Hereditas(Beijing) ›› 2024, Vol. 46 ›› Issue (9): 677-689.doi: 10.16288/j.yczz.24-146
• Review • Previous Articles Next Articles
Progress on SOX9 and its enhancers in mammalian sex determination
Min Yang( ), Siyuan Lin, Changqi Yang, Yaosheng Chen, Zuyong He(
), Siyuan Lin, Changqi Yang, Yaosheng Chen, Zuyong He( )
)
- National Key Laboratory of Aquatic Animal Disease and Healthy Breeding, School of Life Sciences, Sun Yat-sen University, Guangzhou 510275, China
-
Received:2024-05-23Revised:2024-07-04Online:2024-09-20Published:2024-08-01 -
Contact:Zuyong He E-mail:yangmin32@163.com;zuyonghe@foxmail.com -
Supported by:Key R&D Program of Guangdong Province(2018B020203003)
Cite this article
Min Yang, Siyuan Lin, Changqi Yang, Yaosheng Chen, Zuyong He. Progress on SOX9 and its enhancers in mammalian sex determination[J]. Hereditas(Beijing), 2024, 46(9): 677-689.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
| [1] |
Sánchez L, Chaouiya C. Primary sex determination of placental mammals: a modelling study uncovers dynamical developmental constraints in the formation of sertoli and granulosa cells. BMC Syst Biol, 2016, 10(1): 37.
doi: 10.1186/s12918-016-0282-3 pmid: 27229461 |
| [2] |
Bashamboo A, McElreavey K. Human sex-determination and disorders of sex-development (DSD). Semin Cell Dev Biol, 2015, 45: 77-83.
doi: 10.1016/j.semcdb.2015.10.030 pmid: 26526145 |
| [3] | Sinclair AH, Berta P, Palmer MS, Hawkins JR, Griffiths BL, Smith MJ, Foster JW, Frischauf AM, Lovell-Badge R, Goodfellow PN. A gene from the human sex-determining region encodes a protein with homology to a conserved DNA-binding motif. Nature, 1990, 346(6281): 240-244. |
| [4] | Vining B, Ming ZH, Bagheri-Fam S, Harley V. Diverse regulation but conserved function: SOX9 in vertebrate sex determination. Genes (Basel), 2021, 12(4): 486. |
| [5] |
Kwok C, Goodfellow PN, Hawkins JR. Evidence to exclude SOX9 as a candidate gene for XY sex reversal without skeletal malformation. J Med Genet, 1996, 33(9): 800-801.
pmid: 8880588 |
| [6] |
Meyer J, Südbeck P, Held M, Wagner T, Schmitz ML, Bricarelli FD, Eggermont E, Friedrich U, Haas OA, Kobelt A, Leroy JG, Van Maldergem L, Michel E, Mitulla B, Pfeiffer RA, Schinzel A, Schmidt H, Scherer G. Mutational analysis of the SOX9 gene in campomelic dysplasia and autosomal sex reversal: lack of genotype/ phenotype correlations. Hum Mol Genet, 1997, 6(1): 91-98.
pmid: 9002675 |
| [7] | Lavery R, Lardenois A, Ranc-Jianmotamedi F, Pauper E, Gregoire EP, Vigier C, Moreilhon C, Primig M, Chaboissier MC. XY Sox9 embryonic loss-of-function mouse mutants show complete sex reversal and produce partially fertile XY oocytes. Dev Biol, 2011, 354(1): 111-122. |
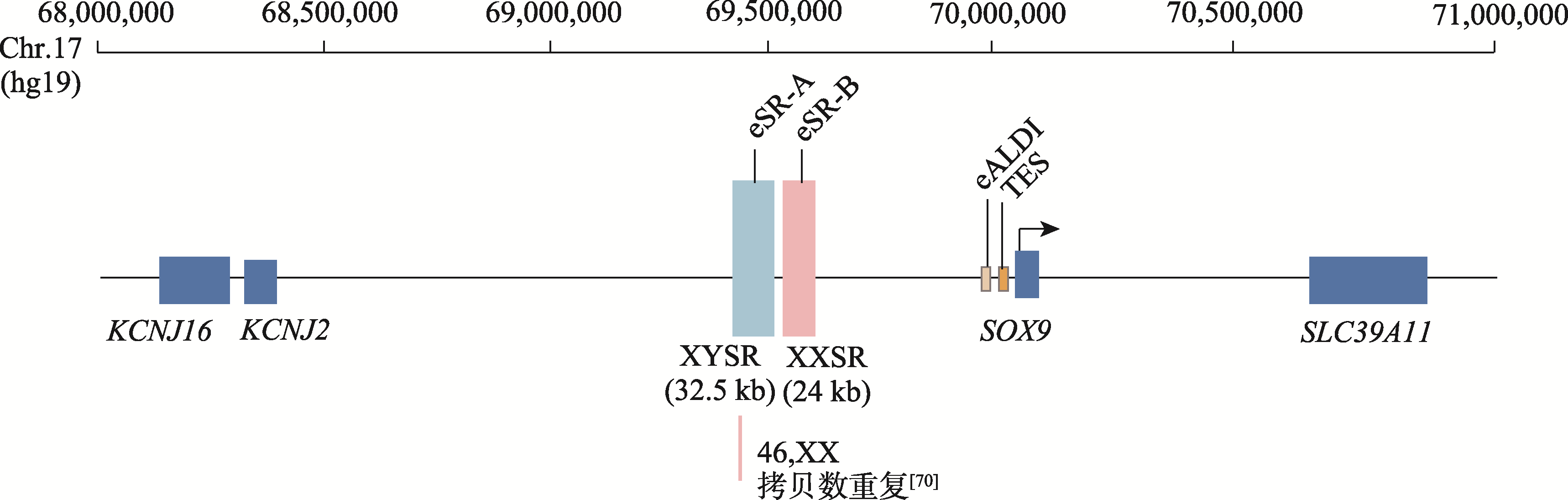
| [8] | Kim GJ, Sock E, Buchberger A, Just W, Denzer F, Hoepffner W, German J, Cole T, Mann J, Seguin JH, Zipf W, Costigan C, Schmiady H, Rostásy M, Kramer M, Kaltenbach S, Rösler B, Georg I, Troppmann E, Teichmann AC, Salfelder A, Widholz SA, Wieacker P, Hiort O, Camerino G, Radi O, Wegner M, Arnold HH, Scherer G. Copy number variation of two separate regulatory regions upstream of SOX9 causes isolated 46,XY or 46,XX disorder of sex development. J Med Genet, 2015, 52(4): 240-247. |
| [9] |
Reyes AP, León NY, Frost ER, Harley VR. Genetic control of typical and atypical sex development. Nat Rev Urol, 2023, 20(7): 434-451.
doi: 10.1038/s41585-023-00754-x pmid: 37020056 |
| [10] | Xie YS, Wu CH, Li ZC, Wu ZF, Hong LJ. Early gonadal development and sex determination in mammal. Int J Mol Sci, 2022, 23(14): 7500. |
| [11] | Svingen T, Koopman P. Building the mammalian testis: origins, differentiation, and assembly of the component cell populations. Genes Dev, 2013, 27(22): 2409-2426. |
| [12] |
Orvis GD, Behringer RR. Cellular mechanisms of Müllerian duct formation in the mouse. Dev Biol, 2007, 306(2): 493-504.
doi: 10.1016/j.ydbio.2007.03.027 pmid: 17467685 |
| [13] |
Ostrer H. Sexual differentiation. Semin Reprod Med, 2000, 18(1): 41-49.
pmid: 11299518 |
| [14] |
Albrecht KH, Eicheer EM. Evidence that Sry is expressed in pre-sertoli cells and sertoli and granulosa cells have a common precursor. Dev Biol, 2001, 240(1): 92-107.
pmid: 11784049 |
| [15] | Tevosian SG, Albrecht KH, Crispino JD, Fujiwara Y, Eicher EM, Orkin SH. Gonadal differentiation, sex determination and normal Sry expression in mice require direct interaction between transcription partners GATA4 and FOG2. Development, 2002, 129(19): 4627-4634. |
| [16] |
Bradford ST, Wilhelm D, Bandiera R, Vidal V, Schedl A, Koopman P. A cell-autonomous role for WT1 in regulating Sry in vivo. Hum Mol Genet, 2009, 18(18): 3429-3438.
doi: 10.1093/hmg/ddp283 pmid: 19549635 |
| [17] | Pilon N, Daneau I, Paradis V, Hamel F, Lussier JG, Viger RS, Silversides DW. Porcine SRY promoter is a target for steroidogenic factor 1. Biol Reprod, 2003, 68(4): 1098- 1106. |
| [18] |
Fujimoto Y, Tanaka SS, Yamaguchi YL, Kobayashi H, Kuroki S, Tachibana M, Shinomura M, Kanai Y, Morohashi KI, Kawakami K, Nishinakamura R. Homeoproteins Six1 and Six4 regulate male sex determination and mouse gonadal development. Dev Cell, 2013, 26(4): 416-430.
doi: 10.1016/j.devcel.2013.06.018 pmid: 23987514 |
| [19] | Nef S, Verma-Kurvari S, Merenmies J, Vassalli JD, Efstratiadis A, Accili D, Parada LF. Testis determination requires insulin receptor family function in mice. Nature, 2003, 426(6964): 291-295. |
| [20] | Pitetti JL, Calvel P, Romero Y, Conne B, Truong V, Papaioannou MD, Schaad O, Docquier M, Herrera PL, Wilhelm D, Nef S. Insulin and IGF1 receptors are essential for XX and XY gonadal differentiation and adrenal development in mice. PLoS Genet, 2013, 9(1): e1003160. |
| [21] |
Sekido R, Bar I, Narvaez V, Penny G, Lovell-Badge R. SOX9 is up-regulated by the transient expression of SRY specifically in sertoli cell precursors. Dev Biol, 2004, 274(2): 271-279.
doi: 10.1016/j.ydbio.2004.07.011 pmid: 15385158 |
| [22] | Sekido R, Lovell-Badge R. Sex determination involves synergistic action of SRY and SF1 on a specific Sox9 enhancer. Nature, 2008, 453(7197): 930-934. |
| [23] |
Sekido R. SRY: a transcriptional activator of mammalian testis determination. Int J Biochem Cell Biol, 2010, 42(3): 417-420.
doi: 10.1016/j.biocel.2009.12.005 pmid: 20005972 |
| [24] |
Bagheri-Fam S, Sim H, Bernard P, Jayakody I, Taketo MM, Scherer G, Harley VR. Loss of Fgfr2 leads to partial XY sex reversal. Dev Biol, 2008, 314(1): 71-83.
pmid: 18155190 |
| [25] |
Hiramatsu R, Harikae K, Tsunekawa N, Kurohmaru M, Matsuo I, Kanai Y. FGF signaling directs a center-to-pole expansion of tubulogenesis in mouse testis differentiation. Development, 2010, 137(2): 303-312.
doi: 10.1242/dev.040519 pmid: 20040496 |
| [26] |
Moniot B, Declosmenil F, Barrionuevo F, Scherer G, Aritake K, Malki S, Marzi L, Cohen-Solal A, Georg I, Klattig J, Englert C, Kim Y, Capel B, Eguchi N, Urade Y, Boizet-Bonhoure B, Poulat F. The PGD2 pathway, independently of FGF9, amplifies SOX9 activity in sertoli cells during male sexual differentiation. Development, 2009, 136(11): 1813-1821.
doi: 10.1242/dev.032631 pmid: 19429785 |
| [27] |
Rotgers E, Jørgensen A, Yao HHC. At the crossroads of fate-somatic cell lineage specification in the fetal gonad. Endocr Rev, 2018, 39(5): 739-759.
doi: 10.1210/er.2018-00010 pmid: 29771299 |
| [28] |
Sekido R, Lovell-Badge R. Sex determination and SRY: down to a wink and a nudge? Trends Genet, 2009, 25(1): 19-29.
doi: 10.1016/j.tig.2008.10.008 pmid: 19027189 |
| [29] | Akiyama H, Lyons JP, Mori-Akiyama Y, Yang XH, Zhang R, Zhang ZP, Deng JM, Taketo MM, Nakamura T, Behringer RR, McCrea PD, de Crombrugghe B. Interactions between Sox9 and beta-catenin control chondrocyte differentiation. Genes Dev, 2004, 18(9): 1072-1087. |
| [30] |
Topol L, Chen W, Song H, Day TF, Yang YZ. Sox9 inhibits Wnt signaling by promoting beta-catenin phosphorylation in the nucleus. J Biol Chem, 2009, 284(5): 3323-3333.
doi: 10.1074/jbc.M808048200 pmid: 19047045 |
| [31] |
Wilhelm D, Martinson F, Bradford S, Wilson MJ, Combes AN, Beverdam A, Bowles J, Mizusaki H, Koopman P. Sertoli cell differentiation is induced both cell- autonomously and through prostaglandin signaling during mammalian sex determination. Dev Biol, 2005, 287(1): 111-124.
pmid: 16185683 |
| [32] |
Georg I, Barrionuevo F, Wiech T, Scherer G. Sox9 and Sox8 are required for basal lamina integrity of testis cords and for suppression of FOXL2 during embryonic testis development in mice. Biol Reprod, 2012, 87(4): 99.
doi: 10.1095/biolreprod.112.101907 pmid: 22837482 |
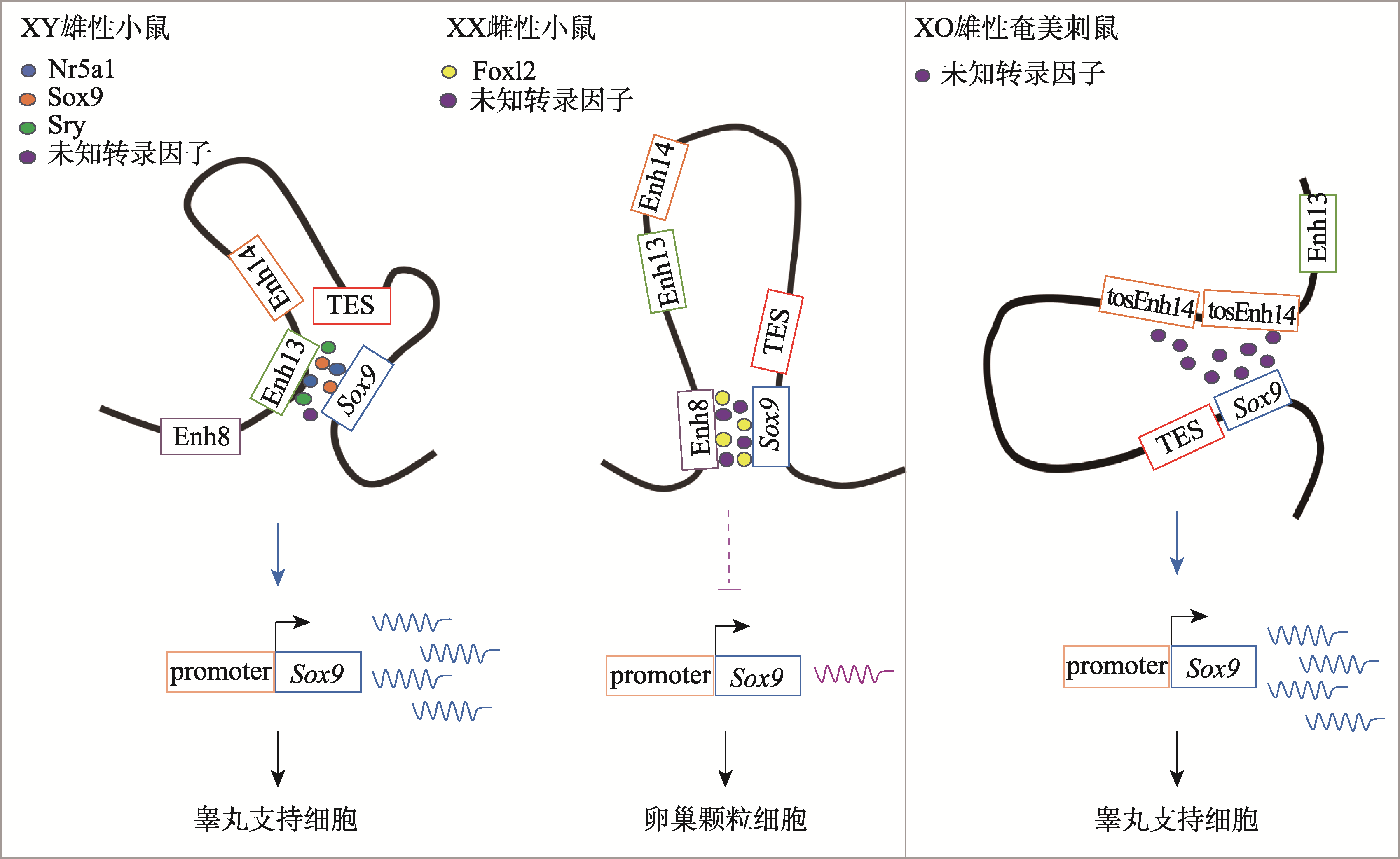
| [33] | Chassot AA, Gillot I, Chaboissier MC. R-spondin1, WNT4, and the CTNNB1 signaling pathway: strict control over ovarian differentiation. Reproduction, 2014, 148(6): R97-R110. |
| [34] |
Ohnesorg T, Vilain E, Sinclair AH. The genetics of disorders of sex development in humans. Sex Dev, 2014, 8(5): 262-272.
doi: 10.1159/000357956 pmid: 24504012 |
| [35] |
Maatouk DM, DiNapoli L, Alvers A, Parker KL, Taketo MM, Capel B. Stabilization of beta-catenin in XY gonads causes male-to-female sex-reversal. Hum Mol Genet, 2008, 17(19): 2949-2955.
doi: 10.1093/hmg/ddn193 pmid: 18617533 |
| [36] |
Arboleda VA, Sandberg DE, Vilain E. DSDs: genetics, underlying pathologies and psychosexual differentiation. Nat Rev Endocrinol, 2014, 10(10): 603-615.
doi: 10.1038/nrendo.2014.130 pmid: 25091731 |
| [37] |
Pannetier M, Chassot AA, Chaboissier MC, Pailhoux E. Involvement of FOXL2 and RSPO1 in ovarian determination, development, and maintenance in mammals. Sex Dev, 2016, 10(4): 167-184.
pmid: 27649556 |
| [38] |
Pannetier M, Fabre S, Batista F, Kocer A, Renault L, Jolivet G, Mandon-Pépin B, Cotinot C, Veitia R, Pailhoux E. FOXL2 activates P450 aromatase gene transcription: towards a better characterization of the early steps of mammalian ovarian development. J Mol Endocrinol, 2006, 36(3): 399-413.
pmid: 16720712 |
| [39] |
Uhlenhaut NH, Jakob S, Anlag K, Eisenberger T, Sekido R, Kress J, Treier AC, Klugmann C, Klasen C, Holter NI, Riethmacher D, Schütz G, Cooney AJ, Lovell-Badge R, Treier M. Somatic sex reprogramming of adult ovaries to testes by FOXL2 ablation. Cell, 2009, 139(6): 1130-1142.
doi: 10.1016/j.cell.2009.11.021 pmid: 20005806 |
| [40] | Wagner T, Wirth J, Meyer J, Zabel B, Held M, Zimmer J, Pasantes J, Bricarelli FD, Keutel J, Hustert E, Wolf U, Tommerup N, Schempp W, Scherer G. Autosomal sex reversal and campomelic dysplasia are caused by mutations in and around the SRY-related gene SOX9. Cell, 1994, 79(6): 1111-1120. |
| [41] |
Lecointre C, Pichon O, Hamel A, Heloury Y, Michel- Calemard L, Morel Y, David A, Familial acampomelic form of campomelic dysplasia caused by a 960 kb deletion upstream of SOX9. Am J Med Genet A, 2009, 149A(6): 1183-1189.
doi: 10.1002/ajmg.a.32830 pmid: 19449405 |
| [42] |
Kamachi Y, Uchikawa M, Kondoh H. Pairing SOX off: with partners in the regulation of embryonic development. Trends Genet, 2000, 16(4): 182-187.
doi: 10.1016/s0168-9525(99)01955-1 pmid: 10729834 |
| [43] | Leung VYL, Gao B, Leung KKH, Melhado IG, Wynn SL, Au TYK, Dung NWF, Lau JYB, Mak ACY, Chan D, Cheah KSE. SOX9 governs differentiation stage-specific gene expression in growth plate chondrocytes via direct concomitant transactivation and repression. PLoS Genet, 2011, 7(11): e1002356. |
| [44] |
Symon A, Harley V. SOX9: a genomic view of tissue specific expression and action. Int J Biochem Cell Biol, 2017, 87: 18-22.
doi: S1357-2725(17)30057-2 pmid: 28323209 |
| [45] |
Kent J, Wheatley SC, Andrews JE, Sinclair AH, Koopman P. A male-specific role for SOX9 in vertebrate sex determination. Development, 1996, 122(9): 2813-2822.
doi: 10.1242/dev.122.9.2813 pmid: 8787755 |
| [46] | Chaboissier MC, Kobayashi A, Vidal VIP, Lützkendorf S, van de Kant HJG, Wegner M, de Rooij DG, Behringer RR, Schedl A. Functional analysis of Sox8 and Sox9 during sex determination in the mouse. Development, 2004, 131(9): 1891-1901. |
| [47] |
Hacker A, Capel B, Goodfellow P, Lovell-Badge R. Expression of Sry, the mouse sex determining gene. Development, 1995, 121(6): 1603-1614.
doi: 10.1242/dev.121.6.1603 pmid: 7600978 |
| [48] |
Morais da Silva S, Hacker A, Harley V, Goodfellow P, Swain A, Lovell-Badge R. Sox9 expression during gonadal development implies a conserved role for the gene in testis differentiation in mammals and birds. Nat Genet, 1996, 14(1): 62-68.
doi: 10.1038/ng0996-62 pmid: 8782821 |
| [49] |
Bullejos M, Koopman P. Delayed Sry and Sox9 expression in developing mouse gonads underlies B6-Y(DOM) sex reversal. Dev Biol, 2005, 278(2): 473-481.
pmid: 15680364 |
| [50] | Wilhelm D, Washburn LL, Truong V, Fellous M, Eicher EM, Koopman P. Antagonism of the testis- and ovary-determining pathways during ovotestis development in mice. Mech Dev, 2009, 126(5-6): 324-336. |
| [51] |
Mansour S, Hall CM, Pembrey ME, Young ID. A clinical and genetic study of campomelic dysplasia. J Med Genet, 1995, 32(6): 415-420.
pmid: 7666392 |
| [52] |
Bi W, Huang W, Whitworth DJ, Deng JM, Zhang Z, Behringer RR, de Crombrugghe B. Haploinsufficiency of Sox9 results in defective cartilage primordia and premature skeletal mineralization. Proc Natl Acad Sci USA, 2001, 98(12): 6698-6703.
doi: 10.1073/pnas.111092198 pmid: 11371614 |
| [53] |
Gonen N, Lovell-Badge R. The regulation of Sox9 expression in the gonad. Curr Top Dev Biol, 2019, 134: 223-252.
doi: S0070-2153(19)30004-3 pmid: 30999977 |
| [54] |
Vidal VP, Chaboissier MC, de Rooij DG, Schedl A. Sox9 induces testis development in XX transgenic mice. Nat Genet, 2001, 28(3): 216-217.
doi: 10.1038/90046 pmid: 11431689 |
| [55] | Lindeman RE, Gearhart MD, Minkina A, Krentz AD, Bardwell VJ, Zarkower D. Sexual cell-fate reprogramming in the ovary by DMRT1. Curr Biol, 2015, 25(6): 764-771. |
| [56] | Zhao L, Svingen T, Ng ET, Koopman P. Female-to-male sex reversal in mice caused by transgenic overexpression of Dmrt1. Development, 2015, 142(6): 1083-1088. |
| [57] | Rockich BE, Hrycaj SM, Shih HP, Nagy MS, Ferguson MAH, Kopp JL, Sander M, Wellik DM, Spence JR. Sox9 plays multiple roles in the lung epithelium during branching morphogenesis. Proc Natl Acad Sci USA, 2013, 110(47): E4456-E4464. |
| [58] |
Poché RA, Furuta Y, Chaboissier MC, Schedl A, Behringer RR. Sox9 is expressed in mouse multipotent retinal progenitor cells and functions in muller glial cell development. J Comp Neurol, 2008, 510(3): 237-250.
doi: 10.1002/cne.21746 pmid: 18626943 |
| [59] |
Scott CE, Wynn SL, Sesay A, Cruz C, Cheung M, Gomez Gaviro MV, Booth S, Gao B, Cheah KSE, Lovell-Badge R, Briscoe J. SOX9 induces and maintains neural stem cells. Nat Neurosci, 2010, 13(10): 1181-1189.
doi: 10.1038/nn.2646 pmid: 20871603 |
| [60] |
Barrionuevo F, Bagheri-Fam S, Klattig J, Kist R, Taketo MM, Englert C, Scherer G. Homozygous inactivation of Sox9 causes complete XY sex reversal in mice. Biol Reprod, 2006, 74(1): 195-201.
doi: 10.1095/biolreprod.105.045930 pmid: 16207837 |
| [61] |
Vidal VPI, Chaboissier MC, Lützkendorf S, Cotsarelis G, Mill P, Hui CC, Ortonne N, Ortonne JP, Schedl A. Sox9 is essential for outer root sheath differentiation and the formation of the hair stem cell compartment. Curr Biol, 2005, 15(15): 1340-1351.
pmid: 16085486 |
| [62] |
Gordon CT, Tan TY, Benko S, Fitzpatrick D, Lyonnet S, Farlie PG. Long-range regulation at the SOX9 locus in development and disease. J Med Genet, 2009, 46(10): 649-656.
doi: 10.1136/jmg.2009.068361 pmid: 19473998 |
| [63] | Cox JJ, Willatt L, Homfray T, Geoffrey Woods C. A SOX9 duplication and familial 46,XX developmental testicular disorder. N Engl J Med, 2011, 364(1): 91-93. |
| [64] |
Vetro A, Ciccone R, Giorda R, Patricelli MG, Della Mina E, Forlino A, Zuffardi O. XX males SRY negative: a confirmed cause of infertility. J Med Genet, 2011, 48(10): 710-712.
doi: 10.1136/jmedgenet-2011-100036 pmid: 21653197 |
| [65] |
Benko S, Gordon CT, Mallet D, Sreenivasan R, Thauvin-Robinet C, Brendehaug A, Thomas S, Bruland O, David M, Nicolino M, Labalme A, Sanlaville D, Callier P, Malan V, Huet F, Molven A, Dijoud F, Munnich A, Faivre L, Amiel J, Harley V, Houge G, Morel Y, Lyonnet S. Disruption of a long distance regulatory region upstream of SOX9 in isolated disorders of sex development. J Med Genet, 2011, 48(12): 825-830.
doi: 10.1136/jmedgenet-2011-100255 pmid: 22051515 |
| [66] | Xiao B, Ji X, Xing Y, Chen YW, Tao J. A rare case of 46, XX SRY-negative male with approximately 74-kb duplication in a region upstream of SOX9. Eur J Med Genet, 2013, 56(12): 695-698. |
| [67] |
Hyon C, Chantot-Bastaraud S, Harbuz R, Bhouri R, Perrot N, Peycelon M, Sibony M, Rojo S, Piguel X, Bilan F, Gilbert-Dussardier B, Kitzis A, McElreavey K, Siffroi JP, Bashamboo A. Refining the regulatory region upstream of SOX9 associated with 46,XX testicular disorders of sex development (DSD). Am J Med Genet A, 2015, 167A(8): 1851-1858.
doi: 10.1002/ajmg.a.37101 pmid: 25900885 |
| [68] |
Croft B, Ohnesorg T, Sinclair AH. The role of copy number variants in disorders of sex development. Sex Dev, 2018, 12(1-3): 19-29.
doi: 10.1159/000481896 pmid: 29145200 |
| [69] |
Ohnesorg T, van den Bergen JA, Belluoccio D, Shankara-Narayana N, Kean AM, Vasilaras A, Ewans L, Ayers KL, Sinclair AH. A duplication in a patient with 46,XX ovo-testicular disorder of sex development refines the SOX9 testis-specific regulatory region to 24 kb. Clin Genet, 2017, 92(3): 347-349.
doi: 10.1111/cge.12976 pmid: 28317102 |
| [70] | Sajan SA, Brown CM, Davis-Keppen L, Burns K, Royer E, Coleman JAC, Hilton BA, DuPont BR, Perry DL, Taft RJ, Kesari A. The smallest likely pathogenic duplication of a SOX9 enhancer identified to date in a family with 46,XX testicular differences of sex development. Am J Med Genet A, 2023, 191(12): 2831-2836. |
| [71] |
Schoenfelder S, Fraser P. Long-range enhancer-promoter contacts in gene expression control. Nat Rev Genet, 2019, 20(8): 437-455.
doi: 10.1038/s41576-019-0128-0 pmid: 31086298 |
| [72] |
Benabdallah NS, Williamson I, Illingworth RS, Kane L, Boyle S, Sengupta D, Grimes GR, Therizols P, Bickmore WA. Decreased enhancer-promoter proximity accompanying enhancer activation. Mol Cell, 2019, 76(3): 473-484.e477.
doi: S1097-2765(19)30593-3 pmid: 31494034 |
| [73] |
Lieberman-Aiden E, van Berkum NL, Williams L, Imakaev M, Ragoczy T, Telling A, Amit I, Lajoie BR, Sabo PJ, Dorschner MO, Sandstrom R, Bernstein B, Bender MA, Groudine M, Gnirke A, Stamatoyannopoulos J, Mirny LA, Lander ES, Dekker J. Comprehensive mapping of long-range interactions reveals folding principles of the human genome. Science, 2009, 326(5950): 289-293.
doi: 10.1126/science.1181369 pmid: 19815776 |
| [74] |
Robson MI, Ringel AR, Mundlos S. Regulatory landscaping: how enhancer-promoter communication is sculpted in 3D. Mol Cell, 2019, 74(6): 1110-1122.
doi: S1097-2765(19)30404-6 pmid: 31226276 |
| [75] |
Misteli T. The self-organizing genome: principles of genome architecture and function. Cell, 2020, 183(1): 28-45.
doi: 10.1016/j.cell.2020.09.014 pmid: 32976797 |
| [76] | Dixon JR, Selvaraj S, Yue F, Kim A, Li Y, Shen Y, Hu M, Liu JS, Ren B. Topological domains in mammalian genomes identified by analysis of chromatin interactions. Nature, 2012, 485(7398): 376-380. |
| [77] | Nora EP, Lajoie BR, Schulz EG, Giorgetti L, Okamoto I, Servant N, Piolot T, van Berkum NL, Meisig J, Sedat J, Gribnau J, Barillot E, Blüthgen N, Dekker J, Heard E. Spatial partitioning of the regulatory landscape of the X-inactivation centre. Nature, 2012, 485(7398): 381-385. |
| [78] | Shen Y, Yue F, McCleary DF, Ye Z, Edsall L, Kuan S, Wagner U, Dixon J, Lee L, Lobanenkov VV, Ren B. A map of the cis-regulatory sequences in the mouse genome. Nature, 2012, 488(7409): 116-120. |
| [79] | Gonen N, Futtner CR, Wood S, Garcia-Moreno SA, Salamone IM, Samson SC, Sekido R, Poulat F, Maatouk DM, Lovell-Badge R. Sex reversal following deletion of a single distal enhancer of Sox9. Science, 2018, 360(6396): 1469-1473. |
| [80] | Croft B, Ohnesorg T, Hewitt J, Bowles J, Quinn A, Tan J, Corbin V, Pelosi E, van den Bergen J, Sreenivasan R, Knarston I, Robevska G, Vu DC, Hutson J, Harley V, Ayers K, Koopman P, Sinclair A. Human sex reversal is caused by duplication or deletion of core enhancers upstream of SOX9. Nat Commun, 2018, 9(1): 5319. |
| [81] | Terao M, Ogawa Y, Takada S, Kajitani R, Okuno M, Mochimaru Y, Matsuoka K, Itoh T, Toyoda A, Kono T, Jogahara T, Mizushima S, Kuroiwa A. Turnover of mammal sex chromosomes in the Sry-deficient Amami spiny rat is due to male-specific upregulation of Sox9. Proc Natl Acad Sci USA, 2022, 119(49): e2211574119. |
| [82] | Ridnik M, Schoenfelder S, Gonen N. Cis-regulatory control of mammalian sex determination. Sex Dev, 2021, 15(5-6): 317-334. |
| [83] |
Bernard P, Ryan J, Sim H, Czech DP, Sinclair AH, Koopman P, Harley VR. Wnt signaling in ovarian development inhibits Sf1 activation of Sox9 via the Tesco enhancer. Endocrinology, 2012, 153(2): 901-912.
doi: 10.1210/en.2011-1347 pmid: 22128028 |
| [84] | Gonen N, Quinn A, O'Neill HC, Koopman P, Lovell-Badge R. Correction: Normal levels of Sox9 expression in the developing mouse testis depend on the TES/TESCO enhancer, but this does not act alone. PLoS Genet, 2017, 13(2): e1006584. |
| [85] |
Ohnesorg T, Croft B, Tan J, Sinclair AH. Using ROADMAP data to identify enhancers associated with disorders of sex development. Sex Dev, 2016, 10(2): 59-65.
doi: 10.1159/000445398 pmid: 27078861 |
| [86] |
Ogawa Y, Terao M, Hara S, Tamano M, Okayasu H, Kato T, Takada S. Mapping of a responsible region for sex reversal upstream of Sox9 by production of mice with serial deletion in a genomic locus. Sci Rep, 2018, 8(1): 17514.
doi: 10.1038/s41598-018-35746-0 pmid: 30504911 |
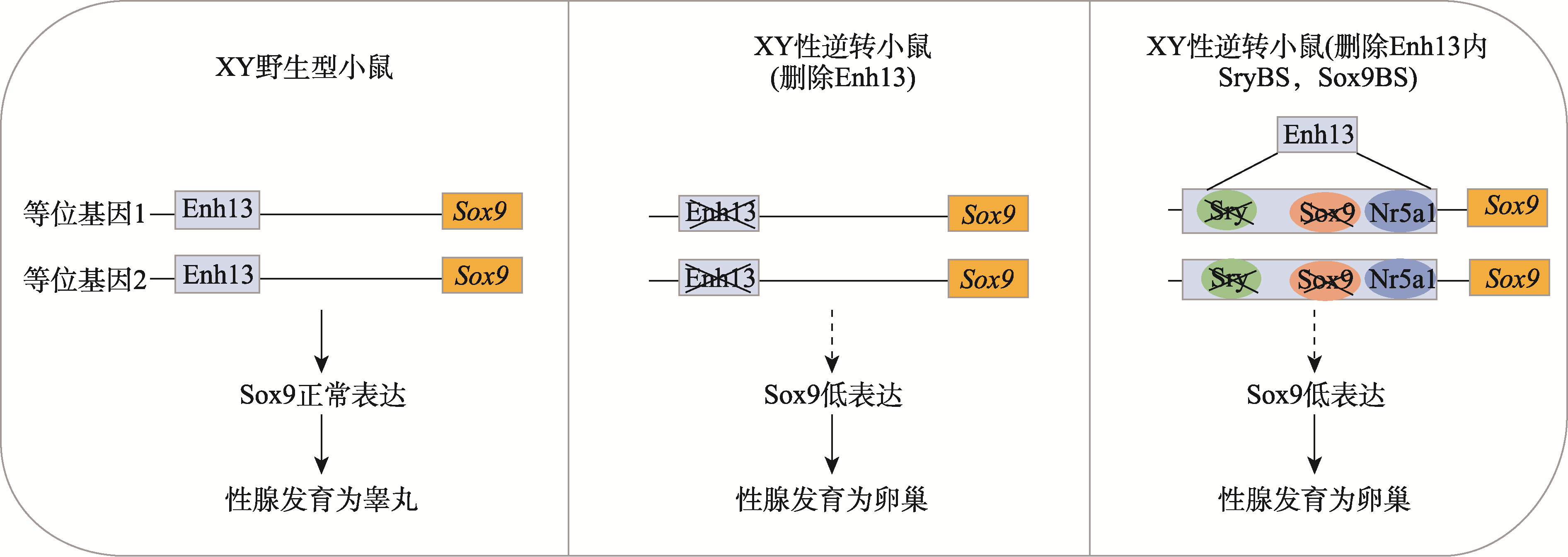
| [87] | Ogawa Y, Terao M, Tsuji-Hosokawa A, Tsuchiya I, Hasegawa M, Takada S. SOX9 and SRY binding sites on mouse mXYSRa/Enh13 enhancer redundantly regulate Sox9 expression to varying degrees. Hum Mol Genet, 2023, 32(1): 55-64. |
| [88] | Ridnik M, Abberbock E, Alipov V, Lhermann SZ, Kaufman S, Lubman M, Poulat F, Gonen N. Two redundant transcription factor binding sites in a single enhancer are essential for mammalian sex determination. Nucleic Acids Res, 2024, 52(10): 5514-5528. |
| [89] |
Zhao L, Wang CW, Lehman ML, He MY, An JY, Svingen T, Spiller CM, Ng ET, Nelson CC, Koopman P. Transcriptomic analysis of mRNA expression and alternative splicing during mouse sex determination. Mol Cell Endocrinol, 2018, 478: 84-96.
doi: S0303-7207(18)30234-X pmid: 30053582 |
| [90] | Georges A, L'Hôte D, Todeschini AL, Auguste A, Legois B, Zider A, Veitia RA. The transcription factor FOXL2 mobilizes estrogen signaling to maintain the identity of ovarian granulosa cells. Elife, 2014, 3: e04207. |
| [91] |
Nicol B, Grimm SA, Gruzdev A, Scott GJ, Ray MK, Yao HHC. Genome-wide identification of FOXL2 binding and characterization of FOXL2 feminizing action in the fetal gonads. Hum Mol Genet, 2018, 27(24): 4273-4287.
doi: 10.1093/hmg/ddy312 pmid: 30212841 |
| [92] |
Rossitto M, Déjardin S, Rands CM, Le Gras S, Migale R, Rafiee MR, Neirijnck Y, Pruvost A, Nguyen AL, Bossis G, Cammas F, Le Gallic L, Wilhelm D, Lovell-Badge R, Boizet-Bonhoure B, Nef S, Poulat F. TRIM28-dependent SUMOylation protects the adult ovary from activation of the testicular pathway. Nat Commun, 2022, 13(1): 4412.
doi: 10.1038/s41467-022-32061-1 pmid: 35906245 |
| [93] | Bolt CC, Duboule D. The regulatory landscapes of developmental genes. Development, 2020, 147(3): dev171736. |
| [1] | Jilong Wang, Qing Li, Tingzheng Zhan. Principle and application of self-transcribing active regulatory region sequencing in enhancer discovery research [J]. Hereditas(Beijing), 2024, 46(8): 589-602. |
| [2] | Yang Yang, Mingxing Chu, Qiuyue Liu. The mechanism of circadian clock and its influence on animal circannual rhythm [J]. Hereditas(Beijing), 2023, 45(5): 409-424. |
| [3] | Xiuli Chen, Haiyan Huang, Qiang Wu. Targeted deletion of 5′HS2 enhancer of β-globin locus control region in K562 cells [J]. Hereditas(Beijing), 2022, 44(9): 783-797. |
| [4] | Siyuan Xu, Jia Shou, Qiang Wu. Additional evidence of HS5-1 enhancer eRNA PEARL for protocadherin alpha gene regulation [J]. Hereditas(Beijing), 2022, 44(8): 695-764. |
| [5] | Sihan Qi, Qilin Wang, Junyou Zhang, Qian Liu, Chunyan Li. The regulatory mechanisms by enhancers during cancer initiation and progression [J]. Hereditas(Beijing), 2022, 44(4): 275-288. |
| [6] | Xingqi Wan, Wanzhen Wei, Shengliang Guo, Yixiao Cui, Xueying Jing, Lujie Huang, Jie Ma. Functional analysis of the long-range regulatory element of BMP2 gene [J]. Hereditas(Beijing), 2022, 44(12): 1141-1147. |
| [7] | Shanshan Gao, Jinliang Li, Jiani Yang, Tong Zhou, Rui Liu, Xiaoping Wang, Li Yu. Progresses on adaptive evolution of gliding and flying ability in mammals [J]. Hereditas(Beijing), 2022, 44(1): 46-58. |
| [8] | Ling Wang, Jinhuan Li, Haiyan Huang, Qiang Wu. Serial deletions of tandem reverse CTCF sites reveal balanced HOXD regulatory landscape of enhancers [J]. Hereditas(Beijing), 2021, 43(8): 775-791. |
| [9] | Qian Liu, Chunyan Li. The identification of enhancers and its application in cancer studies [J]. Hereditas(Beijing), 2020, 42(9): 817-831. |
| [10] | Zhongyong Qin, Xiao Shi, Pingping Cao, Ying Chu, Wei Guan, Nan Yang, He Cheng, Yujie Sun. The NOXA promoter could function as an active enhancer to regulate the expression of BCL2 in the apoptosis response [J]. Hereditas(Beijing), 2020, 42(11): 1110-1121. |
| [11] | Zhiqiang Wu, Zeyun Mi. Research progress of super enhancer in cancer [J]. Hereditas(Beijing), 2019, 41(1): 41-51. |
| [12] | Xiao Cheng,Qiong Yang,Zhendong Tan,Ya Tan,Hongzhou Pu,Xue Zhao,Shunhua Zhang,Li Zhu. The current research status of enhancer RNAs [J]. Hereditas(Beijing), 2017, 39(9): 784-797. |
| [13] | Juntao Li,Wei Zhao,Dandan Li,Jing Feng,Gui Ba,Tianzeng Song,Hongping Zhang. miR-101a targeting EZH2 promotes the differentiation of goat skeletal muscle satellite cells [J]. Hereditas(Beijing), 2017, 39(9): 828-836. |
| [14] | Yi Kang,Guijun Guan,Yunhan Hong. Insights of sex determination and differentiation from medaka as a teleost model [J]. Hereditas(Beijing), 2017, 39(6): 441-454. |
| [15] | Fang Yang, Baoyun Zhang, Guangde Feng, Wei Xiang, Yunxia Ma, Hang Chen, Mingxing Chu, Pingqing Wang. A mechanistic review of how hypoxic mircroenvironment regulates mammalian ovulation [J]. HEREDITAS(Beijing), 2016, 38(2): 109-117. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||