2015年,我国学者屠呦呦因为发现了青蒿素、爱尔兰学者坎贝尔(Campbell)和日本学者大村智(Ōmura)因为发现了阿维菌素而共同分享了当年的诺贝尔生理学或医学奖,这是继青霉素和链霉素之后,以阿维菌素为代表的微生物药物第3次受到诺贝尔奖的青睐。阿维菌素自20世纪80年代由美国默克(Merck)公司投放市场以来,目前已成为全球用量最大、使用技术最成熟的绿色生物农药。阿维菌素不仅在农业生产上有广泛的应用,对许多危害农作物的害虫都有活性,而且其衍生物伊维菌素还可以有效治疗严重威胁人类健康的盘尾丝虫病(又称河盲症)和淋巴丝虫病(又称象皮病)。中国众多微生物学家、药学家、发酵工程专家等从“七五”至“九五”经过连续攻关,已经实现了阿维菌素的产业化。同时,随着遗传学、代谢工程和合成生物学等新技术方法的发展和应用,我国阿维菌素产业化竞争能力不断提升,生产成本不断降低,最终打破了默克公司的垄断,使阿维菌素逐渐从“贵族农药”走向廉价的“平民使用药”,打上了中国“智”造的标签。截至2015年9月,我国已有168项阿维菌素相关专利获得授权,并成为世界上阿维菌素的唯一生产国,年产量超过3500吨[1]。我国科学家对阿维菌素的研发和改造,也成为国内抗生素领域内一个“依靠创新、后来居上、直至引领世界”产业升级的成功范例。本文重点介绍阿维菌素的发现、基础及应用研究的发展历程,尤其是中国“智”造的历史沿革,为我国微生物药物的产业化发展提供参考和借鉴。
1 阿维菌素的发现
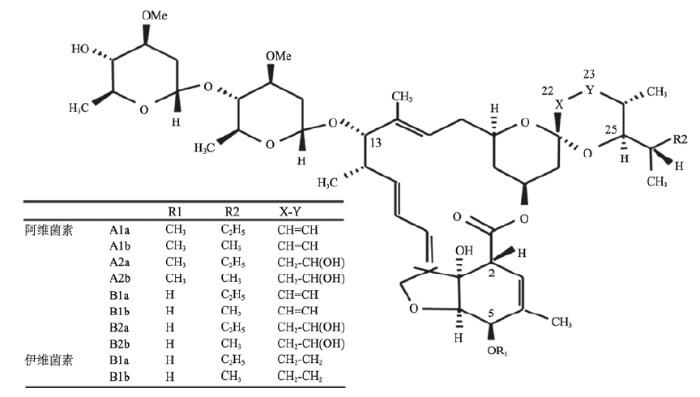
阿维菌素的产生菌最早在1974年由日本北里研究所的大村智从静岗县的一个土壤样品中分离得到,并寄往了美国默克公司。1975年,默克公司的寄生虫学专家坎贝尔和他的团队经小鼠感染模型筛选,发现该菌可产生具有强烈杀虫活性的化合物。经分类鉴定,该菌属于链霉菌属的一个新种,被命名为阿维链霉菌(Streptomyces avermitilis)。之后,默克公司的米勒(Miller)团队从阿维链霉菌的发酵培养物中分离纯化了杀虫活性化合物—阿维菌素,为一组结构相似的十六元环大环内酯类抗生素,共包含8个组分:A1a、A1b、A2a、A2b、B1a、B1b、B2a和B2b,这些组分的区别在于 C5、C22~C23和 C25 位所连接的基团不同(图1)。与此同时,坎贝尔团队对含量最高、活性最强的B1a组分进行了详细研究,发现其对多种动物体内外寄生虫均具有很强的杀虫活性。1979年,大村智、米勒和坎贝尔3个团队在学术期刊Antimicrob Agents Chemother上发表文章,分别报道了阿维菌素产生菌的分离与发酵、活性物质的色谱分离和B1a组分的活性研究成果[2,3,4]。伊维菌素是阿维菌素B1组分在C22, 23位的双氢还原产物(图1)。在后续研究中,坎贝尔团队发现伊维菌素的毒性更低,使用更安全[5,6]。
图1
阿维菌素和伊维菌素具有独特的作用机制,可导致线虫及节肢动物类寄生虫麻痹而死,但对哺乳动物的毒性很低,安全性很高[7,8,9]。作为一种绿色生物农药,阿维菌素几乎可以杀灭所有与农业虫害有关的线虫和节肢动物,而且与其他化学杀虫药无交叉抗性,不易引起耐药性,使用剂量少,效用时间长,可在日光下迅速分解,残留量低,因此对环境友好。由于阿维菌素类化合物具有广谱、高效、低毒的杀虫活性,因此它的发现标志着一类新型杀虫药物的诞生。默克公司首先将阿维菌素和伊维菌素商品化,于1981、1985年分别作为兽药和农药投放市场,都取得了巨大成功。人用伊维菌素也于1987年在法国上市。同年,默克公司CEO瓦杰洛斯(Vagelos)宣布与世界银行等联合组织实施伊维菌素捐赠计划,使之成为消除威胁数亿人河盲症的利器。自阿维菌素和伊维菌素上市后的20余年时间里,默克公司每年获利约8亿美元,并根据合同支付给大村智累计达2.3亿美元的特许权费。
2 遗传学改造揭示阿维菌素的生物合成及其调控机制
由于阿维菌素的重要应用价值,自其发现以来,已形成了一个重要的抗生素研究领域。早期研究以美国默克公司、日本北里研究所和北里大学为主,后期中国学者逐渐加入,从跟跑到并跑再到领跑,共同推动了阿维菌素基础与应用研究的快速发展。
2.1 阿维菌素的复杂生物合成过程
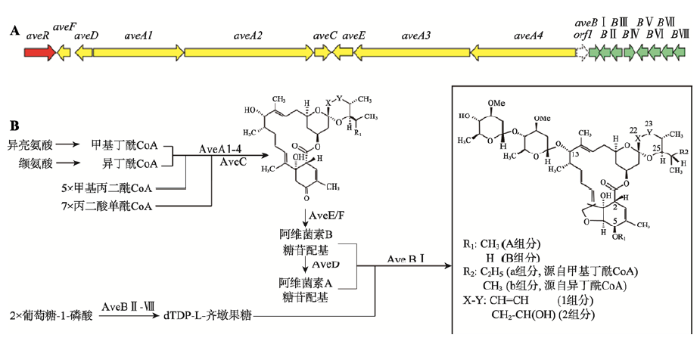
阿维菌素生物合成基因簇全长82 kb,共编码18个ORFs (图2)。其中4个大的ORF—aveA1、aveA2、aveA3和aveA4的编码产物共同组成聚酮合酶,参与阿维菌素合成的第一个步骤,负责阿维菌素起始糖苷配基的合成。阿维菌素“1”组分在C22~C23之间为含有羟基的单键,“2”组分为双键。之前研究表明aveC以某种方式决定“1”和“2”组分的比例[17,18],但其具体功能直到2013年才由中国科学院上海有机化学研究所刘文团队阐明。aveC编码双功能酶AveC,催化C22~C23之间的脱水和C17~C25螺缩醛酮的形成,该酶的两个活性中心独立发挥作用,一个活性中心的改变并不影响另一个活性中心的功能[19]。脱水作用使C22~C23之间的单键变成双键,因此AveC是决定“1”和“2”组分比例的一个关键酶,可通过突变aveC改变“1”和“2” 组分的含量[17,18]。AveC催化之后,由aveE编码的细胞色素P450羟化酶通过引入氧原子使C6和C8a间的呋喃环闭合,再由aveF编码的C5-酮基还原酶使C5位的酮基还原为羟基,形成“B”组分的糖苷配基。紧邻aveF上游的aveD编码C5-O-甲基转移酶,负责将C5位甲基化,形成“A”组分的糖苷配基。糖基化修饰对阿维菌素的杀虫活性至关重要,位于基因簇右侧的aveBⅠ-aveBⅧ负责合成和转移齐墩果糖。首先,AveBⅡ和AveBⅢ催化葡萄糖-1-磷酸形成TDP-4-酮-6-脱氧葡萄糖,然后在AveBⅣ- AveBⅧ的作用下合成dTDP-L-齐墩果糖,最后由糖基转移酶AveBⅠ将dTDP-L-齐墩果糖连接到阿维菌素糖苷配基的C13和C4′位上,最终形成阿维菌素。
图2
图2
阿维菌素的生物合成
A:阿维菌素生物合成基因簇。黄箭头:参与阿维菌素糖苷配基合成的基因;绿箭头:参与糖苷配基糖基化的基因;红箭头:调控基因;虚线箭头:不参与阿维菌素合成的基因。B:阿维菌素生物合成途径。
Fig. 2
Biosynthesis of avermectins
2.2 阿维菌素生物合成的精准调控
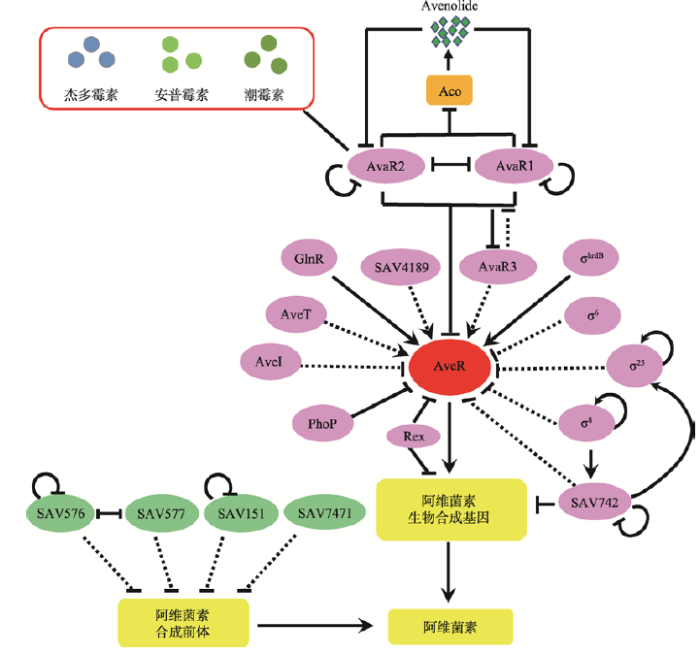
阿维菌素生物合成基因簇内只有一个位于最左侧的CSR基因aveR (图2),编码LuxR家族调控蛋白。AveR不仅正调控基因簇内所有结构基因的表达,还负调控寡霉素的合成,其作用具有多效性[22,23]。已知持家σ因子σhrdB可识别aveR的启动子[24];响应磷酸盐信号的双组份调控系统PhoR/PhoP中的调控蛋白PhoP[25]、响应氧化还原信号的调控因子Rex[26]以及TetR家族转录因子AvaR1[27]和AvaR2[28]直接负调控aveR的表达;响应氮信号的调控因子GlnR直接正调控aveR的表达[29](图3)。其他已知调控阿维菌素合成的转录因子包括:AraC家族的SAV742[30]直接调控基因簇内结构基因的表达;TetR家族的AveI[31,32]、SAV7471[33]、SAV151[34]、SAV576[35]、SAV577[36]、AveT[37]、AvaR3[38],MarR家族的SAV4189[39],ECF家族σ因子σ6[40]、σ25[41]和选择性σ因子σ8[42]以不同方式间接调控阿维菌素的合成(图3)。上述调控因子中的AveT以阿维菌素前体C5-O-B1为配体,通过响应前体浓度变化正调控阿维菌素合成,从而使胞内阿维菌素浓度维持在适当的水平[37]。PhoP、AvaR1、AvaR2、GlnR、Rex、SAV742、AveI和σ8都属于全局调控因子,不仅调控次级代谢,还调控初级代谢、胁迫响应和(/或)形态分化等生理遗传过程,有些调控因子之间还存在交互调控(图3)。
图3
图3
阿维菌素生物合成的调控网络
箭头:正调控或合成产物;平末端:负调控;实线:直接作用;虚线:间接作用或未知途径。
Fig. 3
Regulatory network of avermectin biosynthesis
许多链霉菌抗生素的合成都受到γ-丁酸内酯类信号分子(以灰色链霉菌中A因子为代表)的调控,这些自调控因子扮演着重要的转录开关角色,通过与受体蛋白结合开启下游基因的转录,导致抗生素合成和/或形态分化的发生。但在阿维链霉菌中没有发现γ-丁酸内酯类信号分子,而是发现了一种新型的丁烯羟酸内酯类信号分子avenolide (aco编码其关键合成酶),它在4 nmol/L浓度下即可诱导阿维菌素合成[43]。阿维链霉菌中有3个γ-丁酸内酯受体同源蛋白AvaR1、AvaR2和AvaR3,其中AvaR1与A因子受体蛋白ArpA的同源性最高,AvaR2与天蓝色链霉菌和委内瑞拉链霉菌中响应抗生素信号分子的假γ-丁酸内酯受体ScbR2和JadR2的同源性最高,AvaR3比一般的γ-丁酸内酯受体多出75个氨基酸。研究表明,AvaR1和AvaR2都可作为avenolide受体,通过响应该信号分子直接负调控阿维菌素和avenolide合成。此外,AvaR2还能响应其他链霉菌产生的抗生素信号分子,调控靶基因的表达(图3)。AvaR1与AvaR2既相互竞争又协同作用于相同的靶基因,共同调控阿维菌素和avenolide合成,但它们也有各自不同的靶基因,可交互调控不同的生理过程[27,28]。AvaR3不仅正调控阿维菌素合成,还影响形态分化,但调控机制尚不清楚[38]。
综上可知,阿维菌素的生物是各种复杂的信号传导途径和调控网络协同控制的结果。阐明阿维菌素合成的复杂调控网络,可为理性控制阿维菌素合成、使产生菌作为细胞工厂大量生产阿维菌素奠定理论基础。
3 阿维菌素产生菌的理性化改造
3.1 利用代谢工程提高菌株高产性能
阿维菌素发酵生产的最佳碳源是淀粉,它先在淀粉酶作用下转变为麦芽糖和麦芽糊精再被菌体利用,过表达麦芽糖转运系统malEFG可促进淀粉的利用,从而提高阿维菌素产量[52]。核糖体循环因子参与催化翻译终止后核糖体复合物的解离,使核糖体进入下一轮的循环,过表达核糖体循环因子基因frr可提高核糖体的利用效率,从而促进菌体生长和阿维菌素合成[53]。avtAB位于阿维菌素合成基因簇上游,编码阿维菌素的外排泵,过表达avtAB可减少阿维菌素的反馈抑制从而提高其产量[54]。metK是S-腺苷甲硫氨酸(SAM)合成酶基因,过表达metK可提高SAM的浓度和阿维菌素产量[55]。通过比较代谢组学分析添加SAM导致高产的原因,揭示了阿维菌素合成的关键前体代谢物,合理添加这些代谢物可显著提高产量[56]。
中国科学院微生物研究所张立新团队发现阿维菌素高产菌株中基因簇内正调控基因aveR的表达水平显著提高,由于体外转录实验证实σhrdB可识别aveR的启动子,因此利用体外定向进化构建了hrdB基因的突变库,进一步通过高通量筛选获得了高产重组菌株A56,在180 L发酵罐上阿维菌素B1a产量提高了53%,达到6382 μg/mL[24]。该研究是一个从基础研究走向实际应用,并实现对高产关键基因精确定位的典型范例。
3.2 通过组合生物合成产生阿维菌素衍生物
作为第2代阿维菌素类药物的伊维菌素,由于使用更安全而主要用于兽药和医药上,目前其生产主要采用化学法从阿维菌素B1还原而来,该过程需要昂贵的氯化铑做催化剂。英国剑桥大学Leadlay团队首先尝试了通过组合生物合成技术,用雷帕霉素PKS模块13上的DH-ER-KR(脱水酶-烯基还原 酶-酮基还原酶)结构域取代野生型阿维链霉菌中阿维菌素PKS模块2上的DH-KR结构域(aveDH2- KR2),获得的重组菌株具有直接合成伊维菌素的能力[57]。中国农业大学李季伦团队先构建了不产有毒成分寡霉素而仅产阿维菌素B组分的工程菌Olm73- 12[58],以此为出发菌株,将aveDH2-KR2分别用来自苦霉素PKS模块4和寡霉素PKS模块3上的DH-ER-KR所置换,得到了不产寡霉素而产伊维菌素的工程菌[59,60]。然而上述工程菌伊维菌素的产量极低,无法用于工业生产。米尔贝霉素是与阿维菌素结构类似的杀虫药物,其C22~C23之间为饱和键,与伊维菌素相同。2015年,东北农业大学向文胜团队在阿维菌素高产菌株中用来自冰城链霉菌米尔贝霉素PKS模块2的DH-ER-KR替换aveDH2-KR2,获得的工程菌伊维菌素B1a产量达到3450 μg/mL,可用于工业化生产[61]。
多拉菌素是20世纪90年代由美国辉瑞(Pfizer)公司研发的阿维菌素第3代衍生物,比伊维菌素的杀虫效果更好,其结构为阿维菌素B1的C25位被环己烷基所取代[62]。Cropp 等[62]将山丘链霉菌中的环己酰CoA (CHC-CoA)合成基因转入阿维菌素的前体合成阻断突变株(支链α-酮酸脱氢酶缺陷,不能合成起始单元2-甲基丁酸和异丁酸)中,使突变株获得了合成多拉菌素的能力,同时还产生无效的CHC-B2组分。辉瑞公司研究人员通过对aveC基因进行突变,获得了多拉菌素的比例提高了23倍的突变株[18]。中国科学院上海有机化学研究所唐功利团队则采取另一个策略,将阿维菌素PKS的起始模块替换为可加载环己烷羧酸(CHC)的磷内酯霉素PKS起始模块,同时转入CHC-CoA合成基因,构建了产多拉菌素的工程菌[63]。刘文团队从阿维链霉菌的ΔaveCDE突变株中分离到3个新的阿维菌素衍生物,体外实验发现它们具有抗肿瘤细胞活性[64]。
2015年,张立新团队和中国科学院过程工程研究所郑舰艇团队合作,解析了阿维菌素PKS起始模块中酰基转移酶(AT)结构域的结构与功能,发现其底物特异性并不强,可将40多种羧酸作为起始单元掺入到聚酮链中。通过对AT活性位点的氨基酸残基进行定点突变可改变底物的特异性,有望合成新的活性更高、作用范围更广、毒性更低的阿维菌素衍生物[65]。
3.3 基于合成生物学理念的菌株遗传改造
阿维链霉菌基因组中有38个次级代谢生物合成基因簇[67],但目前仅有阿维菌素、寡霉素、菲律宾菌素等几种主要的产物得到分离鉴定,表明阿维链霉菌不仅具有合成丰富次级代谢产物的潜力,同时可为多种次级代谢产物合成提供充足的前体、能量和还原力,适于开发为合成生物学底盘细胞。2010年,日本Ikeda团队将阿维链霉菌基因组精简,即缺失染色体左臂约1.4 Mb的非必需区域和寡霉素等次级代谢产物合成基因簇,构建了一系列底盘细胞SUKA2~SUKA22,并成功地异源表达了链霉菌来源的链霉素、头霉素、普拉地内酯和植物来源的萜类化合物前体[68]。2013年,他们利用之前改造的底盘细胞SUKA17或 SUKA22对不同类型的20个次级代谢生物合成基因簇进行了异源表达[69]。2015年,该团队又利用底盘细胞SUKA22异源表达了多个在其他链霉菌中处于沉默的萜类化合物基因簇,并获得了13个新的萜类化合物[70]。
由于链霉菌丝状生长的特性,使其基因表达调控难以精确设计、定量和预测。2015年,中国科学院微生物研究所的张立新和娄春波两个团队合作,通过制备单细胞阿维链霉菌的原生质体,首次建立了基于流式细胞仪和报告基因(sfGFP)的对链霉菌调控元件进行单细胞精确定量的方法,通过对近200个天然或人工合成的启动子和核糖体结合位点(RBS)调控元件进行高通量定量表征,极大地丰富了链霉菌调控元件库。然后又成功利用绝缘子RiboJ消除了启动子和RBS之间的干扰作用,提高了这些调控元件的性能。这些调控元件模块也被成功地应用于激活阿维链霉菌中处于沉默的番茄红素基因簇的表达,并通过可预测地替换调控元件使番茄红素产量达到了工业生产的要求[71]。该研究开发的调控元件还可用于调节阿维菌素合成基因的表达水平,通过优化生物合成过程提高阿维菌素产量。
4 阿维菌素的中国“智”造升级
1991年默克公司在我国获得阿维菌素的临时登记,而我国的阿维菌素研究始于1984年,其中沈寅初、李季伦两位院士为阿维菌素的中国“智”造做出了重要贡献,也曾获得了诺贝尔奖提名。
1984年,上海市农药研究所沈寅初团队从广东揭阳土壤中分离到7051菌株,经鉴定为阿维链霉菌,该菌产生的有效杀虫成分7051杀虫素与阿维菌素结构一致,这一技术成果于1992年率先转让于海门制药厂(浙江海正药业股份有限公司前身)进行工业生产,获得1995年上海市一等奖(畜用阿维菌素)和1997年化工部一等奖(农用阿维菌素),并于1999年获得国家科技进步二等奖。李季伦团队于1986年开始研究阿维菌素,阿维菌素的研究及工业化生产被列为国家“七五”、“八五”、“九五”重点科技攻关项目。经过连续攻关,阿维菌素的摇瓶发酵单位从最初的50~100 μg/mL提高到3500~4000 μg/mL。1996年,齐鲁制药厂与李季伦团队合作完成了阿维菌素的中试生产工作,在30吨发酵罐上的发酵单位达到3000 μg/mL,并采用直接结晶法提取阿维菌素B1,提取率达到61.5%,提前完成了攻关任务。1998年,阿维菌素在齐鲁制药厂正式投入工业化大生产。2006年,李季伦等的阿维菌素研究成果获得了国家科技进步二等奖。
科技进步的推动和市场需求的拉动,使阿维菌素产业在我国不断壮大,成为中国农药领域中发展最快的品种。至2005年,阿维菌素原药登记企业有14家,年产能超过1000吨,原药销售价格由最初的2万元/公斤降到1000~1300元/公斤,我国阿维菌素生产能力占世界的90%,成为名副其实的阿维菌素生产大国。从2007年起,阿维菌素这一“贵族产品”彻底走下神坛,成为家喻户晓的农民用得起的生物农药,正式进入“平民阶段”。
巨大的产业需要以科技创新为坚实基础。华东理工大学张嗣良团队不断对阿维菌素发酵工艺进行优化[44,45,46,47],张立新团队利用响应面法优化了发酵培养基,建立了利用96孔板培养细胞和紫外检测阿维菌素产量的高通量筛选体系,利用该体系结合新的诱变手段筛选到了具有核心竞争力的高效生产菌 株[48,49,50,51]。2010年4月,中国13家阿维菌素主要生产企业成立了阿维菌素产业联盟,使产业联盟与新技术相结合,以形成优势互补,通过科技创新带动整个产业的发展,提升该产业的国际竞争能力。张立新团队与产业联盟组长单位威远生物化工有限公司建立了长期合作关系,合作的阿维菌素项目于2011年获得内蒙古自治区科技进步一等奖。
2013年1月,中国科学院微生物研究所主持的973项目“合成微生物体系的适配性研究”正式启动,旨在运用新兴的合成生物学手段大幅度提高阿维菌素的发酵单位。该项目以阿维链霉菌为底盘细胞,从元件挖掘(Mining)、建模(Modeling)、元件组装与通路搭建(Manipulation)、系统测试(Measurement)、绿色工业制造(Manufacture) 5个方面(简称“5M”策略)[16,72]展开研究,实现了阿维菌素高产关键元件的精确定位、改造和高产菌株的发酵、提取工艺优化,在120~550立方米发酵罐中,提高了阿维菌素B1a的产量至9000 μg/mL以上,达到了原始菌株的1000倍。同时还建立一种简单的分离提取工艺,使阿维菌素B1的回收率高达90%,生产效率较曾经垄断阿维菌素国际市场的默克公司提高了60%以上,市场价格降低至500元/公斤,对提升我国阿维菌素产业整体技术水平、引领行业技术进步,起到了积极的促进作用。2015年9月,中国成为阿维菌素原药的唯一生产国,默克公司全面停产阿维菌素原药,转而向中国采购。张立新团队的“阿维菌素的微生物高效合成及其生物制造”项目成果荣获了2016年度国家科技进步奖二等奖。
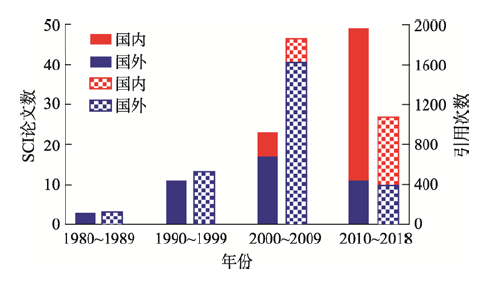
图4
图4
与阿维菌素产量有关的SCI论文及引用次数
Fig. 4
Number of SCI articles and citations related to avermectin production
目前国内阿维菌素相关的登记证超过2500个, 涉及的企业超过1000家,其中原药生产企业29家,产生了4个上市公司,形成了一个巨大的产业链。阿维菌素是目前唯一一个年产值达到30亿元的生物农药,原料药远销世界各国,创造了巨大的社会和经济效益。
以上数据充分显示了中国科研工作者在阿维菌素研发中从跟跑到并跑再到领跑的过程,最终使中国从阿维菌素“发酵大国”转变成为“发酵强国”,为用遗传学方法提高其他微生物药物的产量和效率提供了宝贵的经验和借鉴。
5 结语与展望
阿维菌素从发现至今已有40多年的历史,如今仍在杀虫剂舞台上独具魅力,备受市场宠爱,尚无其他药物可以替代它的重要地位。随着研究的深入,将会有更多结构和作用机制新颖的阿维菌素衍生物产品问世,使阿维菌素类药物不断升级换代,降低其耐药性,提高其广谱性,并开发出具有抗真菌、抗病毒、抗结核、抗肿瘤等活性的新药。最近发现,阿维菌素B2的杀虫谱不同于B1,对根结线虫、根腐线虫、胞囊线虫、茎线虫和松材线虫等多种植物线虫有特别高的杀灭活性,有望作为一种阿维菌素新产品推广应用,具有广阔的市场前景。此外,还可以将阿维菌素高产工业菌株打造成为新的合成生物学底盘细胞,利用它异源表达有价值的但产量较低的微生物活性物质,为新药开发做出贡献。期待阿维菌素再续新的篇章。
(责任编委: 刘钢)
参考文献
Avermectin, from winning the Nobel Prize to "innovation in China"
纵观世界历史,大国崛起无不伴随着科技的兴起和机制体制的突破。来自大自然的天然产物对于人类的健康起到非常重要的作用,从抗肿瘤明星分子紫杉醇到挽救了无数人生命的抗感染药物青霉素,从治疗代谢疾病到营养保健,都离不开天然产物。此外,还有大量天然产物资源没有被开发过。而随着阿维菌素的发现者Satoshi Ōmura教授和William C. Campbell博士,及青蒿素的发现者屠呦呦研究员因为这两种天然产物在治疗寄生虫感染病和疟疾上的应用而获得2015年诺贝尔生理与医学奖后,天然产物有望迎来其发展的第二个黄金时代。我国是世界工厂也是天然药物的资源大国,为了实现产业升级,弯道超车,实现大国崛起的中国梦,我国科学家在“十二五”期间围绕着天然产物的高产和创新两大主题开展了富有成效的合成生物学研究。阿维菌素是由阿维链霉菌产生的高效低毒生物杀虫剂,其原料产能占国际市场的100%。但我国原有生产菌株的单位发酵产量较低,高消耗、高污染、片面追求生产规模的粗放型发展模式已经成了阻碍低碳经济持续高速发展的瓶颈。如何从根本上解决问题,提高生产菌株的单位发酵产量和原料利用率,降低能耗和生产成本,减少环境污染,是促进我国从“发酵大国”向“发酵强国”转变的关键。本文以阿维菌素为例,综述其基础研究的技术发展,特别是中国科学院微生物研究所引入合成生物学技术,将阿维菌素的单位产量提高了1000倍,至9 g/L,在内蒙古新威远与阿维菌素产业联盟的公司应用,迫使默克公司全面退出阿维菌素历史舞台,从而引领产业迅速发展的过程,为我国其它天然产物生物制造品种的改良提供思路和方法。
从阿维菌素获得诺贝尔奖到中国创造
纵观世界历史,大国崛起无不伴随着科技的兴起和机制体制的突破。来自大自然的天然产物对于人类的健康起到非常重要的作用,从抗肿瘤明星分子紫杉醇到挽救了无数人生命的抗感染药物青霉素,从治疗代谢疾病到营养保健,都离不开天然产物。此外,还有大量天然产物资源没有被开发过。而随着阿维菌素的发现者Satoshi Ōmura教授和William C. Campbell博士,及青蒿素的发现者屠呦呦研究员因为这两种天然产物在治疗寄生虫感染病和疟疾上的应用而获得2015年诺贝尔生理与医学奖后,天然产物有望迎来其发展的第二个黄金时代。我国是世界工厂也是天然药物的资源大国,为了实现产业升级,弯道超车,实现大国崛起的中国梦,我国科学家在“十二五”期间围绕着天然产物的高产和创新两大主题开展了富有成效的合成生物学研究。阿维菌素是由阿维链霉菌产生的高效低毒生物杀虫剂,其原料产能占国际市场的100%。但我国原有生产菌株的单位发酵产量较低,高消耗、高污染、片面追求生产规模的粗放型发展模式已经成了阻碍低碳经济持续高速发展的瓶颈。如何从根本上解决问题,提高生产菌株的单位发酵产量和原料利用率,降低能耗和生产成本,减少环境污染,是促进我国从“发酵大国”向“发酵强国”转变的关键。本文以阿维菌素为例,综述其基础研究的技术发展,特别是中国科学院微生物研究所引入合成生物学技术,将阿维菌素的单位产量提高了1000倍,至9 g/L,在内蒙古新威远与阿维菌素产业联盟的公司应用,迫使默克公司全面退出阿维菌素历史舞台,从而引领产业迅速发展的过程,为我国其它天然产物生物制造品种的改良提供思路和方法。
Avermectins, new family of potent anthelmintic agents: producing organism and fermentation
Abstract The avermectins are a complex of chemically related agents which exhibit extraordinarily potent anthelmintic activity. They are produced by a novel species of actinomycete, NRRL 8165, which we have named Streptomyces avermitilis. The morphological and cultural characteristics which differentiate the producing organism from other species are described. The avermectins have been identified as a series of macrocyclic lactone derivatives which, in contrast to the macrolide or polyene antibiotics, lack significant antibacterial or antifungal activity. The avermectin complex is fully active against the gastrointestinal nematode Nematospiroides dubius when fed to infected mice for 6 days at 0.0002% of the diet. Fermentation development, including medium modification and strain selection, resulted in increasing the broth yields from 9 to 500 mug/ml.
Avermectins, new family of potent anthelmintic agents: isolation and chromatographic properties
Avermectins, new family of potent anthelmintic agents: efficacy of the B1a component
Ivermectin, a new broad-spectrum antiparasitic agent
Abstract 22,23-Dihydroavermectin B1, ivermectin, derived from avermectin B1 by selective hydrogenation using Wilkinson's homogenous catalyst [Ph3P)3RhCl], was shown to be a highly effective drug for the treatment of a wide variety of metazoan parasitic diseases in animals.
Ivermectin: A potent new antiparasitic agent
Ivermectin is the 22,23-dihydro derivative of avermectin B1, a macrocyclic lactone produced by an actinomycete, Streptomyces avermitilis. It is active at extremely low dosage against a wide variety of nematode and arthropod parasites, apparently by virtue of its action on the mediation of neurotransmission by gamma-aminobutyric acid. It is now in commercial use in various countries for the treatment and control of parasites in cattle, horses, and sheep, and is expected to become available for use in swine and dogs. Since studies with the drug in man are in a preliminary stage, it is not yet known whether ivermectin will be useful in human medicine.
Avermectin B1a, a paralysing anthelmintic that affects interneurons and inhibiting motoneurons in Ascaris
Avermectins and milbemycins
Glutamate-gated chloride channels and the mode of action of the avermectin/ milbemycin anthelmintics
A serine protease was purified 942-fold from culture supernatant of L. amazonensis promastigotes using (NH4)2SO4 precipitation followed by affinity chromatography on aprotinin-agarose and continuous elution electrophoresis by Prep Cell, yielding a total recovery of 61%. The molecular mass of the active enzyme estimated by SDS-PAGE under conditions of reduction was 56 kDa and 115 kDa under conditions of non-reduction, suggesting that the protease is a dimeric protein. Additionally, it was found to be a non-glycosylated enzyme, with a pI of 5.0. The optimal pH and temperature of the enzyme were 7.5 and 28 degrees C respectively, using alpha-N-rho-tosyl-L-arginine-methyl ester (L-TAME) as substrate. Assays of thermal stability indicated that 61% of the enzyme activity was preserved after 1 h of pre-treatment at 42 degrees C. Haemoglobin, bovine serum albumin (BSA), ovalbumin, fibrinogen, collagen, gelatin and peptide substrates containing arginine in an ester bond and amide substrates containing hydrophobic residues at the P1 site were hydrolysed by this extracellular protease. The insulin beta-chain was also hydrolysed by the enzyme and many peptidic bonds were susceptible to the protease action, and 4 of them (L11-V12, E3-A14, L15-Y16 and Y16-L17) were identified. Inhibition studies suggested that the enzyme belongs to the serine protease class inhibited by calcium and manganese and activated by zinc. These findings show that this enzyme of L. amazonensis is a novel serine protease, which differs from all known flagellate proteases characterized.
Anthelmintic avermectins kill Mycobacterium tuberculosis, including multidrug-resistant clinical strains
Reversal of meticillin resistance in Staphylococcus aureus by the anthelmintic avermectin
Ivermectin: Panacea for resource-poor communities
The multitargeted drug ivermectin: from an antiparasitic agent to a repositioned cancer drug
Cachexia is a multifactorial paraneoplastic syndrome commonly associated with advanced stages of cancer. Cachexia is responsible for poor responses to antitumoral treatment and death in close to one-third of affected patients. There is still an incomplete understanding of the metabolic dysregulation induced by a tumor that leads to the appearance and persistence of cachexia. Furthermore,... [Show full abstract]
Organization of the biosynthetic gene cluster for the polyketide anthelmintic macrolide avermectin in Streptomyces avermitilis
Interrogation of Streptomyces avermitilis for efficient production of avermectins
Construction of a single-component producer from the wild-type avermectin producer Streptomyces avermitilis
Semi-synthetic DNA shuffling of aveC leads to improved industrial scale production of doramectin by Streptomyces avermitilis
Spiroketal formation and modification in avermectin biosynthesis involves a dual activity of AveC
Genome sequence of an industrial microorganism Streptomyces avermitilis: deducing the ability of producing secondary metabolites
Complete genome sequence and comparative analysis of the industrial microorganism Streptomyces avermitilis
The pathway- specific regulator AveR from Streptomyces avermitilis positively regulates avermectin production while it negatively affects oligomycin biosynthesis
Characterization of a regulatory gene, aveR, for the biosynthesis of avermectin in Streptomyces avermitilis
Reverse biological engineering of hrdB to enhance the production of avermectins in an industrial strain of Streptomyces avermitilis
The PhoP transcription factor negatively regulates avermectin biosynthesis in Streptomyces avermitilis
Redox-sensing regulator Rex regulates aerobic metabolism, morphological differentiation, and avermectin production in Streptomyces avermitilis
AvaR1, a butenolide-type autoregulator receptor in Streptomyces avermitilis, directly represses avenolide and avermectin biosynthesis and multiple physiological responses
AvaR2, a pseudo γ-butyrolactone receptor homologue from Streptomyces avermitilis, is a pleiotropic repressor of avermectin and avenolide biosynthesis and cell growth
Direct involvement of the master nitrogen metabolism regulator GlnR in antibiotic biosynthesis in Streptomyces
SAV742, a novel AraC-family regulator from Streptomyces avermitilis, controls avermectin biosynthesis, cell growth and development
Characterization of a negative regulator AveI for avermectin biosynthesis in Streptomyces avermitilis NRRL8165
Transcriptomics analyses reveal global roles of the regulator AveI in Streptomyces avermitilis
Characterization of SAV7471, a TetR-family transcriptional regulator involved in the regulation of coenzyme A metabolism in Streptomyces avermitilis
Engineering of the TetR family transcriptional regulator SAV151 and its target genes increases avermectin production in Streptomyces avermitilis
A novel TetR family transcriptional regulator, SAV576, negatively controls avermectin biosynthesis in Streptomyces avermitilis
Two adjacent and similar TetR family transcriptional regulator genes, SAV577 and SAV576, co-regulate avermectin production in Streptomyces avermitilis
Increasing avermectin production in Streptomyces avermitilis by manipulating the expression of a novel TetR-family regulator and its target gene product
The autoregulator receptor homologue AvaR3 plays a regulatory role in antibiotic production, mycelial aggregation and colony development of Streptomyces avermitilis
SAV4189, a MarR-family regulator in Streptomyces avermitilis, activates avermectin biosynthesis
Inactivation of the extracytoplasmic function sigma factor Sig6 stimulates avermectin production in Streptomyces avermitilis
An extracytoplasmic function sigma factor, σ 25, differentially regulates avermectin and oligomycin biosynthesis in Streptomyces avermitilis
An alternative σ factor, σ 8, controls avermectin production and multiple stress responses in Streptomyces avermitilis
Avenolide, a Streptomyces hormone controlling antibiotic production in Streptomyces avermitilis
Ethanol evolution rate: a new parameter to determine the feeding rate for the production of avermectins by Streptomyces avermitilis
Optimization of avermectin fermentation process based on parameter association analysis
阿维菌素发酵过程参数相关特性研究及过程优化
Effect of inoculation methods on avermectin fermentation by streptomyces avermilitis
在摇瓶发酵中对除虫链霉菌的孢子接种工艺进行了初步研究,同时比较了孢子接种、常规的挖块接种和菌丝接种3种不同接种方式对除虫链霉菌菌体生长和细胞代谢参数的影响。实验结果表明:孢子接种的最适孢子接种量在4~6×10^6个/mL范围内,而不同接种方式导致了发酵过程除虫链霉菌营养物质消耗、PH值变化、菌丝形态以及效价等方面差异。孢子接种方式在摇瓶发酵中表现出明显优势,采用孢子接种方式获得最终阿维菌素的效价比常规的挖块接种方式提高10%以上,比菌丝接种方式提高了20%以上。
接种方式对阿维菌素发酵过程的影响
在摇瓶发酵中对除虫链霉菌的孢子接种工艺进行了初步研究,同时比较了孢子接种、常规的挖块接种和菌丝接种3种不同接种方式对除虫链霉菌菌体生长和细胞代谢参数的影响。实验结果表明:孢子接种的最适孢子接种量在4~6×10^6个/mL范围内,而不同接种方式导致了发酵过程除虫链霉菌营养物质消耗、PH值变化、菌丝形态以及效价等方面差异。孢子接种方式在摇瓶发酵中表现出明显优势,采用孢子接种方式获得最终阿维菌素的效价比常规的挖块接种方式提高10%以上,比菌丝接种方式提高了20%以上。
Medium optimization for the production of avermectin B1a by Streptomyces avermitilis 14-12A using response surface methodology
Identification of avermectin-high-producing strains by high-throughput screening methods
Avermectins produced by Streptomyces avermitilis are potent against a broad spectrum of nematode and arthropod parasites with low-level side effects on the host organisms. This study was designed to investigate a high-throughput screening strategy for the efficient identification of avermectin high-yield strains. The production protocol was miniaturized in 96 deep-well microplates. UV absorbance at 245nm was used to monitor avermectin production. A good correlation between fermentation results in both 96 deep-well microplates and conventional Erlenmeyer flasks was observed. With this protocol, the production of avermectins was determined in less than 10min for a full plate without compromising accuracy. The high-yield strain selected through this protocol was also tested in 360m 3 batch fermentation with 1.6-fold improved outcome. Thus, the development of this protocol is expected to accelerate the selection of superior avermectin-producing strains.
Assessing the potential of an induced-mutation strategy for avermectin overproducers
Mutant libraries of avermectin-producing Streptomyces avermitilis strains were constructed by different mutagenesis strategies. A metric was applied to assess the mutation spectrum by calculating the distribution of average phenotypic distance of each population. The results showed for the first time that a microgravity environment could introduce larger phenotype distribution and diversity than UV and N-methyl-N-nitro-N-nitrosoguanidine (NTG) could.
Magnetic field is the dominant factor to induce the response of Streptomyces avermitilis in altered gravity simulated by diamagnetic levitation
Enhancement of avermectin and ivermectin production by overexpression of the maltose ATP-binding cassette transporter in Streptomyces avermiilis
Overexpression of ribosome recycling factor causes increased production of avermectin in Streptomyces avermitilis strains
Overexpression of the ABC transporter AvtAB increases avermectin production in Streptomyces avermitilis
Overexpression of metK shows different effects on avermectin production in various Streptomyces avermitilis strains
Comparative metabolomics reveals the mechanism of avermectin production enhancement by S-adenosylmethionine
Direct production of ivermectin- like drugs after domain exchange in the avermectin polyketide synthase of Streptomyces avermitilis ATCC31272
Deletion analysis of oligomycin PKS genes ( olmA) in Streptomyces avermitilis
Construction of ivermectin producer by domain swaps of avermectin polyketide synthase in Streptomyces avermitilis
Engineering of avermectin biosynthetic genes to improve production of ivermectin in Streptomyces avermitilis
Designed biosynthesis of 25-methyl and 25-ethyl ivermectin with enhanced insecticidal activity by domain swap of avermectin polyketide synthase
Identification of a cyclohexylcarbonyl CoA biosynthetic gene cluster and application in the production of doramectin
Production of doramectin by rational engineering of the avermectin biosynthetic pathway
Abstract In an attempt to construct a strain that produces doramectin, the loading module of Ave polyketide synthase (PKS) from Streptomyces avermitilis M1 was replaced with a cyclohexanecarboxylic (CHC) unique loading module from phoslactomycin PKS. Additionally, the CHC-CoA biosynthetic gene cassette was introduced into the engineered strain, which provided the precursor for directed biosynthesis of doramectin. The doramectin production ability of the final mutant S. avermitilis TG2002 was increased about six times and the ratio of Dor to Ave was enhanced 300 times more than the original strain. Copyright 2011 Elsevier Ltd. All rights reserved.
1,19-seco- avermectin analogues from a ΔaveCDE mutant Streptomyces avermectinius strain
Structural and functional analysis of the loading acyltransferase from avermectin modular polyketide synthase
Synthetic biology of avermectin for production improvement and structure diversification
Natural products are still key sources of current clinical drugs and innovative therapeutic agents. Since wild-type microorganisms only produce natural products in very small quantities, yields of production strains need to be improved by breaking down the precise genetic and biochemical circuitry. Herein, we use avermectins as an example of production improvement and chemical structure diversification by synthetic biology. Avermectins are macrocyclic lactones produced by Streptomyces avermitilis and are well known and widely used for antiparasitic therapy. Given the importance of this molecule and its derivatives, many efforts and strategies were employed to improve avermectin production and generate new active analogues. This review describes the current status of synthetic strategies successfully applied for developing natural-product-producing strains and discusses future prospects for the application of enhanced avermectin production.
Genome mining of the Streptomyces avermitilis genome and development of genome-minimized hosts for heterologous expression of biosynthetic gene clusters
Genome-minimized Streptomyces host for the heterologous expression of secondary metabolism
To construct a versatile model host for heterologous expression of genes encoding secondary metabolite biosynthesis, the genome of the industrial microorganism Streptomyces avermitilis was systematically deleted to remove nonessential genes. A region of more than 1.4 Mb was deleted stepwise from the 9.02-Mb S. avermitilis linear chromosome to generate a series of defined deletion mutants, corresponding to 83.12-81.46% of the wild-type chromosome, that did not produce any of the major endogenous secondary metabolites found in the parent strain. The suitability of the mutants as hosts for efficient production of foreign metabolites was shown by heterologous expression of three different exogenous biosynthetic gene clusters encoding the biosynthesis of streptomycin (from S. griseus Institute for Fermentation, Osaka [IFO] 13350), cephamycin C (from S. clavuligerus American type culture collection (ATCC) 27064), and pladienolide (from S. platensis Mer-11107). Both streptomycin and cephamycin C were efficiently produced by individual transformants at levels higher than those of the native-producing species. Although pladienolide was not produced by a deletion mutant transformed with the corresponding intact biosynthetic gene cluster, production of the macrolide was enabled by introduction of an extra copy of the regulatory gene pldR expressed under control of an alternative promoter. Another mutant optimized for terpenoid production efficiently produced the plant terpenoid intermediate, amorpha-4,11-diene, by introduction of a synthetic gene optimized for Streptomyces codon usage. These findings highlight the strength and flexibility of engineered S. avermitilis as a model host for heterologous gene expression, resulting in the production of exogenous natural and unnatural metabolites.
Engineered Streptomyces avermitilis host for heterologous expression of biosynthetic gene cluster for secondary metabolites
Shin-ya K, Cane DE, Ikeda H. Novel terpenes generated by heterologous expression of bacterial terpene synthase genes in an engineered Streptomyces host
Exploiting a precise design of universal synthetic modular regulatory elements to unlock the microbial natural products in Streptomyces
Learn from microbial intelligence for avermectins overproduction
Abstract Microbial strains are amazingly clever by homeostasis of their own survival and optimization for the overproduction of a desired phenotype, for example drugable secondary metabolites through coordination of key genes overexpression and media optimizations. Besides their pesticide activities, avermectins (AVMs) are identified as potent antibiotic agents for a wide range of drug-resistant pathogens by a high-throughput synergy screening strategy. To rewire the genetic circuitry controlling low yields, we summarized the work on balancing the biological chassis with functional parts, and optimized their dynamical process, as well as predicted favorable effective overproduction of AVMs by 5Ms strategy. AVMs are exclusively made in China now and intelligences learned from the success of AVMs will help transform microbes into a true power-house of innovation. Copyright 2017. Published by Elsevier Ltd.