinology, Changzhou Second People's Hospital affiliated to Nanjing Medical University, Changzhou 213000, China
-
Received:2022-08-12Revised:2022-09-30Online:2022-12-20Published:2022-10-14 -
Contact:Yuan Xiaoqing E-mail:huijiexiaojie@126.com;lide93207@sina.com;adiposeyy@126.com -
Supported by:Support by Jiangsu Innovation Team Fund Project(CXTDC2016005)
Cite this article
Huijie Yang, De Li, Huiling Bai, Ming Zhang, Jun Huang, Xiaoqing Yuan. Diagnosis, treatment and genetic analysis of a case of Alstrom syndrome caused by compoud heterozygous mutation of ALMS1[J]. Hereditas(Beijing), 2022, 44(12): 1148-1157.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
Table 1
DNA variation information"
| 基因 | 染色体位置 | 转录本外显子 | 突变位点 | 纯合/ 杂合 | 正常人中频率 | 预测 | 致病性 分析 | 遗传 方式 | 疾病/ 表型 | 变异 来源 |
|---|---|---|---|---|---|---|---|---|---|---|
| ALM S1 | Chr.2: 73679191- 73679192 | NM_0151 20;exon 8 | c.5535delG (p.S1847Lfs*24) | het | - | - | 可能致病 | AR | Alstrom 综合征 | 母亲 |
| ALM S1 | Chr.2: 73799826 | NM_0151 20;exon 16 | c.10819C>T (p.R3607X) | het | - | - | 可能致病 | AR | Alstrom 综合征 | 父亲 |
Table 2
Clinical data of the patient under the treatment and follow-up"
| 日期 | 2018年2月 | 2018年11月 | 2019年1月 | 2020年1月 | 2020年9月 | 2021年2月 | 2022年5月 |
|---|---|---|---|---|---|---|---|
| 年龄(岁) | 21 | 22 | 22 | 23 | 24 | 24 | 25 |
| 体重(kg) | 80 | 80 | - | - | - | 76 | 74.2 |
| 体重指数(kg/m2) | 30.1 | 30.1 | - | - | - | 28.6 | 27.9 |
| 血压(mmHg) | 115/78 | 132/74 | - | - | - | 131/65 | 115/78 |
| 血红蛋白(130~175 g/L) | 170 | 176 | - | - | - | 176 | 189 |
| 谷丙转氨酶(9~50 U/L) | 64 | 81.6 | 64 | - | 77 | 36 | 55.6 |
| 谷草转氨酶 (15~40 U/L) | 52 | 90.7 | 39 | 99 | 61 | 31.9 | 51.1 |
| r-谷氨酰转肽酶 (10~60 U/L) | 240 | 449 | 143 | - | 202 | 131 | 290 |
| 空腹血糖 (3.9~6.1 mmol/L) | 12.93 | 10.15 | 8.27 | 15.7 | 15.41 | 4.94 | 12.91 |
| 尿素氮 (3.1~8.0 mmol/L) | 7.3 | 7.8 | 7.3 | 8 | 7.2 | 9.5 | 10.7 |
| 肌酐(45~105 μmol/L) | 101.2 | 140 | 144 | 129.9 | 139 | 155 | 182 |
| 肾小球滤过率 (80~120 ml/min1.73m2) | 85.77 | 58.42 | - | - | - | 51.04 | 42.05 |
| 尿微白蛋白/肌酐 (<30 mg/g) | 270 | 462.2 | 400.5 | - | - | 414.4 | >300 |
| 尿酸(90~420 μmol/L) | 524.8 | 653 | 687 | - | 835 | 315 | 439 |
| 甘油三酯 (0.11~2.26 mmol/L) | 8.87 | 19.51 | 2.96 | - | 12.76 | 3.25 | 8.58 |
| 总胆固醇 (2~5.2 mmol/L) | 6.41 | 8.18 | 5.85 | - | 7.34 | 5.15 | 8.41 |
| 低密度胆固醇 (0~3.12 mmol/L) | 5 | 4.5 | 3.88 | - | 2.67 | 3.25 | 5.02 |
| 糖化血红蛋白(4%~6%) | 11.4 | 13.1 | - | - | 11.1 | 11.3 | 12.3 |
| C-肽0 min (370~1470 pmol/L) | 2617 | 2584 | - | - | 2859 | 3890 | 2903 |
| C-肽60 min | 4083 | 3993 | - | - | - | 5242 | 3000 |
| C-肽120 min | 4992 | 3923 | - | - | - | 5728 | 4013 |
| 胰岛素敏感指数 (钳夹实验) | - | 0.216 | - | - | - | 1.045 | - |
| 内脏脂肪(<100 cm2) | 96 | 86 | - | - | - | 84 | 80 |
| 皮下脂肪(cm2) | 138 | 172 | - | - | - | 142 | 139 |
| 肝脏脂肪含量(<5%) | - | 14%~17% | - | - | - | 14%~17% | 16% |
| 肝脏硬度(<9.7 kPa) | - | - | - | - | - | 8.6 | 7.3 |
| 脂肪衰减(<240 dM/m) | - | - | - | - | - | 309 | 313 |
| 降糖方案 | 地特36 IU天; 赖脯42 IU/天; 二甲双胍; 吡格列酮; | 二甲双胍; 吡格列酮; 达格列净; | 二甲双胍; 吡格列酮; 达格列净; | 二甲双胍; 吡格列酮; 达格列净; | 二甲双胍; 吡格列酮; 达格列净; | 地特20 IU/天; 二甲双胍; 吡格列酮; 达格列净; 度拉糖肽; | 地特20 IU/天; 吡格列酮; 达格列净; 度拉糖肽; |
| [1] | Zhou C, Xiao YY, Xie HB, Liu SL, Wang J. A novel variant in ALMS1 in a patient with Alström syndrome and prenatal diagnosis for the fetus in the family:a case report and literature review. Mol Med Rep, 2020, 22(4): 3271- 3276. |
| [2] | Marshall JD, Maffei P, Collin GB, Naggert JK. Alström syndrome: genetics and clinical overview. Curr Genomics, 2011, 12(3): 225-235. |
| [3] | Zhang JJ, Wang JQ, Sun MQ, Xu D, Xiao Y, Lu WL, Dong ZY. Alström syndrome with a novel mutation of ALMS1and Graves’ hyperthyroidism:a case report and review of the literature. World J Clin Cases, 2021, 9(13): 3200- 3211. |
| [4] | Shenje LT, Andersen P, Halushka MK, Lui C, Fernandez L, Collin GB, Amat-Alarcon N, Meschino W, Cutz E, Chang K, Yonescu R, Batista DA, Chen Y, Chelko S, Crosson JE, Scheel J, Vricella L, Craig BD, Marosy BA, Mohr DW, Hetrick KN, Romm JM, Scott AF, Valle D, Naggert JK, Kwon C, Doheny KF, Judge DP.Mutations in Alström protein impair terminal differentiation of cardiomyocytes. Nat Commun, 2014, 5: 3416. |
| [5] |
Dassie F, Favaretto F, Bettini S, Parolin M, Valenti M, Reschke F, Danne T, Vettor R, Milan G, Maffei P. Alstrom syndrome: an ultra-rare monogenic disorder as a model for insulin resistance, type 2 diabetes mellitus and obesity. Endocrine, 2021, 71(3): 618-625.
doi: 10.1007/s12020-021-02643-y |
| [6] | Marshall JD, Bronson RT, Collin GB, Nordstrom AD, Maffei P, Paisey RB, Carey C, Macdermott S, Russell-Eggitt I, Shea SE, Davis J, Beck S, Shatirishvili G, Mihai GM, Hoeltzenbein M, Pozzan GB, Hopkinson I, Sicolo N, Naggert JK, Nishina PM.New Alström syndrome phenotypes based on the evaluation of 182 cases. Arch Intern Med, 2005, 165(6): 675-683. |
| [7] | Collin GB, Marshall JD, Ikeda A, So WV, Russell-Eggitt I, Maffei P, Beck S, Boerkoel CF, Sicolo N, Martin M, Nishina PM, Naggert JK.2 diabetes and neurosensory degeneration in Alström syndrome. Nat Genet, 2002, 31(1): 74-78. |
| [8] |
Hearn T, Renforth GL, Spalluto C, Hanley NA, Piper K, Brickwood S, White C, Connolly V, Taylor JFN, Russell-Eggitt I, Bonneau D, Walker M, Wilson DI. Mutation of ALMS1, a large gene with a tandem repeat encoding 47 amino acids, causes Alström syndrome. Nat Genet, 2002, 31(1): 79-83.
pmid: 11941370 |
| [9] |
Wang CM, Luo XN, Wang YL, Liu Z, Wu SN, Wang SM, Lan XP, Xu QM, Xu WH, Yuan F, Wang AQ, Zeng FY, Jia J, Chen Y. Novel mutations of the ALMS1 gene in patients with Alström syndrome. Intern Med, 2021, 60(23): 3721-3728.
doi: 10.2169/internalmedicine.6467-20 |
| [10] |
Hearn T. ALMS1 and Alström syndrome: a recessive form of metabolic, neurosensory and cardiac deficits. J Mol Med (Berl), 2018, 97(1): 1-17.
doi: 10.1007/s00109-018-1714-x |
| [11] |
Álvarez-Satta M, Lago-Docampo M, Bea-Mascato B, Solarat C, Castro-Sánchez S, Christensen ST, Valverde D. ALMS 1 regulates TGF-β signaling and morphology of primary cilia. Front Cell Dev Biol, 2021, 9: 623829.
doi: 10.3389/fcell.2021.623829 |
| [12] |
Leitch CC, Lodh S, Prieto-Echagüe V, Badano JL, Zaghloul NA. Basal body proteins regulate notch signaling through endosomal trafficking. J Cell Sci, 2014, 127(Pt 11): 2407-2419.
doi: 10.1242/jcs.130344 pmid: 24681783 |
| [13] | Zulato E, Favaretto F, Veronese C, Campanaro S, Marshall JD, Romano S, Cabrelle A, Collin GB, Zavan B, Belloni AS, Rampazzo E, Naggert JK, Abatangelo G, Sicolo N, Maffei P, Milan G, Vettor R.ALMS1-deficient fibroblasts over-express extra-cellular matrix components, display cell cycle delay and are resistant to apoptosis. PLoS One, 2011, 6(4): e19081. |
| [14] |
Oh EC, Vasanth S, Katsanis N. Metabolic regulation and energy homeostasis through the primary cilium. Cell Metab, 2015, 21(1): 21-31.
doi: 10.1016/j.cmet.2014.11.019 pmid: 25543293 |
| [15] |
Fraser AM, Davey MG. TALPID 3 in Joubert syndrome and related ciliopathy disorders. Curr Opin Genet Dev, 2019, 56: 41-48.
doi: S0959-437X(19)30019-X pmid: 31326647 |
| [16] |
Geets E, Meuwissen MEC, Van Hul W. Clinical, molecular genetics and therapeutic aspects of syndromic obesity. Clin Genet, 2018, 95(1): 23-40.
doi: 10.1111/cge.13367 |
| [17] |
Gathercole LL, Hazlehurst JM, Armstrong MJ, Crowley R, Boocock S, O'Reilly MW, Round M, Brown R, Bolton S, Cramb R, Newsome PN, Semple RT, Paisey R, Tomlinson JW, Geberhiwot T. Advanced non-alcoholic fatty liver disease and adipose tissue fibrosis in patients with Alström syndrome. Liver Int, 2016, 36(11): 1704-1712.
doi: 10.1111/liv.13163 pmid: 27178444 |
| [18] |
Girard D, Petrovsky N. Alström syndrome: insights into the pathogenesis of metabolic disorders. Nat Rev Endocrinol, 2010, 7(2): 77-88.
doi: 10.1038/nrendo.2010.210 |
| [19] |
Han JC, Reyes-Capo DP, Liu CY, Reynolds JC, Turkbey E, Turkbey IB, Bryant J, Marshall JD, Naggert JK, Gahl WA, Yanovski JA, Gunay-Aygun M. Comprehensive endocrine-metabolic evaluation of patients with Alstrom syndrome compared with BMI-matched controls. J Clin Endocrinol Metab, 2018, 103(7): 2707-2719.
doi: 10.1210/jc.2018-00496 |
| [20] | Romano S, Milan G, Veronese C, Collin GB, Marshall JD, Centobene C, Favaretto F, Dal Pra C, Scarda A, Leandri S, Naggert JK, Maffei P, Vettor R.Regulation of Alström syndrome gene expression during adipogenesis and its relationship with fat cell insulin sensitivity. Int J Mol Med, 2008, 21(6): 731-736. |
| [21] |
Huang-Doran I, Semple PK.Knockdown of the Alström syndrome-associated gene Alms1 in 3T3-L1 preadipocytes impairs adipogenesis but has no effect on cell-autonomous insulin action. Int J Obes (Lond), 2010, 34(10): 1554-1558.
doi: 10.1038/ijo.2010.92 |
| [22] | Collin GB, Marshall JD, King BL, Milan G, Maffei P, Jagger DJ, Naggert JK.The Alström syndrome protein, ALMS1, interacts with α-Actinin and components of the endosome recycling pathway. PLoS One, 2012, 7(5): e37925. |
| [23] | Favaretto F, Milan G, Collin GB, Marshall JD, Stasi F, Maffei P, Vettor R, Naggert JK. GLUT4 defects in adipose tissue are early signs of metabolic alterations in Alms1GT/ GT, a mouse model for obesity and insulin resistance. PLoS One, 2014, 9(10): e109540. |
| [24] |
Nesmith JE, Hostelley TL, Leitch CC, Matern MS, Sethna S, McFarland R, Lodh S, Westlake CJ, Hertzano R, Ahmed ZM, Zaghloul NA. Genomic knockout of alms1 in zebrafish recapitulates Alström syndrome and provides insight into metabolic phenotypes. Hum Mol Genet, 2019. 28(13): 2212-2223.
doi: 10.1093/hmg/ddz053 pmid: 31220269 |
| [25] |
Geberhiwot T, Baig S, Obringer C, Girard D, Dawson C, Manolopoulos K, Messaddeq N, Bel Lassen P, Clement K, Tomlinson JW, Steeds RP, Dollfus H, Petrovsky N, Marion V. Relative adipose tissue failure in Alström syndrome drives obesity-induced insulin resistance. Diabetes, 2021, 70(2): 364-376.
doi: 10.2337/db20-0647 pmid: 32994277 |
| [26] | Bettini S, Bombonato G, Dassie F, Favaretto F, Piffer L, Bizzotto P, Busetto L, Chemello L, Senzolo M, Merkel C, Angeli P, Vettor R, Milan G, Maffei P. Liver fibrosis and steatosis in Alström syndrome: a genetic model for metabolic syndrome. Diagnostics (Basel), 2021, 11(5): 797. |
| [27] |
Waldman M, Han JC, Reyes-Capo DP, Bryant J, Carson KA, Turkbey B, Choyke P, Naggert JK, Gahl WA, Marshall JD, Gunay-Aygun M. Alstrom syndrome: renal findings in correlation with obesity, insulin resistance, dyslipidemia and cardiomyopathy in 38 patients prospectively evaluated at the NIH clinical center. Mol Genet Metab, 2018, 125(1-2): 181-191.
doi: S1096-7192(18)30337-8 pmid: 30064963 |
| [28] |
Choudhury AR, Munonye I, Sanu KP, Islam N, Gadaga C. A review of Alström syndrome: a rare monogenic ciliopathy. Intractable Rare Dis Res, 2021, 10(4): 257-262.
doi: 10.5582/irdr.2021.01113 |
| [29] |
Poli L, Arroyo G, Garofalo M, Choppin de Janvry E, Intini G, Saracino A, Pretagostini R, Della Pietra F, Berloco PB. Kidney transplantation in Alström syndrome: case report. Transplant Proc, 2017, 49(4): 733-735.
doi: 10.1016/j.transproceed.2017.02.018 |
| [30] |
Baig S, Veeranna V, Bolton S, Edwards N, Tomlinson JW, Manolopoulos K, Moran J, Steeds RP, Geberhiwot T. Treatment with PBI-4050 in patients with Alström syndrome: study protocol for a phase 2, single-centre, single-arm, open-label trial. BMC Endocr Disord, 2018, 18(1): 88.
doi: 10.1186/s12902-018-0315-6 pmid: 30477455 |
| [31] |
Tahani N, Maffei P, Dollfus H, Paisey R, Valverde D, Milan G, Han JC, Favaretto F, Madathil SC, Dawson C, Armstrong MJ, Warfield AT, Duzenli S, Francomano CA, Gunay-Aygun M, Dassie F, Marion V, Valenti M, Leeson-Beevers K, Chivers A, Steeds R, Barrett T, Geberhiwot T. Consensus clinical management guidelines for Alström syndrome. Orphanet J Rare Dis, 2020, 15(1): 253.
doi: 10.1186/s13023-020-01468-8 pmid: 32958032 |
| [32] |
Kaneto H, Obata A, Kimura T, Shimoda M, Okauchi S, Shimo N, Matsuoka TA, Kaku K. Beneficial effects of sodium-glucose cotransporter 2 inhibitors for preservation of pancreatic β-cell function and reduction of insulin resistance. J Diabetes, 2017, 9(3): 219-225.
doi: 10.1111/1753-0407.12494 pmid: 27754601 |
| [33] |
Xu L, Nagata N, Nagashimada M, Zhuge F, Ni YH, Chen GL, Mayoux E, Kaneko S, Ota T. SGLT2 inhibition by empagliflozin promotes fat utilization and browning and attenuates inflammation and insulin resistance by polarizing M2 macrophages in diet-induced obese mice. EBioMedicine, 2017, 20: 137-149.
doi: S2352-3964(17)30226-8 pmid: 28579299 |
| [1] | Liwen Zhang, Meihua Ruan, Jialan Liu, Caihong He, Jianrong Yu. Progress on research and development in diabetes mellitus [J]. Hereditas(Beijing), 2022, 44(10): 824-839. |
| [2] | Yuzhuo Wang, Yiming Zhang, Xiaolian Dong, Xuecai Wang, Jianfu Zhu, Na Wang, Feng Jiang, Yue Chen, Qingwu Jiang, Chaowei Fu. Modification effects of T2DM-susceptible SNPs on the reduction of blood glucose in response to lifestyle interventions [J]. Hereditas(Beijing), 2020, 42(5): 483-492. |
| [3] | Xian Gong, Chao Zhang, Aisa Yiliyasi, Ying Shi, Xuewei Yang, Aosiman Nuersimanguli, Yaqun Guan, Shuhua Xu. A comparative analysis of genetic diversity of candidate genes associated with type 2 diabetes in worldwide populations [J]. Hereditas(Beijing), 2016, 38(6): 543-559. |
| [4] | Ri Wu,Chao Ma,Xiaodan Li,Huikun Duan,Yanli Ji,Yu Wang,Pingzhe Jiang,Haisong Wang,Peipei Tu,Miao Li,Ganggang Ni,Baicheng Ma,Minggang Li. Construction of yeast strains expressing long-acting glucagon-like peptide-1 (GLP-1) and their therapeutic effects on type 2 diabetes mellitus mouse model [J]. HEREDITAS(Beijing), 2015, 37(2): 183-191. |
| [5] | TANG Lin-Lin LIU Qiong BU Shi-Zhong XU Lei-Ting WANG Qin-Wen MAI Yi-Feng DUAN Shi-Wei. The effect of environmental factors and DNA methylation on type 2 diabetes mellitus [J]. HEREDITAS, 2013, 35(10): 1143-1152. |
| [6] | PU Lian-Mei, NAN Nan, YANG Ze, JIN Ze-Ning. Association between SUMO4 polymorphisms and type 2 diabetes mellitus [J]. HEREDITAS, 2012, 34(3): 315-325. |
| [7] | TANG Xiao-Li, DENG Li-Bin, LI Gui-Lin, LIU Shuang-Mei, LIN Jia-Ri, XIE Jin-Yan, LIU Jun, KONG Fan-Jun, LIANG Shang-Dong. Analysis of gene expression profile of peripheral ganglia in early stage type Ⅱ diabetic rats [J]. HEREDITAS, 2012, 34(2): 198-207. |
| [8] | HOU Yu-Ting, LI Jin-Na, LIN Gui-Ping, LIU Ming-Yao, SUN Guo-Feng, WANG Wen-Fei, LI De-Shan. Cloning, expression and glucose regulation activity of human FGF-21 [J]. HEREDITAS, 2010, 32(6): 583-587. |
| [9] | CHEN Fang-Jian, YU Hong, FAN Fan, LU Jian-Xin. Mitochondrial D-Loop gene polymorphisms in the patients with type 2 diabetes mellitus [J]. HEREDITAS, 2009, 31(3): 265-272. |
| [10] | . Study on a new point mutation of nt7444 G→A of mitochondrial DNA in a type 2 diabetes mellitus family [J]. HEREDITAS, 2007, 29(4): 433-437. |
| [11] | WANG Wei, LAI Mao-De. Alternative Splicing of Insulin Receptor mRNA in Cancer and Type 2 Diabetes Mellitus: A Review [J]. HEREDITAS, 2006, 28(2): 226-230. |
| [12] | ZHAO Jing, JI Jing-Zhang, WANG Da-Wang, ZHANG Jie, WU Hui-Jie, LU Jian-Xin. Detecting of mtDNA Mutations at Position A3243G and G3316A in Patients with type 2 Diabetes Mellitus in Wenzhou [J]. HEREDITAS, 2006, 28(10): 1206-1212. |
| [13] | WANG Chen, WANG Li, YANG Ze. Role of Protein Tyrosine Phosphatase 1B in the Type 2 Diabetes and Obesity [J]. HEREDITAS, 2004, 26(6): 941-946. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||