遗传 ›› 2025, Vol. 47 ›› Issue (7): 711-728.doi: 10.16288/j.yczz.25-022
收稿日期:2025-01-20
修回日期:2025-05-08
出版日期:2025-05-09
发布日期:2025-05-09
通讯作者:
田烨,博士,研究员,研究方向:线粒体应激反应和衰老。E-mail: ytian@genetics.ac.cn作者简介:张宁,博士,研究方向:细胞生物学。E-mail: zn19951211@163.com
基金资助:Received:2025-01-20
Revised:2025-05-08
Published:2025-05-09
Online:2025-05-09
Supported by:摘要:
荧光蛋白的发现为细胞生物学研究带来了革命性进展。通过将荧光蛋白与目标蛋白融合,构建荧光生物探针,可以在活细胞和生物体内实时监测细胞事件的动态变化。荧光蛋白的独特理化特性,如光谱范围、发色团成熟速度、pH敏感性和稳定性等,为探针的设计提供了多样化的选择。基于这些特性,研究者开发了用于监测不同分子事件的多种荧光生物探针。本文系统总结了荧光蛋白探针设计的主要策略及其在生物学研究中的典型应用,为开发更高效、更专用的新型荧光生物探针以应对复杂生物学问题提供了参考。
张宁, 田烨. 基于荧光蛋白的生物探针设计策略及其应用[J]. 遗传, 2025, 47(7): 711-728.
Ning Zhang, Ye Tian. Design strategies and applications of fluorescent protein-based probes[J]. Hereditas(Beijing), 2025, 47(7): 711-728.
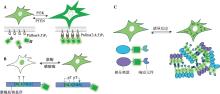
图1
利用单一荧光蛋白的空间信息构建的生物探针示例 A:PtdIns(3,4,5)P3荧光探针动态变化示意图。在细胞膜上,PI3K催化PtdIns(4,5)P2生成PtdIns(3,4,5)P3,PTEN可拮抗这一过程。通过设计能特异结合PtdIns(3,4,5)P3的荧光探针,可以实时监测其动态变化。B:指示激酶活性的荧光探针示意图。激酶底物序列由底物识别基序、NLS、NES和荧光蛋白组成。NLS和NES上带有激酶磷酸化位点(如T位点),当激酶介导底物磷酸化时,会抑制NLS的活性并激活NES的功能,导致荧光探针从细胞核转移至细胞质。通过观察荧光信号的空间分布变化,可评估激酶的活性。C:基于液-液相分离的荧光探针响应分子事件的示意图。通过将荧光蛋白与响应分子事件的蛋白或多肽标记融合,再与具有液-液相分离能力的分子融合构建探针。当目标分子事件发生时,响应分子间相互作用会促成液-液相分离,形成含有强荧光信号的液滴。通过观察液滴的出现及分布,可实现对分子事件的灵敏检测。"
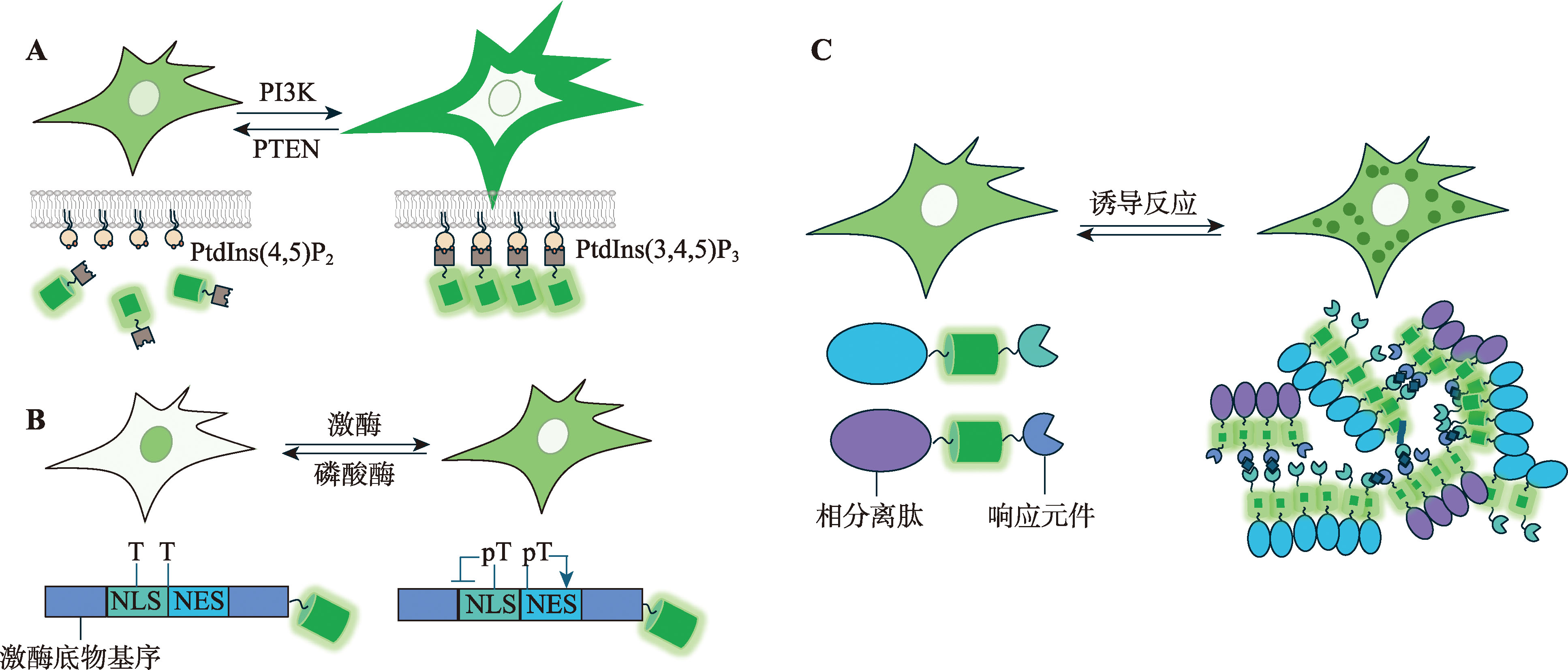
图2
利用FT、pH敏感荧光蛋白、cpFP和BiFC设计生物探针的示意图 A:荧光时钟随时间变化的示意图。B:基于pH敏感荧光蛋白设计的探针示意图。通过将pH敏感的GFP与pH不敏感的mRFP串联并融合LC3构建探针,用于监测自噬过程。当自噬体成熟时,其内部pH降低,导致GFP荧光强度下降,而mRFP的荧光强度保持不变,从而反映自噬过程的动态变化。C:基于cpFP设计探针的示意图。目标信号分子诱导探针构象发生变化,从而增强cpFP的荧光强度,实现对目标分子活动的灵敏监测。D:基于cpFP设计的检测GPCR激活的探针示意图。GPCR激活后发生构象改变,驱动cpFP的荧光强度增加,指示GPCR信号的激活状态。E:基于BiFC设计探针的示意图。当目标蛋白发生寡聚化后,分离的BiFC片段(如VN和VC)通过复合重构,生成可检测的荧光信号,用于监测蛋白相互作用或寡聚化过程。F:基于TriFC设计探针的示意图。FP 10和FP 11单独时无法与FP 1~9重组。当FP 10和FP 11分别与目标蛋白融合后,通过目标蛋白的相互作用使它们相邻,随后与FP 1~9结合,重构完整的荧光蛋白并产生荧光信号,从而实现对目标蛋白相互作用的检测。"
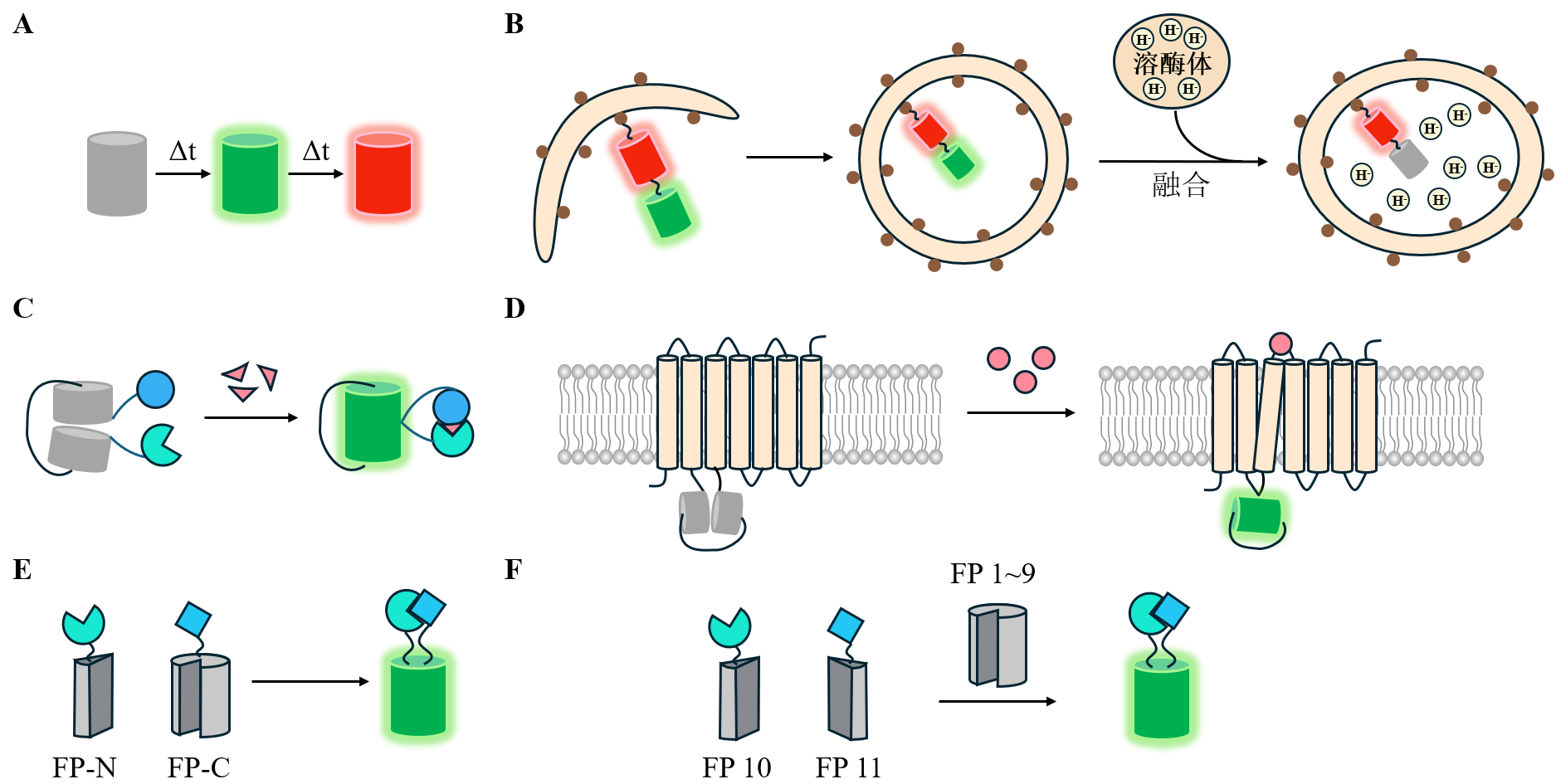
| [64] |
Asanova AN, Subach OM, Myachina SA, Evteeva MA, Gunitseva NM, Borisova AA, Patrushev MV, Vlaskina AV, Nikolaeva AY, Yang LN, Gabdulkhakov A, Dronova E, Samygina VR, Xiao X, Zhao H, Piatkevich KD, Subach FV. NeIle, a genetically encoded indicator for branched-chain amino acids based on mNeonGreen fluorescent protein and LIVBP protein. ACS Sens, 2024, 9(10): 5135-5147.
pmid: 39400357 |
| [65] |
Lee HM, Choi DW, Kim S, Lee A, Kim M, Roh YJ, Jo YH, Cho HY, Lee HJ, Lee SR, Tarrago L, Gladyshev VN, Kim JH, Lee BC. Biosensor-linked immunosorbent assay for the quantification of methionine oxidation in target proteins. ACS Sens, 2022, 7(1): 131-141.
pmid: 35145320 |
| [66] |
Marvin JS, Scholl B, Wilson DE, Podgorski K, Kazemipour A, Müller JA, Schoch S, Quiroz FJU, Rebola N, Bao H, Little JP, Tkachuk AN, Cai E, Hantman AW, Wang SSH, DePiero VJ, Borghuis BG, Chapman ER, Dietrich D, DiGregorio DA, Fitzpatrick D, Looger LL. Stability, affinity, and chromatic variants of the glutamate sensor iGluSnFR. Nat Methods, 2018, 15(11): 936-939.
pmid: 30377363 |
| [67] |
Marvin JS, Shimoda Y, Magloire V, Leite M, Kawashima T, Jensen TP, Kolb I, Knott EL, Novak O, Podgorski K, Leidenheimer NJ, Rusakov DA, Ahrens MB, Kullmann DM, Looger LL. A genetically encoded fluorescent sensor for in vivo imaging of GABA. Nat Methods, 2019, 16(8): 763-770.
pmid: 31308547 |
| [68] |
Kubitschke M, Müller M, Wallhorn L, Pulin M, Mittag M, Pollok S, Ziebarth T, Bremshey S, Gerdey J, Claussen KC, Renken K, Groß J, Gneiße P, Meyer N, Wiegert JS, Reiner A, Fuhrmann M, Masseck OA. Next generation genetically encoded fluorescent sensors for serotonin. Nat Commun, 2022, 13(1): 7525.
pmid: 36473867 |
| [69] |
Chamberland S, Yang HH, Pan MM, Evans SW, Guan SH, Chavarha M, Yang Y, Salesse C, Wu HD, Wu JC, Clandinin TR, Toth K, Lin MZ, St-Pierre F. Fast two-photon imaging of subcellular voltage dynamics in neuronal tissue with genetically encoded indicators. eLife, 2017, 6: e25690.
pmid: 28749338 |
| [70] |
St-Pierre F, Marshall JD, Yang Y, Gong YY, Schnitzer MJ, Lin MZ. High-fidelity optical reporting of neuronal electrical activity with an ultrafast fluorescent voltage sensor. Nat Neurosci, 2014, 17(6): 884-889.
pmid: 24755780 |
| [71] |
Villette V, Chavarha M, Dimov IK, Bradley J, Pradhan L, Mathieu B, Evans SW, Chamberland S, Shi DQ, Yang RZ, Kim BB, Ayon A, Jalil A, St-Pierre F, Schnitzer MJ, Bi GQ, Toth K, Ding J, Dieudonné S, Lin MZ. Ultrafast two-photon imaging of a high-gain voltage indicator in awake behaving mice. Cell, 2019, 179(7): 1590-1608.e23.
pmid: 31835034 |
| [72] |
Hsu YY, Resto Irizarry AM, Fu JP, Liu AP. Mechanosensitive channel-based optical membrane tension reporter. ACS Sens, 2023, 8(1): 12-18.
pmid: 36608338 |
| [73] |
Weis WI, Kobilka BK. The molecular basis of G protein-coupled receptor activation. Annu Rev Biochem, 2018, 87: 897-919.
pmid: 29925258 |
| [74] |
Sun FM, Zeng JZ, Jing M, Zhou JH, Feng JS, Owen SF, Luo YC, Li FN, Wang H, Yamaguchi T, Yong ZH, Gao YJ, Peng WL, Wang LZ, Zhang SY, Du JL, Lin DY, Xu M, Kreitzer AC, Cui GH, Li YL. A genetically encoded fluorescent sensor enables rapid and specific detection of dopamine in flies, fish, and mice. Cell, 2018, 174(2): 481-496.e19.
pmid: 30007419 |
| [75] |
Patriarchi T, Cho JR, Merten K, Howe MW, Marley A, Xiong WH, Folk RW, Broussard GJ, Liang RQ, Jang MJ, Zhong HN, Dombeck D, Von Zastrow M, Nimmerjahn A, Gradinaru V, Williams JT, Tian L. Ultrafast neuronal imaging of dopamine dynamics with designed genetically encoded sensors. Science, 2018, 360(6396): eaat4422.
pmid: 29853555 |
| [76] |
Jing M, Zhang P, Wang GF, Feng JS, Mesik L, Zeng JZ, Jiang HQ, Wang SH, Looby JC, Guagliardo NA, Langma LW, Lu J, Zuo Y, Talmage DA, Role LW, Barrett PQ, Zhang LI, Luo MM, Song Y, Zhu JJ, Li YL. A genetically encoded fluorescent acetylcholine indicator for in vitro and in vivo studies. Nat Biotechnol, 2018, 36(8): 726-737.
pmid: 29985477 |
| [77] |
Feng JS, Zhang CM, Lischinsky JE, Jing M, Zhou JH, Wang H, Zhang YJ, Dong A, Wu ZF, Wu H, Chen WY, Zhang P, Zou J, Hires SA, Zhu JJ, Cui GH, Lin DY, Du JL, Li YL. A genetically encoded fluorescent sensor for rapid and specific in vivo detection of norepinephrine. Neuron, 2019, 102(4): 745-761.e8.
pmid: 30922875 |
| [78] |
Wu ZF, Cui YT, Wang H, Wu H, Wan Y, Li BH, Wang L, Pan SL, Peng WL, Dong A, Yuan ZW, Jing M, Xu M, Luo MM, Li YL. Neuronal activity-induced, equilibrative nucleoside transporter-dependent, somatodendritic adenosine release revealed by a GRAB sensor. Proc Natl Acad Sci USA, 2023, 120(14): e2212387120.
pmid: 36996110 |
| [79] |
Wan JX, Peng WL, Li XL, Qian TR, Song K, Zeng JZ, Deng F, Hao SY, Feng JS, Zhang P, Zhang YJ, Zou J, Pan SL, Shin MM, Venton BJ, Zhu JJ, Jing M, Xu M, Li YL. A genetically encoded sensor for measuring serotonin dynamics. Nat Neurosci, 2021, 24(5): 746-752.
pmid: 33821000 |
| [80] |
Duffet L, Williams ET, Gresch A, Chen SM, Bhat MA, Benke D, Hartrampf N, Patriarchi T. Optical tools for visualizing and controlling human GLP-1 receptor activation with high spatiotemporal resolution. eLife, 2023, 12: RP86628.
pmid: 37265064 |
| [1] |
Gest AMM, Sahan AZ, Zhong YH, Lin W, Mehta S, Zhang J. Molecular spies in action: genetically encoded fluorescent biosensors light up cellular signals. Chem Rev, 2024, 124(22): 12573-12660.
pmid: 39535501 |
| [2] |
Reja SI, Minoshima M, Hori Y, Kikuchi K. Recent advancements of fluorescent biosensors using semisynthetic probes. Biosens Bioelectron, 2024, 247: 115862.
pmid: 38147718 |
| [3] |
Syed AJ, Anderson JC. Applications of bioluminescence in biotechnology and beyond. Chem Soc Rev, 2021, 50(9): 5668-5705.
pmid: 33735357 |
| [4] |
Wu ZS, Du YT, Kirchhausen T, He KM. Probing and imaging phospholipid dynamics in live cells. Life Metab, 2024, 3(4): loae014.
pmid: 39872507 |
| [5] |
Rosen SAJ, Gaffney PRJ, Spiess B, Gould IR. Understanding the relative affinity and specificity of the pleckstrin homology domain of protein kinase B for inositol phosphates. Phys Chem Chem Phys, 2012, 14(2): 929-936.
pmid: 22121510 |
| [6] |
Dickson EJ, Hille B. Understanding phosphoinositides: rare, dynamic, and essential membrane phospholipids. Biochem J, 2019, 476(1): 1-23.
pmid: 30617162 |
| [7] |
Thallmair V, Schultz L, Zhao WC, Marrink SJ, Oliver D, Thallmair S. Two cooperative binding sites sensitize PI(4,5)P2 recognition by the tubby domain. Sci Adv, 2022, 8(36): eabp9471.
pmid: 36070381 |
| [8] |
Wang J, An ZY, Wu ZS, Zhou W, Sun PY, Wu PY, Dang S, Xue R, Bai X, Du YT, Chen RM, Wang WX, Huang P, Lam SM, Ai YW, Liu SL, Shui GH, Zhang Z, Liu Z, Huang JY, Fang XH, He KM. Spatial organization of PI3K-PI(3,4,5)P3-AKT signaling by focal adhesions. Mol Cell, 2024, 84(22): 4401-4418.e9.
pmid: 39488211 |
| [9] |
Zhang F, Wang ZQ, Lu M, Yonekubo Y, Liang X, Zhang YQ, Wu P, Zhou Y, Grinstein S, Hancock JF, Du GW. Temporal production of the signaling lipid phosphatidic acid by phospholipase D2 determines the output of extracellular signal-regulated kinase signaling in cancer cells. Mol Cell Biol, 2014, 34(1): 84-95.
pmid: 24164897 |
| [10] |
Chung J, Torta F, Masai K, Lucast L, Czapla H, Tanner LB, Narayanaswamy P, Wenk MR, Nakatsu F, De Camilli P. Intracellular transport. PI4P/phosphatidylserine countertransport at ORP5- and ORP8-mediated ER-plasma membrane contacts. Science, 2015, 349(6246): 428-432.
pmid: 26206935 |
| [81] |
Yang F, Moss LG, Phillips Jr GN. The molecular structure of green fluorescent protein. Nat Biotechnol, 1996, 14(10): 1246-1251.
pmid: 9631087 |
| [82] |
Ghosh I, Hamilton AD, Regan L. Antiparallel leucine zipper-directed protein reassembly: application to the green fluorescent protein. J Am Chem Soc, 2000, 122(23): 5658-5659.
pmid: ja994421w |
| [83] |
Hu CD, Chinenov Y, Kerppola TK. Visualization of interactions among bZIP and Rel family proteins in living cells using bimolecular fluorescence complementation. Mol Cell, 2002, 9(4): 789-798.
pmid: 11983170 |
| [84] |
Shyu YJ, Liu H, Deng XH, Hu CD. Identification of new fluorescent protein fragments for bimolecular fluorescence complementation analysis under physiological conditions. Biotechniques, 2006, 40(1): 61-66.
pmid: 16454041 |
| [85] |
Chu J, Zhang ZH, Zheng Y, Yang J, Qin LS, Lu JL, Huang ZL, Zeng SQ, Luo QM. A novel far-red bimolecular fluorescence complementation system that allows for efficient visualization of protein interactions under physiological conditions. Biosens Bioelectron, 2009, 25(1): 234-239.
pmid: 19596565 |
| [86] |
Fan JY, Cui ZQ, Wei HP, Zhang ZP, Zhou YF, Wang YP, Zhang XE. Split mCherry as a new red bimolecular fluorescence complementation system for visualizing protein-protein interactions in living cells. Biochem Biophys Res Commun, 2008, 367(1): 47-53.
pmid: 18158915 |
| [87] |
Jach G, Pesch M, Richter K, Frings S, Uhrig JF. An improved mRFP1 adds red to bimolecular fluorescence complementation. Nat Methods, 2006, 3(8): 597-600.
pmid: 16862132 |
| [88] |
Henderson MX, Trojanowski JQ, Lee VMY. α-Synuclein pathology in Parkinson's disease and related α-synucleinopathies. Neurosci Lett, 2019, 709: 134316.
pmid: 31170426 |
| [89] |
Bae EJ, Lee HJ, Lee SJ. Cell models to study cell-to-cell transmission of α-synuclein. Methods Mol Biol, 2016, 1345: 291-298.
pmid: 26453220 |
| [90] |
Frey B, AlOkda A, Jackson MP, Riguet N, Duce JA, Lashuel HA. Monitoring alpha-synuclein oligomerization and aggregation using bimolecular fluorescence complementation assays: what you see is not always what you get. J Neurochem, 2021, 157(4): 872-888.
pmid: 32772367 |
| [91] |
Tak H, Haque MM, Kim MJ, Lee JH, Baik JH, Kim Y, Kim DJ, Grailhe R, Kim YK. Bimolecular fluorescence complementation; lighting-up tau-tau interaction in living cells. PLoS One, 2013, 8(12): e81682.
pmid: 24312574 |
| [11] |
Kudo T, Jeknić S, Macklin DN, Akhter S, Hughey JJ, Regot S, Covert MW. Live-cell measurements of kinase activity in single cells using translocation reporters. Nat Protoc, 2017, 13(1): 155-169.
pmid: 29266096 |
| [12] |
Zhang Q, Huang H, Zhang LQ, Wu R, Chung CI, Zhang SQ, Torra J, Schepis A, Coughlin SR, Kornberg TB, Shu XK. Visualizing dynamics of cell signaling in vivo with a phase separation-based kinase reporter. Mol Cell, 2018, 69(2): 347.
pmid: 29351851 |
| [13] |
Chung CI, Zhang Q, Shu XK. Dynamic imaging of small molecule induced protein-protein interactions in living cells with a fluorophore phase transition based approach. Anal Chem, 2018, 90(24): 14287-14293.
pmid: 30431263 |
| [14] |
Brown MC, Turner CE. Paxillin: adapting to change. Physiol Rev, 2004, 84(4): 1315-1339.
pmid: 15383653 |
| [15] |
Hirano M, Ando R, Shimozono S, Sugiyama M, Takeda N, Kurokawa H, Deguchi R, Endo K, Haga K, Takai- Todaka R, Inaura S, Matsumura Y, Hama H, Okada Y, Fujiwara T, Morimoto T, Katayama K, Miyawaki A. A highly photostable and bright green fluorescent protein. Nat Biotechnol, 2022, 40(7): 1132-1142.
pmid: 35468954 |
| [16] |
Ando R, Shimozono S, Ago H, Takagi M, Sugiyama M, Kurokawa H, Hirano M, Niino Y, Ueno G, Ishidate F, Fujiwara T, Okada Y, Yamamoto M, Miyawaki A. StayGold variants for molecular fusion and membrane- targeting applications. Nat Methods, 2024, 21(4): 648-656.
pmid: 38036853 |
| [17] |
Matz MV, Fradkov AF, Labas YA, Savitsky AP, Zaraisky AG, Markelov ML, Lukyanov SA. Fluorescent proteins from nonbioluminescent Anthozoa species. Nat Biotechnol, 1999, 17(10): 969-973.
pmid: 10504696 |
| [18] |
Terskikh A, Fradkov A, Ermakova G, Zaraisky A, Tan P, Kajava AV, Zhao X, Lukyanov S, Matz M, Kim S, Weissman I, Siebert P. "Fluorescent timer": protein that changes color with time. Science, 2000, 290(5496): 1585-1588.
pmid: 11090358 |
| [19] |
Subach OM, Tashkeev A, Vlaskina AV, Petrenko DE, Gaivoronskii FA, Nikolaeva AY, Ivashkina OI, Anokhin KV, Popov VO, Boyko KM, Subach FV. The mRubyFT protein, genetically encoded blue-to-red fluorescent timer. Int J Mol Sci, 2022, 23(6): 3208.
pmid: 35328628 |
| [92] |
Shin S, Kim D, Song JY, Jeong H, Hyeon SJ, Kowall NW, Ryu H, Pae AN, Lim S, Kim YK. Visualization of soluble tau oligomers in TauP301L-BiFC transgenic mice demonstrates the progression of tauopathy. Prog Neurobiol, 2020, 101782.
pmid: 32105751 |
| [93] |
Feng SY, Sekine S, Pessino V, Li H, Leonetti MD, Huang B. Improved split fluorescent proteins for endogenous protein labeling. Nat Commun, 2017, 8(1): 370.
pmid: 28851864 |
| [94] |
To TL, Schepis A, Ruiz-González R, Zhang Q, Yu D, Dong ZQ, Coughlin SR, Shu XK. Rational design of a GFP-based fluorogenic caspase reporter for imaging apoptosis in vivo. Cell Chem Biol, 2016, 23(7): 875-882.
pmid: 27447051 |
| [95] |
Cabantous S, Nguyen HB, Pedelacq JD, Koraïchi F, Chaudhary A, Ganguly K, Lockard MA, Favre G, Terwilliger TC, Waldo GS. A new protein-protein interaction sensor based on tripartite split-GFP association. Sci Rep, 2013, 3: 2854.
pmid: 24092409 |
| [96] |
Zhang Q, Zheng YW, Coughlin SR, Shu XK. A rapid fluorogenic GPCR-β-arrestin interaction assay. Protein Sci, 2018, 27(4): 874-879.
pmid: 29411438 |
| [97] |
Hu CD, Kerppola TK. Simultaneous visualization of multiple protein interactions in living cells using multicolor fluorescence complementation analysis. Nat Biotechnol, 2003, 21(5): 539-545.
pmid: 12692560 |
| [98] |
Yin J, Zhu DH, Zhang ZP, Wang W, Fan JY, Men D, Deng JY, Wei HP, Zhang XE, Cui ZQ. Imaging of mRNA-protein interactions in live cells using novel mCherry trimolecular fluorescence complementation systems. PLoS One, 2013, 8(11): e80851.
pmid: 24260494 |
| [99] |
Han Y, Wang SF, Zhang ZP, Ma XH, Li W, Zhang XW, Deng JY, Wei HP, Li ZY, Zhang XE, Cui ZQ. In vivo imaging of protein-protein and RNA-protein interactions using novel far-red fluorescence complementation systems. Nucleic Acids Res, 2014, 42(13): e103.
pmid: 24813442 |
| [100] |
Chen M, Sui TT, Yang L, Qian YQ, Liu ZQ, Liu YS, Wang GR, Lai LX, Li ZJ. Live imaging of RNA and RNA splicing in mammalian cells via the dcas13a- SunTag-BiFC system. Biosens Bioelectron, 2022, 204: 114074.
pmid: 35149451 |
| [101] |
Hu H, Yang XJ, Tang C. Visualization of genomic loci in living cells with BiFC-TALE. Curr Protoc Cell Biol, 2019, 82(1): e78.
pmid: 30375749 |
| [20] |
Subach OM, Vlaskina AV, Agapova YK, Nikolaeva AY, Anokhin KV, Piatkevich KD, Patrushev MV, Boyko KM, Subach FV. Blue-to-red TagFT, mTagFT, mTsFT, and green-to-farred mNeptusFT2 proteins, genetically encoded true and tandem fluorescent timers. Int J Mol Sci, 2023, 24(4): 3279.
pmid: 36834686 |
| [21] |
Tsuboi T, Kitaguchi T, Karasawa S, Fukuda M, Miyawaki A. Age-dependent preferential dense-core vesicle exocytosis in neuroendocrine cells revealed by newly developed monomeric fluorescent timer protein. Mol Biol Cell, 2009, 21(1): 87-94.
pmid: 19889833 |
| [22] |
Terada Y, Obara K, Yoshioka Y, Ochiya T, Bito H, Tsuchida K, Ageta H, Ageta-Ishihara N. Intracellular dynamics of ubiquitin-like 3 visualized using an inducible fluorescent timer expression system. Biol Open, 2024, 13(11): bio060345.
pmid: 39498724 |
| [23] |
Subach FV, Subach OM, Gundorov IS, Morozova KS, Piatkevich KD, Cuervo AM, Verkhusha VV. Monomeric fluorescent timers that change color from blue to red report on cellular trafficking. Nat Chem Biol, 2009, 5(2): 118-126.
pmid: 19136976 |
| [24] |
Yau B, Hays L, Liang C, Laybutt DR, Thomas HE, Gunton JE, Williams L, Hawthorne WJ, Thorn P, Rhodes CJ, Kebede MA. A fluorescent timer reporter enables sorting of insulin secretory granules by age. J Biol Chem, 2020, 295(27): 8901-8911.
pmid: 32341128 |
| [25] |
Ono M. Unraveling T-cell dynamics using fluorescent timer: insights from the Tocky system. Biophys Physicobiol, 2024, 21(Supplemental): e211010.
pmid: 39175859 |
| [26] |
Elliot TAE, Jennings EK, Lecky DAJ, Rouvray S, Mackie GM, Scarfe L, Sheriff L, Ono M, Maslowski KM, Bending D. Nur77-Tempo mice reveal T cell steady state antigen recognition. Discov Immunol, 2022, 1(1): kyac009.
pmid: 36704407 |
| [27] |
Khmelinskii A, Keller PJ, Bartosik A, Meurer M, Barry JD, Mardin BR, Kaufmann A, Trautmann S, Wachsmuth M, Pereira G, Huber W, Schiebel E, Knop M. Tandem fluorescent protein timers for in vivo analysis of protein dynamics. Nat Biotechnol, 2012, 30(7): 708-714.
pmid: 22729030 |
| [28] |
Fung JJ, Blöcher-Juárez K, Khmelinskii A. High- throughput analysis of protein turnover with tandem fluorescent protein timers. Methods Mol Biol, 2022, 2378: 85-100.
pmid: 34985695 |
| [102] |
Zhu JQ, He X, Bernard D, Shen JN, Su Y, Wolek A, Issacs B, Mishra N, Tian XC, Garmendia A, Tang Y. Identification of new compounds against PRRSV infection by directly targeting CD163. J Virol, 2023, 97(5): e0005423.
pmid: 37133376 |
| [103] |
Zych C, Domling A, Ayyavoo V. Development of a robust cell-based high-throughput screening assay to identify targets of HIV-1 viral protein R dimerization. Drug Des Devel Ther, 2013, 7: 403-412.
pmid: 23737660 |
| [104] |
Poe JA, Vollmer L, Vogt A, Smithgall TE. Development and validation of a high-content bimolecular fluorescence complementation assay for small-molecule inhibitors of HIV-1 Nef dimerization. J Biomol Screen, 2014, 19(4): 556-565.
pmid: 24282155 |
| [105] |
Xu Y, Liu R, Leu NA, Zhang L, Ibragmova I, Schultz DC, Wang PJ. A cell-based high-content screen identifies isocotoin as a small molecule inhibitor of the meiosis- specific MEIOB-SPATA22 complex†. Biol Reprod, 2020, 103(2): 333-342.
pmid: 32463099 |
| [106] |
Alford SC, Abdelfattah AS, Ding YD, Campbell RE. A fluorogenic red fluorescent protein heterodimer. Chem Biol, 2012, 19(3): 353-360.
pmid: 22444590 |
| [107] |
Alford SC, Ding YD, Simmen T, Campbell RE. Dimerization-dependent green and yellow fluorescent proteins. ACS Synth Biol, 2012, 1(12): 569-575.
pmid: 23656278 |
| [108] |
Miner GE, Smith SY, Showalter WK, So CM, Ragusa JV, Powers AE, Zanellati MC, Hsu CH, Marchan MF, Cohen S. Contact-FP: a dimerization-dependent fluorescent protein toolkit for visualizing membrane contact site dynamics. Contact (Thousand Oaks), 2024, 7: 25152564241228911.
pmid: 38327561 |
| [109] |
Son S, Nagahama K, Lee J, Jung K, Kwak C, Kim J, Noh YW, Kim E, Lee S, Kwon HB, Heo WD. Real-time visualization of structural dynamics of synapses in live cells in vivo. Nat Methods, 2024, 21(2): 353-360.
pmid: 38191933 |
| [110] |
Koh DHZ, Naito T, Na M, Yeap YJ, Rozario P, Zhong FL, Lim KL, Saheki Y. Visualization of accessible cholesterol using a GRAM domain-based biosensor. Nat Commun, 2023, 14(1): 6773.
pmid: 37880244 |
| [111] |
Ding YD, Li J, Enterina JR, Shen Y, Zhang I, Tewson PH, Mo GCH, Zhang J, Quinn AM, Hughes TE, Maysinger D, Alford SC, Zhang Y, Campbell RE. Ratiometric biosensors based on dimerization-dependent fluorescent protein exchange. Nat Methods, 2015, 12(3): 195-198.
pmid: 25622108 |
| [29] |
Chattoraj M, King BA, Bublitz GU, Boxer SG. Ultra-fast excited state dynamics in green fluorescent protein: multiple states and proton transfer. Proc Natl Acad Sci USA, 1996, 93(16): 8362-8367.
pmid: 8710876 |
| [30] |
Miesenböck G, De Angelis DA, Rothman JE. Visualizing secretion and synaptic transmission with pH-sensitive green fluorescent proteins. Nature, 1998, 394(6689): 192-195.
pmid: 9671304 |
| [31] |
Sankaranarayanan S, De Angelis D, Rothman JE, Ryan TA. The use of pHluorins for optical measurements of presynaptic activity. Biophys J, 2000, 79(4): 2199-2208.
pmid: 11023924 |
| [32] |
Li YL, Tsien RW. pHTomato, a red, genetically encoded indicator that enables multiplex interrogation of synaptic activity. Nat Neurosci, 2012, 15(7): 1047-1053.
pmid: 22634730 |
| [33] |
Shen Y, Rosendale M, Campbell RE, Perrais D. pHuji, a pH-sensitive red fluorescent protein for imaging of exo- and endocytosis. J Cell Biol, 2014, 207(3): 419-432.
pmid: 25385186 |
| [34] |
Li SA, Meng XY, Zhang YJ, Chen CL, Jiao YX, Zhu YQ, Liu PP, Sun W. Progress in pH-sensitive sensors: essential tools for organelle pH detection, spotlighting mitochondrion and diverse applications. Front Pharmacol, 2023, 14: 1339518.
pmid: 38269286 |
| [35] |
Liu AY, Huang XS, He WT, Xue FD, Yang YR, Liu JJ, Chen LY, Yuan L, Xu PY. pHmScarlet is a pH-sensitive red fluorescent protein to monitor exocytosis docking and fusion steps. Nat Commun, 2021, 12(1): 1413.
pmid: 33658493 |
| [36] |
Seidenthal M, Jánosi B, Rosenkranz N, Schuh N, Elvers N, Willoughby M, Zhao XD, Gottschalk A. pOpsicle: an all-optical reporter system for synaptic vesicle recycling combining pH-sensitive fluorescent proteins with optogenetic manipulation of neuronal activity. Front Cell Neurosci, 2023, 17: 1120651.
pmid: 37066081 |
| [37] |
Tanaka H, Funahashi J, Hirano T. Live-cell imaging of endocytosed synaptophysin around individual hippocampal presynaptic active zones. Front Cell Neurosci, 2023, 17: 1277729.
pmid: 37927445 |
| [38] |
Blaustein M, Wirth S, Saldaña G, Piantanida AP, Bogetti ME, Martin ME, Colman-Lerner A, Uchitel OD. A new tool to sense pH changes at the neuromuscular junction synaptic cleft. Sci Rep, 2020, 10(1): 20480.
pmid: 33235222 |
| [112] | Kawski A. Zwischenmolekulare energiewanderung und konzentrationsdepolarisation der fluoreszenz. Annalen der Physik, 1961, 463(1-2): 116-119. |
| [113] |
Bajar BT, Wang ES, Zhang S, Lin MZ, Chu J. A guide to fluorescent protein FRET pairs. Sensors (Basel), 2016, 16(9): 1488.
pmid: 27649177 |
| [114] |
Terai K, Imanishi A, Li CJ, Matsuda M. Two decades of genetically encoded biosensors based on förster resonance energy transfer. Cell Struct Funct, 2019, 44(2): 153-169.
pmid: 30905922 |
| [115] |
Miyawaki A. Development of probes for cellular functions using fluorescent proteins and fluorescence resonance energy transfer. Annu Rev Biochem, 2011, 80: 357-373.
pmid: 21529159 |
| [116] |
Kim H, Ju J, Lee HN, Chun H, Seong J. Genetically encoded biosensors based on fluorescent proteins. Sensors (Basel), 2021, 21(3): 795.
pmid: 33504068 |
| [117] |
O'Connor CL, Anguissola S, Huber HJ, Dussmann H, Prehn JHM, Rehm M. Intracellular signaling dynamics during apoptosis execution in the presence or absence of X-linked-inhibitor-of-apoptosis-protein. Biochim Biophys Acta, 2008, 1783(10): 1903-1913.
pmid: 18590777 |
| [118] |
Hellwig CT, Kohler BF, Lehtivarjo AK, Dussmann H, Courtney MJ, Prehn JHM, Rehm M. Real time analysis of tumor necrosis factor-related apoptosis-inducing ligand/cycloheximide-induced Caspase activities during apoptosis initiation. J Biol Chem, 2008, 283(31): 21676-21685.
pmid: 18522940 |
| [119] |
Takemoto K, Nagai T, Miyawaki A, Miura M. Spatio- temporal activation of caspase revealed by indicator that is insensitive to environmental effects. J Cell Biol, 2003, 160(2): 235-243.
pmid: 12527749 |
| [120] |
VanEngelenburg SB, Palmer AE. Fluorescent biosensors of protein function. Curr Opin Chem Biol, 2008, 12(1): 60-65.
pmid: 18282482 |
| [121] |
Grashoff C, Hoffman BD, Brenner MD, Zhou RB, Parsons M, Yang MT, McLean MA, Sligar SG, Chen CS, Ha T, Schwartz MA. Measuring mechanical tension across vinculin reveals regulation of focal adhesion dynamics. Nature, 2010, 466(7303): 263-266.
pmid: 20613844 |
| [39] |
Pescosolido MF, Ouyang Q, Liu JS, Morrow EM. Live-imaging detection of multivesicular body-plasma membrane fusion and exosome release in cultured primary neurons. Methods Mol Biol, 2023, 2683: 213-220.
pmid: 37300778 |
| [40] |
Sung BH, von Lersner A, Guerrero J, Krystofiak ES, Inman D, Pelletier R, Zijlstra A, Ponik SM, Weaver AM. A live cell reporter of exosome secretion and uptake reveals pathfinding behavior of migrating cells. Nat Commun, 2020, 11(1): 2092.
pmid: 32350252 |
| [41] |
Parzych KR, Klionsky DJ. An overview of autophagy: morphology, mechanism, and regulation. Antioxid Redox Signal, 2013, 20(3): 460-473.
pmid: 23725295 |
| [42] |
Maulucci G, Chiarpotto M, Papi M, Samengo D, Pani G, De Spirito M. Quantitative analysis of autophagic flux by confocal pH-imaging of autophagic intermediates. Autophagy, 2015, 11(10): 1905-1916.
pmid: 26506895 |
| [43] |
Tanida I, Ueno T, Uchiyama Y. A super-ecliptic, pHluorin-mKate2, tandem fluorescent protein-tagged human LC3 for the monitoring of mammalian autophagy. PLoS One, 2014, 9(10): e110600.
pmid: 25340751 |
| [44] |
Zhou CH, Zhong W, Zhou J, Sheng FG, Fang ZY, Wei Y, Chen YY, Deng XY, Xia B, Lin J. Monitoring autophagic flux by an improved tandem fluorescent-tagged LC3 (mTagRFP-mWasabi-LC3) reveals that high-dose rapamycin impairs autophagic flux in cancer cells. Autophagy, 2012, 8(8): 1215-1226.
pmid: 22647982 |
| [45] |
Kimura S, Noda T, Yoshimori T. Dissection of the autophagosome maturation process by a novel reporter protein, tandem fluorescent-tagged LC3. Autophagy, 2007, 3(5): 452-460.
pmid: 17534139 |
| [46] |
Kim H, Kim H, Choi J, Inn KS, Seong J. Visualization of autophagy progression by a red-green-blue autophagy sensor. ACS Sens, 2020, 5(12): 3850-3861.
pmid: 33261316 |
| [47] |
Montava-Garriga L, Singh F, Ball G, Ganley IG. Semi-automated quantitation of mitophagy in cells and tissues. Mech Ageing Dev, 2020, 185: 111196.
pmid: 31843465 |
| [48] |
Calleja-Felipe M, Wojtas MN, Diaz-González M, Ciceri D, Escribano R, Ouro A, Morales M, Knafo S. FORTIS: a live-cell assay to monitor AMPA receptors using pH-sensitive fluorescence tags. Transl Psychiatry, 2021, 11(1): 324.
pmid: 34045447 |
| [49] |
Baird GS, Zacharias DA, Tsien RY. Circular permutation and receptor insertion within green fluorescent proteins. Proc Natl Acad Sci USA, 1999, 96(20): 11241-11246.
pmid: 10500161 |
| [122] |
Rothenberg KE, Scott DW, Christoforou N, Hoffman BD. Vinculin force-sensitive dynamics at focal adhesions enable effective directed cell migration. Biophys J, 2018, 114(7): 1680-1694.
pmid: 29642037 |
| [123] |
Boersma AJ, Zuhorn IS, Poolman B. A sensor for quantification of macromolecular crowding in living cells. Nat Methods, 2015, 12(3): 227-229, 1 p following 229.
pmid: 25643150 |
| [124] |
Miyagi T, Yamanaka Y, Harada Y, Narumi S, Hayamizu Y, Kuroda M, Kanekura K. An improved macromolecular crowding sensor CRONOS for detection of crowding changes in membrane-less organelles under stressed conditions. Biochem Biophys Res Commun, 2021, 583: 29-34.
pmid: 34717122 |
| [125] |
Zadran S, Standley S, Wong K, Otiniano E, Amighi A, Baudry M. Fluorescence resonance energy transfer (FRET)-based biosensors: visualizing cellular dynamics and bioenergetics. Appl Microbiol Biotechnol, 2012, 96(4): 895-902.
pmid: 23053099 |
| [126] |
Shrestha D, Jenei A, Nagy P, Vereb G, Szöllősi J. Understanding FRET as a research tool for cellular studies. Int J Mol Sci, 2015, 16(4): 6718-6756.
pmid: 25815593 |
| [127] |
Kogure T, Karasawa S, Araki T, Saito K, Kinjo M, Miyawaki A. A fluorescent variant of a protein from the stony coral Montipora facilitates dual-color single-laser fluorescence cross-correlation spectroscopy. Nat Biotechnol, 2006, 24(5): 577-581.
pmid: 16648840 |
| [128] |
Shcherbakova DM, Hink MA, Joosen L, Gadella TWJ, Verkhusha VV. An orange fluorescent protein with a large Stokes shift for single-excitation multicolor FCCS and FRET imaging. J Am Chem Soc, 2012, 134(18): 7913-7923.
pmid: 22486524 |
| [129] |
Shcherbakova DM, Verkhusha VV. Chromophore chemistry of fluorescent proteins controlled by light. Curr Opin Chem Biol, 2014, 20: 60-68.
pmid: 24819887 |
| [130] |
Patterson GH, Lippincott-Schwartz J. A photoactivatable GFP for selective photolabeling of proteins and cells. Science, 2002, 297(5588): 1873-1877.
pmid: 12228718 |
| [131] |
Wiedenmann J, Ivanchenko S, Oswald F, Schmitt F, Röcker C, Salih A, Spindler KD, Nienhaus GU. EosFP, a fluorescent marker protein with UV-inducible green-to- red fluorescence conversion. Proc Natl Acad Sci USA, 2004, 101(45): 15905-15910.
pmid: 15505211 |
| [50] |
Nakai J, Ohkura M, Imoto K. A high signal-to-noise Ca2+ probe composed of a single green fluorescent protein. Nat Biotechnol, 2001, 19(2): 137-141.
pmid: 11175727 |
| [51] |
Miyawaki A, Llopis J, Heim R, McCaffery JM, Adams JA, Ikura M, Tsien RY. Fluorescent indicators for Ca2+ based on green fluorescent proteins and calmodulin. Nature, 1997, 388(6645): 882-887.
pmid: 9278050 |
| [52] |
Zou YJ, Wang AX, Huang L, Zhu XD, Hu QX, Zhang YN, Chen XJ, Li FW, Wang QH, Wang H, Liu RM, Zuo FT, Li T, Yao J, Qian YJ, Shi M, Yue X, Chen WC, Zhang Z, Wang CR, Zhou Y, Zhu LY, Ju ZY, Loscalzo J, Yang Y, Zhao YZ. Illuminating NAD+ metabolism in live cells and in vivo using a genetically encoded fluorescent sensor. Dev Cell, 2020, 53(2): 240-252.e7.
pmid: 32197067 |
| [53] |
Zhao YZ, Jin J, Hu QX, Zhou HM, Yi J, Yu ZH, Xu L, Wang X, Yang Y, Loscalzo J. Genetically encoded fluorescent sensors for intracellular NADH detection. Cell Metab, 2011, 14(4): 555-566.
pmid: 21982715 |
| [54] |
Harada K, Ito M, Wang XW, Tanaka M, Wongso D, Konno A, Hirai H, Hirase H, Tsuboi T, Kitaguchi T. Red fluorescent protein-based cAMP indicator applicable to optogenetics and in vivo imaging. Sci Rep, 2017, 7(1): 7351.
pmid: 28779099 |
| [55] |
Liu WF, Liu C, Ren PG, Chu J, Wang L. An improved genetically encoded fluorescent cAMP indicator for sensitive cAMP imaging and fast drug screening. Front Pharmacol, 2022, 13: 902290.
pmid: 35694242 |
| [56] |
Wang L, Wu CL, Peng WL, Zhou ZL, Zeng JZ, Li XL, Yang YN, Yu SG, Zou Y, Huang M, Liu C, Chen YF, Li Y, Ti PP, Liu WF, Gao YF, Zheng W, Zhong HN, Gao SB, Lu ZH, Ren PG, Ng HL, He J, Chen SD, Xu M, Li YL, Chu J. A high-performance genetically encoded fluorescent indicator for in vivo cAMP imaging. Nat Commun, 2022, 13(1): 5363.
pmid: 36097007 |
| [57] |
Lobas MA, Tao RK, Nagai J, Kronschläger MT, Borden PM, Marvin JS, Looger LL, Khakh BS. A genetically encoded single-wavelength sensor for imaging cytosolic and cell surface ATP. Nat Commun, 2019, 10(1): 711.
pmid: 30755613 |
| [58] |
Marvin JS, Kokotos AC, Kumar M, Pulido C, Tkachuk AN, Yao JS, Brown TA, Ryan TA. iATPSnFR2: a high-dynamic-range fluorescent sensor for monitoring intracellular ATP. Proc Natl Acad Sci USA, 2024, 121(21): e2314604121.
pmid: 38748581 |
| [59] |
Smith JJ, Valentino TR, Ablicki AH, Banerjee R, Colligan AR, Eckert DM, Desjardins GA, Diehl KL. A genetically-encoded fluorescent biosensor for visualization of acetyl-CoA in live cells. Cell Chem Biol, 2025, 32(2): 325-337.e10.
pmid: 38260544 |
| [60] |
Li X, Zhang YN, Xu LY, Wang AX, Zou YJ, Li T, Huang L, Chen WC, Liu SN, Jiang K, Zhang XZ, Wang DM, Zhang LJ, Zhang Z, Zhang ZY, Chen XJ, Jia W, Zhao AH, Yan XF, Zhou HM, Zhu LY, Ma XR, Ju ZY, Jia WP, Wang CR, Loscalzo J, Yang Y, Zhao YZ. Ultrasensitive sensors reveal the spatiotemporal landscape of lactate metabolism in physiology and disease. Cell Metab, 2023, 35(1): 200-211.e9.
pmid: 36309010 |
| [61] |
Nasu Y, Aggarwal A, Le GNT, Vo CT, Kambe Y, Wang XX, Beinlich FRM, Lee AB, Ram TR, Wang FY, Gorzo KA, Kamijo Y, Boisvert M, Nishinami S, Kawamura G, Ozawa T, Toda H, Gordon GR, Ge SY, Hirase H, Nedergaard M, Paquet ME, Drobizhev M, Podgorski K, Campbell RE. Lactate biosensors for spectrally and spatially multiplexed fluorescence imaging. Nat Commun, 2023, 14(1): 6598.
pmid: 37891202 |
| [62] |
Lara-Rojas F, Juárez-Verdayes MA, Wu HM, Cheung AY, Montiel J, Pascual-Morales E, Ryken SE, Bezanilla M, Cardenas L. Using Hyper as a molecular probe to visualize hydrogen peroxide in living plant cells: an updated method. Methods Enzymol, 2023, 683: 265-289.
pmid: 37087192 |
| [63] |
Berndt A, Lee J, Won W, Kimball K, Neiswanger C, Schattauer S, Wang YH, Yeboah F, Ruiz M, Evitts K, Rappleye M, Bremner S, Chun C, Smith N, Mack D, Young J, Lee CJ, Chavkin C. Ultra-fast genetically encoded sensor for precise real-time monitoring of physiological and pathophysiological peroxide dynamics. Res Sq, 2024, rs.3.rs-4048855.
pmid: 38585715 |
| [132] |
Ando R, Mizuno H, Miyawaki A. Regulated fast nucleocytoplasmic shuttling observed by reversible protein highlighting. Science, 2004, 306(5700): 1370-1373.
pmid: 15550670 |
| [133] |
Gaytán P, Roldán-Salgado A. Photoactivatable blue fluorescent protein. ACS Omega, 2024, 9(26): 28577-28582.
pmid: 38973932 |
| [134] |
Khan FI, Hassan F, Anwer R, Juan F, Lai D. Comparative analysis of bacteriophytochrome Agp2 and its engineered photoactivatable NIR fluorescent proteins PAiRFP1 and PAiRFP2. Biomolecules, 2020, 10(9): 1286.
pmid: 32906690 |
| [135] |
Ganguly A, Roy S. Imaging diversity in slow axonal transport. Methods Mol Biol, 2022, 2431: 163-179.
pmid: 35412276 |
| [136] |
Boyer NP, Julien JP, Jung P, Brown A. Neurofilament transport is bidirectional in vivo. eNeuro, 2022, 9(4): ENEURO.0138-22.2022.
pmid: 35896389 |
| [137] |
Yan XW, Stuurman N, Ribeiro SA, Tanenbaum ME, Horlbeck MA, Liem CR, Jost M, Weissman JS, Vale RD. High-content imaging-based pooled CRISPR screens in mammalian cells. J Cell Biol, 2021, 220(2): e202008158.
pmid: 33465779 |
| [138] |
Betzig E, Patterson GH, Sougrat R, Lindwasser OW, Olenych S, Bonifacino JS, Davidson MW, Lippincott- Schwartz J, Hess HF. Imaging intracellular fluorescent proteins at nanometer resolution. Science, 2006, 313(5793): 1642-1645.
pmid: 16902090 |
| [139] |
Hess ST, Girirajan TPK, Mason MD. Ultra-high resolution imaging by fluorescence photoactivation localization microscopy. Biophys J, 2006, 91(11): 4258-4272.
pmid: 16980368 |
| [140] |
Zhang MS, Chang H, Zhang YD, Yu JW, Wu LJ, Ji W, Chen JJ, Liu B, Lu JZ, Liu YF, Zhang JL, Xu PY, Xu T. Rational design of true monomeric and bright photoactivatable fluorescent proteins. Nat Methods, 2012, 9(7): 727-729.
pmid: 22581370 |
| [141] |
Marathe P, H S MS, Nair D, Bhattacharyya D. mEosBrite are bright variants of mEos3.2 developed by semirational protein engineering. J Fluoresc, 2020, 30(3): 703-715.
pmid: 32385659 |
| [142] |
Osuga M, Nishimura T, Suetsugu S. Development of a green reversibly photoswitchable variant of Eos fluorescent protein with fixation resistance. Mol Biol Cell, 2021, 32(21): br7.
pmid: 34495704 |
| [143] |
Pennacchietti F, Alvelid J, Morales RA, Damenti M, Ollech D, Oliinyk OS, Shcherbakova DM, Villablanca EJ, Verkhusha VV, Testa I. Blue-shift photoconversion of near-infrared fluorescent proteins for labeling and tracking in living cells and organisms. Nat Commun, 2023, 14(1): 8402.
pmid: 38114484 |
| [144] |
McKinney SA, Murphy CS, Hazelwood KL, Davidson MW, Looger LL. A bright and photostable photoconvertible fluorescent protein. Nat Methods, 2009, 6(2): 131-133.
pmid: 19169260 |
| [145] |
Tomura M. In vivo tracking of dendritic cell migration. Methods Mol Biol, 2023, 2618: 39-53.
pmid: 36905507 |
| [146] |
Tanaka Y, Morozumi A, Hirokawa N. Nodal flow transfers polycystin to determine mouse left-right asymmetry. Dev Cell, 2023, 58(16): 1447-1461.e6.
pmid: 37413993 |
| [147] |
Fosque BF, Sun Y, Dana H, Yang CT, Ohyama T, Tadross MR, Patel R, Zlatic M, Kim DS, Ahrens MB, Jayaraman V, Looger LL, Schreiter ER. Neural circuits. labeling of active neural circuits in vivo with designed calcium integrators. Science, 2015, 347(6223): 755-760.
pmid: 25678659 |
| [148] |
Hertel F, Mo GCH, Duwé S, Dedecker P, Zhang J. RefSOFI for mapping nanoscale organization of protein- protein interactions in living cells. Cell Rep, 2016, 14(2): 390-400.
pmid: 26748717 |
| [149] |
Huang WZ, Huang S, Fang YP, Zhu TY, Chu FY, Liu QH, Yu KQ, Chen F, Dong J, Zeng WB. AI-powered mining of highly customized and superior ESIPT-based fluorescent probes. Adv Sci (Weinh), 2024, 11(35): e2405596.
pmid: 39021325 |
| [150] |
Abramson J, Adler J, Dunger J, Evans R, Green T, Pritzel A, Ronneberger O, Willmore L, Ballard AJ, Bambrick J, Bodenstein SW, Evans DA, Hung CC, O'Neill M, Reiman D, Tunyasuvunakool K, Wu Z, Žemgulytė A, Arvaniti E, Beattie C, Bertolli O, Bridgland A, Cherepanov A, Congreve M, Cowen-Rivers AI, Cowie A, Figurnov M, Fuchs FB, Gladman H, Jain R, Khan YA, Low CMR, Perlin K, Potapenko A, Savy P, Singh S, Stecula A, Thillaisundaram A, Tong C, Yakneen S, Zhong ED, Zielinski M, Žídek A, Bapst V, Kohli P, Jaderberg M, Hassabis D, Jumper JM. Accurate structure prediction of biomolecular interactions with AlphaFold 3. Nature, 2024, 630(8016): 493-500.
pmid: 38718835 |
| [151] |
Chan J, Dodani SC, Chang CJ. Reaction-based small- molecule fluorescent probes for chemoselective bioimaging. Nat Chem, 2012, 4(12): 973-984.
pmid: 23174976 |
| [152] |
Jun JV, Chenoweth DM, Petersson EJ. Rational design of small molecule fluorescent probes for biological applications. Org Biomol Chem, 2020, 18(30): 5747-5763.
pmid: 32691820 |
| [153] | Leake MC, Quinn SD. A guide to small fluorescent probes for single-molecule biophysics. Chem Phys Rev, 2023, 4(1): 011302. |
| [154] |
Wang X, Ding Q, Groleau RR, Wu LL, Mao YT, Che FD, Kotova O, Scanlan EM, Lewis SE, Li P, Tang B, James TD, Gunnlaugsson T. Fluorescent probes for disease diagnosis. Chem Rev, 2024, 124(11): 7106-7164.
pmid: 38760012 |
| [155] | Zuo FT, Zhang YQ, Yang HM, Yang Y, Chen XJ. Progress on fluorescent RNA and fluorescent RNA-based biosensing technology. Hereditas(Beijing), 2024, 46(2): 92-108. |
| 左方婷, 张雅强, 杨慧敏, 杨弋, 陈显军. 荧光RNA及其生物传感技术研究进展. 遗传, 2024, 46(2): 92-108. | |
| [156] |
Berrones Reyes J, Kuimova MK, Vilar R. Metal complexes as optical probes for DNA sensing and imaging. Curr Opin Chem Biol, 2021, 61: 179-190.
pmid: 33784589 |
| [157] |
García de Arquer FP, Talapin DV, Klimov VI, Arakawa Y, Bayer M, Sargent EH. Semiconductor quantum dots: technological progress and future challenges. Science, 2021, 373(6555): eaaz8541.
pmid: 34353926 |
| [158] |
Keefe AD, Pai S, Ellington A. Aptamers as therapeutics. Nat Rev Drug Discov, 2010, 9(7): 537-550.
pmid: 20592747 |
| [159] | Dunn MR, Jimenez RM, Chaput JC. Analysis of aptamer discovery and technology. Nat Rev Chem, 2017, 1: 0076. |
| [160] |
Tsao KK, Imai S, Chang M, Hario S, Terai T, Campbell RE. The best of both worlds: chemigenetic fluorescent sensors for biological imaging. Cell Chem Biol, 2024, 31(9): 1652-1664.
pmid: 39236713 |
| [161] | Saimi D, Chen ZX. Chemical tags and beyond: live-cell protein labeling technologies for modern optical imaging. Smart Mol, 2023, 1(2): e20230002. |
| [1] | 吴仲胜, 高誉, 杜勇涛, 党颂, 何康敏. CRISPR-Cas9基因编辑技术对细胞内源蛋白进行荧光标记的实验操作[J]. 遗传, 2023, 45(2): 165-175. |
| [2] | 严婷婷, 张蕾, 李余动, 梁新乐. 基于微信的“微生物遗传育种实验”混合式教学模式探究[J]. 遗传, 2018, 40(7): 601-606. |
| [3] | 李智方, 冯冲, 纪慧丽, 石宁宁, 宋小凤, 赵勤丽, 龙川, 潘登科, 杨小淦. 绿色荧光蛋白在α-1,3半乳糖基转移酶敲除猪组织器官的表达分析[J]. 遗传, 2015, 37(12): 1211-1217. |
| [4] | 王明强 武金霞 张玉红 韩凝 边红武 朱睦元. 蛋白质相互作用实验技术的最新进展[J]. 遗传, 2013, 35(11): 1274-1283. |
| [5] | 张鹏,杨珍珍,窦红伟,李伟杭,律波,Bolund Lars,杜玉涛,谭萍萍,马润林. 利用改进的手工克隆技术生产转GFP基因猪克隆胚胎[J]. 遗传, 2011, 33(5): 527-532. |
| [6] | 李姗姗,雍静茹,齐云玲,章颖,赵亮,夏士林,李东,王慧利,包其郁,李佩珍. 螺旋藻耐盐相关基因启动子区功能分析[J]. 遗传, 2011, 33(10): 1134-1140. |
| [7] | 刘长青,郭俣,刘帅,包阿东,陆涛峰,刘洪坤,关伟军,马月辉 . 北京油鸡α1-AGP基因结构与表达特征研究[J]. 遗传, 2009, 31(6): 620-628. |
| [8] | 徐纪明,向太和. 含gfp植物转基因表达载体的构建及在矮牵牛转基因不定根中的高效表达[J]. 遗传, 2008, 30(8): 1069-1074. |
| [9] | 沈卫锋,翁宏飚,牛宝龙,何丽华,刘岩,齐晓朋,孟智启. 根癌农杆菌介导的灰葡萄孢菌遗传转化研究[J]. 遗传, 2008, 30(4): 515-520. |
| [10] | 许文明,张思仲,邱为民,何国平,刘运强,马用信,孙岩. ZNF230/荧光蛋白融合基因表达载体的构建及其在Cos细胞中的表达与定位[J]. 遗传, 2004, 26(4): 451454-451454. |
| [11] | 刘和,陈英旭,张文波,金勇丰. PCR一步法构建融合蛋白基因fpg[J]. 遗传, 2004, 26(4): 525-528. |
| [12] | 龙华,木下政人. GFP标记在转基因青鳉同系繁殖纯化中的应用[J]. 遗传, 2003, 25(4): 409-413. |
| [13] | 李扬,吴凯峰,郭旭东,郭继彤,旭日干. 脂质体介导外源基因体外转染牛胎儿成纤维细胞条件的优化 [J]. 遗传, 2002, 24(6): 653-655. |
| [14] | 黄国存,张寒霜,高鹏,李俊兰,朱生伟,孙敬三. GFP基因在棉花转化中的应用[J]. 遗传, 2001, 23(2): 131-200. |
| [15] | 陈智毅,李清兵,陈列辉,廖森泰,李宝瑜,廖琼香. 利用CLSM检测GFP基因在家蚕的瞬时表达[J]. 遗传, 2000, 22(5): 303-304. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||

www.chinagene.cn
备案号:京ICP备09063187号-4
总访问:,今日访问:,当前在线:

