Hereditas(Beijing) ›› 2025, Vol. 47 ›› Issue (9): 979-991.doi: 10.16288/j.yczz.24-332
• Review • Previous Articles Next Articles
Lipid metabolism imbalance: potential pathological mechanism and new intervention ideas for recurrent miscarriage
Wenhui Nan( ), Xunsi Qin(
), Xunsi Qin( ), Rong Li(
), Rong Li( )
)
- Center for Reproductive Medicine, Department of Obstetrics and Gynecology, Peking University Third Hospital, Beijing 100191, China
-
Received:2025-02-11Revised:2025-05-26Online:2025-05-28Published:2025-05-28 -
Contact:Xunsi Qin, Rong Li E-mail:nanwenhui81@outlook.com;qinxunsi@126.com;roseli001@sina.com -
Supported by:National Key R&D Program of China(2022YFC2702500);National Key R&D Program of China(2021YFC2700601)
Cite this article
Wenhui Nan, Xunsi Qin, Rong Li. Lipid metabolism imbalance: potential pathological mechanism and new intervention ideas for recurrent miscarriage[J]. Hereditas(Beijing), 2025, 47(9): 979-991.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
Table 1
Effects of abnormal expression of lipid metabolism-related genes on early pregnancy"
| 基因 | 物种 | 编码分子 | 基因敲除/缺陷的雌性个体表型 | 参考文献 |
|---|---|---|---|---|
| Napepld | 小鼠(Mus musculus) | N-酰基磷脂酰乙醇胺磷脂酶D | 自发性妊娠失败;子宫容受性和囊胚活化缺陷 | [ |
| Sphk1/2 | 小鼠 | 鞘氨醇激酶1/2 | 子宫出血和早期胚胎致死;在胎母界面形成过多的中性粒细胞胞外陷阱 | [ |
| Pla2g10 | 小鼠 | 磷脂酶A2-X | 减少植入部位的数量 | [ |
| Acadl | 小鼠 | 长链酰基辅酶A脱氢酶 | 胚胎发育异常和囊胚率降低 | [ |
| Ptgs2 | 小鼠 | 环氧合酶2 | 植入和蜕膜化缺陷;血管生成反应减少 | [ |
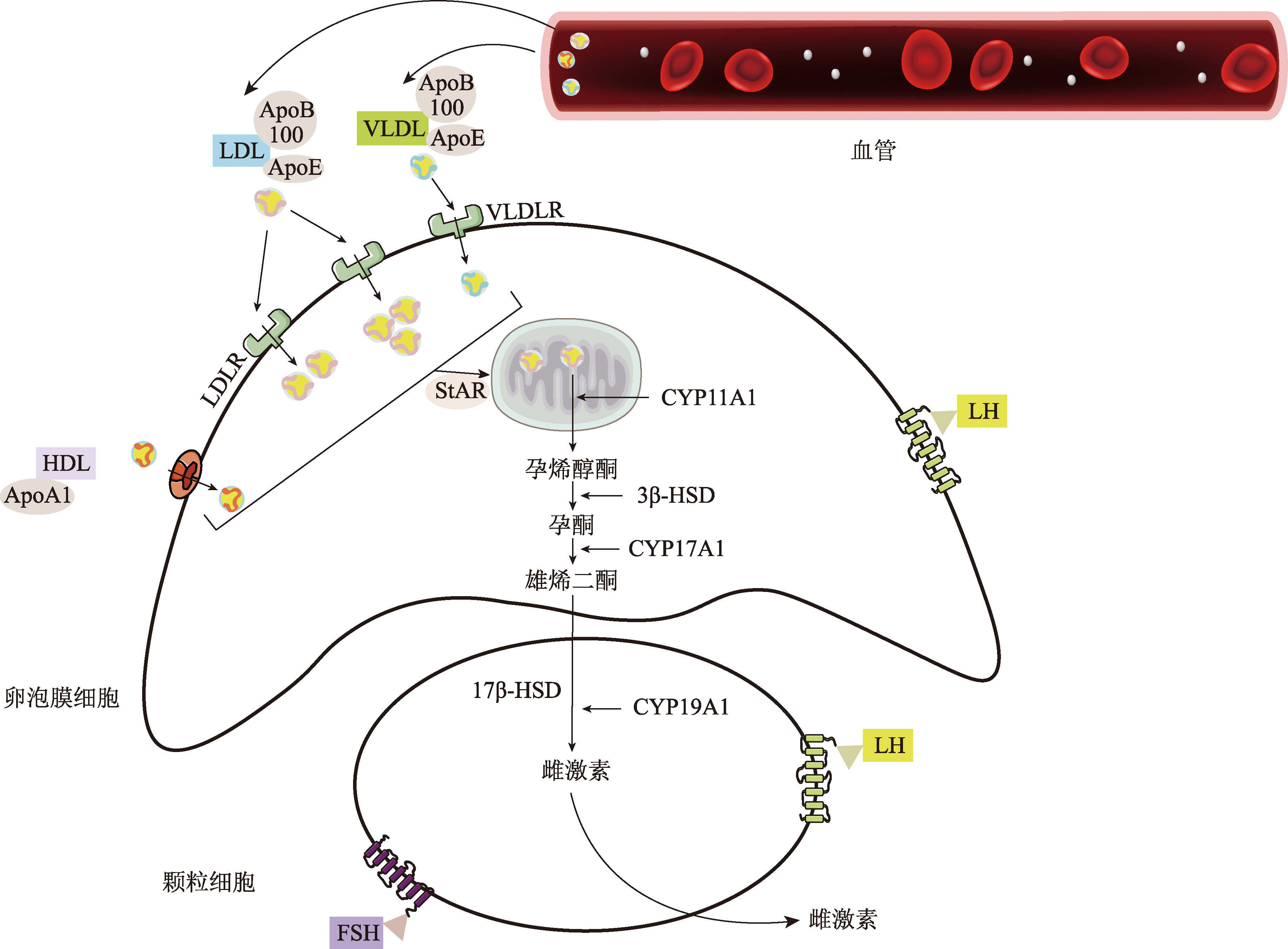
| Ldlr | 小鼠 | 低密度脂蛋白受体 | 黄体细胞内胆固醇和类固醇激素水平降低 | [ |
Table 2
Effects of fatty acids on early embryo development, steroidogenesis, and embryo implantation"
| 脂肪酸 | 物种 | 影响 | 参考文献 |
|---|---|---|---|
| 硬脂酸 | 牛(Bos taurus) | 硬脂酸导致脂质储存减少和受精后发育能力降低 | [ |
| 棕榈酸 | 牛、 猪(Sus scrofa) | 棕榈酸导致脂质储存减少和受精后发育能力降低 | [ |
| 油酸 | 牛 | 油酸浓度升高,卵母细胞成熟对发育能力或囊胚总细胞数没有影响,但提高了囊胚凋亡细胞指数;油酸导致脂滴中脂质储存的增加和卵母细胞发育能力的提高 | [ |
| 非酯化脂肪酸 | 牛 | 与非酯化脂肪酸暴露的卵母细胞和胚胎的基础水平相比,高水平的非酯化脂肪酸暴露卵母细胞和胚胎发育成囊胚的能力较低,并导致囊胚具有改变的DNA甲基化和与脂质和碳水化合物代谢、细胞死亡、免疫反应和代谢紊乱相关的转录组指纹 | [ |
| 丁酸盐 | 小鼠(Mus musculus)、 大鼠(Rattus norvegicus) | 母体丁酸盐增加大鼠植入胚胎的数量并改善卵巢孕酮合成;丁酸钠中断卵母细胞的成熟并促进植入前胚胎的发育 | [ |
| 二十二碳五烯酸 | 人(Homo sapiens) | 二十二碳五烯酸与无排卵风险降低有关 | [ |
| [1] | Xu YJ, Wang LY. Research progress on the association between genetic variations in lipid metabolism and premature coronary artery disease. Hereditas(Beijing), 2008, 30(6): 671-676. |
| 许瑛杰, 王绿娅. 脂质代谢相关基因变异在早发冠心病中作用的研究进展. 遗传, 2008, 30(6): 671-676. | |
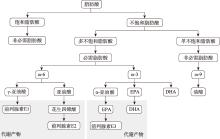
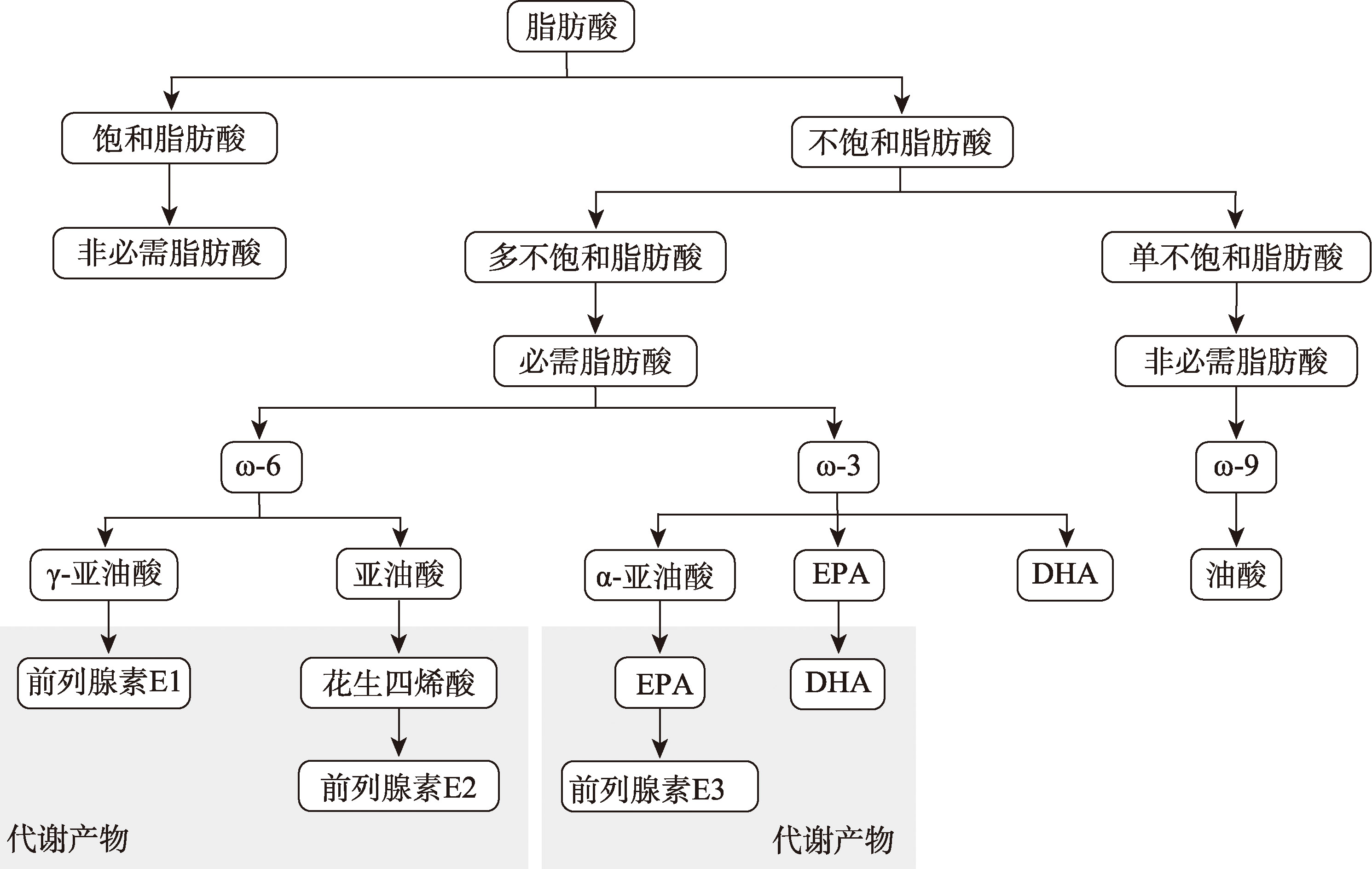
| [2] |
Canella PRBC, Vinces SS, Silva ÁAR, Sanches PHG, Barini R, de Melo Porcari A, Razolli DS, de Oliveira Carvalho P. Altered profile of plasma phospholipids in woman with recurrent pregnancy loss and recurrent implantation failure treated with lipid emulsion therapy. Am J Reprod Immunol, 2023, 89(3): e13673.
pmid: 36585861 |
| [3] |
Yang TL, Zhao J, Liu F, Li YP. Lipid metabolism and endometrial receptivity. Hum Reprod Update, 2022, 28(6): 858-889.
pmid: 35639910 |
| [4] |
Liu K, Xu XJ, Sun L, Li HX, Jin Y, Ma XL, Shen BR, Martin C. Proteomics profiling reveals lipid metabolism abnormalities during oogenesis in unexplained recurrent pregnancy loss. Front Immunol, 2024, 15: 1397633.
pmid: 39176081 |
| [5] |
Wang LL, Liu H, Zhao SJ, Shen L, Xie T, Luo J, Mor G, Liao AH. The metabolic landscape of decidua in recurrent pregnancy loss using a global metabolomics approach. Placenta, 2021, 112: 45-53.
pmid: 34273713 |
| [6] |
Sfakianoudis K, Rapani A, Grigoriadis S, Pantou A, Maziotis E, Kokkini G, Tsirligkani C, Bolaris S, Nikolettos K, Chronopoulou M, Pantos K, Simopoulou M. The role of uterine natural killer cells on recurrent miscarriage and recurrent implantation failure: from pathophysiology to treatment. Biomedicines, 2021, 9(10): 1425.
pmid: 34680540 |
| [7] |
Peng L, Chelariu-Raicu A, Ye Y, Ma Z, Yang HX, Ishikawa-Ankerhold H, Rahmeh M, Mahner S, Jeschke U, von Schönfeldt V. Prostaglandin E2 receptor 4 (EP4) affects trophoblast functions via activating the cAMP- PKA-pCREB signaling pathway at the maternal-fetal interface in unexplained recurrent miscarriage. Int J Mol Sci, 2021, 22(17): 9134.
pmid: 34502044 |
| [8] |
Twig G, Shina A, Amital H, Shoenfeld Y. Pathogenesis of infertility and recurrent pregnancy loss in thyroid autoimmunity. J Autoimmun, 2012, 38(2-3): J275-J281.
pmid: 22218218 |
| [9] |
Tang HY, Pan LQ, Xiong Y, Wang LL, Cui YG, Liu JY, Tang LS. Down-regulation of the Sp1 transcription factor by an increase of microRNA-4497 in human placenta is associated with early recurrent miscarriage. Reprod Biol Endocrinol, 2021, 19(1): 21.
pmid: 33579314 |
| [10] |
Horn J, Tanz LJ, Stuart JJ, Markovitz AR, Skurnik G, Rimm EB, Missmer SA, Rich-Edwards JW. Early or late pregnancy loss and development of clinical cardiovascular disease risk factors: a prospective cohort study. BJOG, 2019, 126(1): 33-42.
pmid: 30144277 |
| [11] |
Catov JM, Ness RB, Wellons MF, Jacobs DR, Roberts JM, Gunderson EP. Prepregnancy lipids related to preterm birth risk: the coronary artery risk development in young adults study. J Clin Endocrinol Metab, 2010, 95(8): 3711-3718.
pmid: 20501685 |
| [12] |
Vrijkotte TGM, Krukziener N, Hutten BA, Vollebregt KC, van Eijsden M, Twickler MB. Maternal lipid profile during early pregnancy and pregnancy complications and outcomes: the ABCD study. J Clin Endocrinol Metab, 2012, 97(11): 3917-3925.
pmid: 22933545 |
| [13] |
Wiznitzer A, Mayer A, Novack V, Sheiner E, Gilutz H, Malhotra A, Novack L. Association of lipid levels during gestation with preeclampsia and gestational diabetes mellitus: a population-based study. Am J Obstet Gynecol, 2009, 201(5): 482.e1-482.e8.
pmid: 19631920 |
| [14] |
Han ES, Krauss RM, Xu F, Sridhar SB, Ferrara A, Quesenberry CP, Hedderson MM. Prepregnancy adverse lipid profile and subsequent risk of gestational diabetes. J Clin Endocrinol Metab, 2016, 101(7): 2721-2727.
pmid: 27045641 |
| [15] |
Adank MC, Benschop L, Kors AW, Peterbroers KR, Smak Gregoor AM, Mulder MT, Schalekamp- Timmermans S, Roeters Van Lennep JE, Steegers EAP. Maternal lipid profile in early pregnancy is associated with foetal growth and the risk of a child born large-for-gestational age: a population-based prospective cohort study: maternal lipid profile in early pregnancy and foetal growth. BMC Med, 2020, 18(1): 276.
pmid: 33004027 |
| [16] |
Tamura I, Shiroshita A, Fujimura T, Tanaka-Doi Y, Shirafuta Y, Taketani T, Sato S, Sugino N. Glucose and lipid metabolisms in human endometrial stromal cells during decidualization. Endocr J, 2023, 70(5): 465-472.
pmid: 37081638 |
| [17] |
Paczkowski M, Schoolcraft WB, Krisher RL. Fatty acid metabolism during maturation affects glucose uptake and is essential to oocyte competence. Reproduction, 2014, 148(4): 429-439.
pmid: 25062802 |
| [18] |
Calabuig-Navarro V, Haghiac M, Minium J, Glazebrook P, Ranasinghe GC, Hoppel C, Hauguel de-Mouzon S, Catalano P, O’Tierney-Ginn P. Effect of maternal obesity on placental lipid metabolism. Endocrinology, 2017, 158(8): 2543-2555.
pmid: 28541534 |
| [19] |
Guo Y, Wang HB, Okamoto Y, Ueda N, Kingsley PJ, Marnett LJ, Schmid HHO, Das SK, Dey SK. N-acylphosphatidylethanolamine-hydrolyzing phospholipase D is an important determinant of uterine anandamide levels during implantation. J Biol Chem, 2005, 280(25): 23429-23432.
pmid: 15890658 |
| [20] |
Mizugishi K, Li CL, Olivera A, Bielawski J, Bielawska A, Deng CX, Proia RL. Maternal disturbance in activated sphingolipid metabolism causes pregnancy loss in mice. J Clin Invest, 2007, 117(10): 2993-3006.
pmid: 17885683 |
| [21] |
Mizugishi1 K, Yamashita K. Neutrophil extracellular traps are critical for pregnancy loss in sphingosine kinase-deficient mice on 129Sv/C57BL/6 background. FASEB J, 2017, 31(12): 5577-5591.
pmid: 28842426 |
| [22] |
Park HK, Park SH, Lee M, Kim GR, Park M, Yang SC, Kim YS, Lim HJ, Kim HR, Song H. Secretory phospholipase A2-X (Pla2g10) is a novel progesterone receptor target gene exclusively induced in uterine luminal epithelium for uterine receptivity in mice. Cell Biosci, 2020, 10(1): 132.
pmid: 33292460 |
| [23] |
Berger PS, Wood PA. Disrupted blastocoele formation reveals a critical developmental role for long-chain acyl-CoA dehydrogenase. Mol Genet Metab, 2004, 82(4): 266-272.
pmid: 15308124 |
| [24] |
Lim H, Paria BC, Das SK, Dinchuk JE, Langenbach R, Trzaskos JM, Dey SK. Multiple female reproductive failures in cyclooxygenase 2-deficient mice. Cell, 1997, 91(2): 197-208.
pmid: 9346237 |
| [25] |
Chang XL, Liu LS, Wang NQ, Chen ZJ, Zhang C. The function of high-density lipoprotein and low-density lipoprotein in the maintenance of mouse ovarian steroid balance. Biol Reprod, 2017, 97(6): 862-872.
pmid: 29092018 |
| [26] |
Liu HH, Li JJ. Aging and dyslipidemia: a review of potential mechanisms. Ageing Res Rev, 2015, 19: 43-52.
pmid: 25500366 |
| [27] |
Guleken Z, Bahat PY, Toto ÖF, Bulut H, Jakubczyk P, Cebulski J, Paja W, Pancerz K, Wosiak A, Depciuch J. Blood serum lipid profiling may improve the management of recurrent miscarriage: a combination of machine learning of mid-infrared spectra and biochemical assays. Anal Bioanal Chem, 2022, 414(29-30): 8341-8352.
pmid: 36227296 |
| [28] |
Cai WY, Luo X, Chen EXD, Lv HY, Fu KY, Wu XK, Xu J. Serum lipid levels and treatment outcomes in women undergoing assisted reproduction: a retrospective cohort study. Front Endocrinol (Lausanne), 2021, 12: 633766.
pmid: 33763032 |
| [29] |
Nicotra M, Muttinelli C, Sbracia M, Rolfi G, Passi S. Blood levels of lipids, lipoperoxides, vitamin E and glutathione peroxidase in women with habitual abortion. Gynecol Obstet Invest, 1994, 38(4): 223-236.
pmid: 7851805 |
| [30] |
Jamro EL, Bloom MS, Browne RW, Kim K, Greenwood EA, Fujimoto VY. Preconception serum lipids and lipophilic micronutrient levels are associated with live birth rates after IVF. Reprod Biomed Online, 2019, 39(4): 665-673.
pmid: 31405720 |
| [31] |
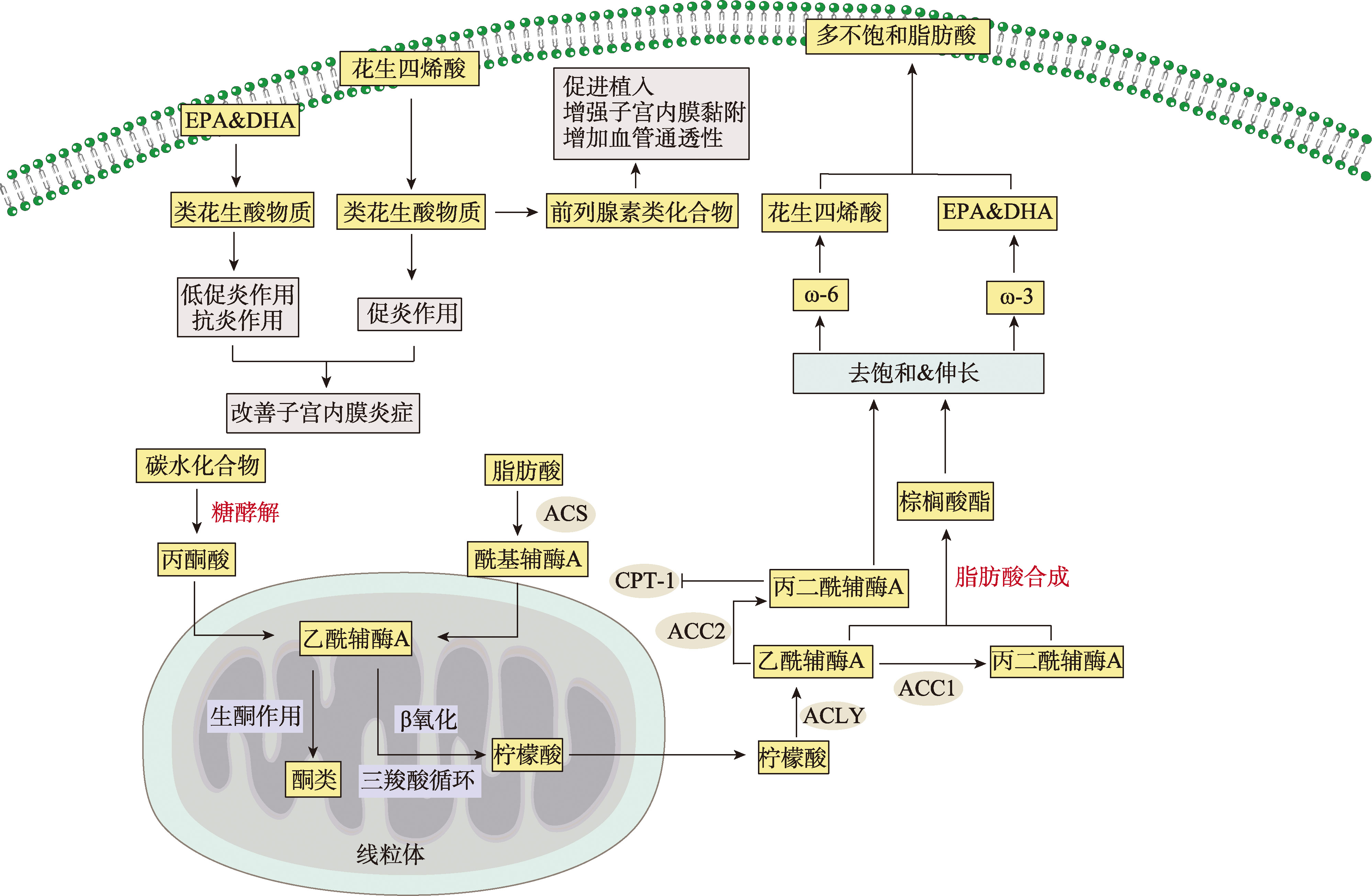
Chen JJ, Yin TX, Hu XY, Chang LY, Sang YF, Xu L, Zhao WJ, Liu L, Xu CF, Lin YK, Li Y, Wu QY, Li DJ, Li YH, Du MR. CD36-mediated arachidonic acid influx from decidual stromal cells increases inflammatory macrophages in miscarriage. Cell Reports, 2024, 43(11): 114881.
pmid: 39427314 |
| [32] |
Shi QY, Chen JH, Zou XD, Tang XC. Intracellular cholesterol synthesis and transport. Front Cell Dev Biol, 2022, 10: 819281.
pmid: 35386193 |
| [33] |
Fernández C, Martín M, Gómez-Coronado D, Lasunción MA. Effects of distal cholesterol biosynthesis inhibitors on cell proliferation and cell cycle progression. J Lipid Res, 2005, 46(5): 920-929.
pmid: 15687348 |
| [34] |
Zhang W, Wang RF, Yang X, Cheng ZY, Wang F. Correlation of dyslipidemia characterized by abnormal cholesterol in first trimester with early pregnancy loss: a retrospective study. Arch Gynecol Obstet, 2025, 311(2): 543-553.
pmid: 39828774 |
| [35] |
Yang TL, Zhao J, Zhang Q, Liu DG, Liu NH, Li YM, Yao ZY, Zhang YQ, Tian F, Liao TT, Tang HY, Li YP. Associations between dyslipidaemia and pregnancy outcomes in the first complete cycle of IVF/ICSI: a real-world analysis. Reprod Biomed Online, 2021, 43(6): 1095-1105.
pmid: 34764017 |
| [36] |
Shetty SS, Kumari S. Fatty acids and their role in type-2 diabetes (Review). Exp Ther Med, 2021, 22(1): 706.
pmid: 34007315 |
| [37] |
Kimura I, Ichimura A, Ohue-Kitano R, Igarashi M. Free fatty acid receptors in health and disease. Physiol Rev, 2020, 100(1): 171-210.
pmid: 31487233 |
| [38] |
Agostoni C, Bruzzese MG. Fatty acids: their biochemical and functional classification. Pediatr Med Chir, 1992, 14(5): 473-479.
pmid: 1488301 |
| [39] |
Jungheim ES, Macones GA, Odem RR, Patterson BW, Moley KH. Elevated serum α-linolenic acid levels are associated with decreased chance of pregnancy after in vitro fertilization. Fertil Steril, 2011, 96(4): 880-883.
pmid: 21840520 |
| [40] |
Jungheim ES, Frolova AI, Jiang H, Riley JK. Relationship between serum polyunsaturated fatty acids and pregnancy in women undergoing in vitro fertilization. J Clin Endocrinol Metab, 2013, 98(8): E1364-E1368.
pmid: 23780371 |
| [41] |
Mirabi P, Chaichi MJ, Esmaeilzadeh S, Ali Jorsaraei SG, Bijani A, Ehsani M, Hashemi Karooee SF. The role of fatty acids on ICSI outcomes: a prospective cohort study. Lipids Health Dis, 2017, 16(1): 18.
pmid: 28109274 |
| [42] |
Chiu YH, Karmon AE, Gaskins AJ, Arvizu M, Williams PL, Souter I, Rueda BR, Hauser R, Chavarro JE, EARTH Study Team. Serum omega-3 fatty acids and treatment outcomes among women undergoing assisted reproduction. Hum Reprod, 2018, 33(1): 156-165.
pmid: 29136189 |
| [43] |
Calabuig-Navarro V, Puchowicz M, Glazebrook P, Haghiac M, Minium J, Catalano P, Hauguel deMouzon S, O’Tierney-Ginn P. Effect of ω-3 supplementation on placental lipid metabolism in overweight and obese women. Am J Clin Nutr, 2016, 103(4): 1064-1072.
pmid: 26961929 |
| [44] |
Aardema H, Vos PLAM, Lolicato F, Roelen BAJ, Knijn HM, Vaandrager AB, Helms JB, Gadella BM. Oleic acid prevents detrimental effects of saturated fatty acids on bovine oocyte developmental competence. Biol Reprod, 2011, 85(1): 62-69.
pmid: 21311036 |
| [45] |
Shibahara H, Ishiguro A, Inoue Y, Koumei S, Kuwayama T, Iwata H. Mechanism of palmitic acid-induced deterioration of in vitro development of porcine oocytes and granulosa cells. Theriogenology, 2020, 141: 54-61.
pmid: 31518729 |
| [46] |
Desmet KLJ, Van Hoeck V, Gagné D, Fournier E, Thakur A, O’Doherty AM, Walsh CP, Sirard MA, Bols PEJ, Leroy JLMR. Exposure of bovine oocytes and embryos to elevated non-esterified fatty acid concentrations: integration of epigenetic and transcriptomic signatures in resultant blastocysts. BMC Genomics, 2016, 17(1): 1004.
pmid: 27931182 |
| [47] |
Van Hoeck V, Rizos D, Gutierrez-Adan A, Pintelon I, Jorssen E, Dufort I, Sirard MA, Verlaet A, Hermans N, Bols PEJ, Leroy JLMR. Interaction between differential gene expression profile and phenotype in bovine blastocysts originating from oocytes exposed to elevated non-esterified fatty acid concentrations. Reprod Fertil Dev, 2015, 27(2): 372-384.
pmid: 24360349 |
| [48] |
Yu MF, Wang JL, Yi JM, Ma L. Sodium butyrate interrupts the maturation of oocytes and enhances the development of preimplantation embryos. PLoS One, 2019, 14(7): e0220479.
pmid: 31356635 |
| [49] |
Ye QH, Cai S, Wang S, Zeng XZ, Ye CC, Chen MX, Zeng XF, Qiao SY. Maternal short and medium chain fatty acids supply during early pregnancy improves embryo survival through enhancing progesterone synthesis in rats. J Nutr Biochem, 2019, 69: 98-107.
pmid: 31063920 |
| [50] |
Mumford SL, Chavarro JE, Zhang CL, Perkins NJ, Sjaarda LA, Pollack AZ, Schliep KC, Michels KA, Zarek SM, Plowden TC, Radin RG, Messer LC, Frankel RA, Wactawski-Wende J. Dietary fat intake and reproductive hormone concentrations and ovulation in regularly menstruating women. Am J Clin Nutr, 2016, 103(3): 868-877.
pmid: 26843151 |
| [51] |
Sano M, Shimazaki S, Kaneko Y, Karasawa T, Takahashi M, Ohkuchi A, Takahashi H, Kurosawa A, Torii Y, Iwata H, Kuwayama T, Shirasuna K. Palmitic acid activates NLRP3 inflammasome and induces placental inflammation during pregnancy in mice. J Reprod Dev, 2020, 66(3): 241-248.
pmid: 32101829 |
| [52] |
Cao XY, Li MY, Shao CX, Shi JL, Zhang T, Xie F, Peng T, Li MQ. Fatty acid metabolism disruptions: a subtle yet critical factor in adverse pregnancy outcomes. Int J Biol Sci, 2024, 20(15): 6018-6037.
pmid: 39664564 |
| [53] |
Gibreel A, Badawy A, El-Refai W, El-Adawi N. Endometrial scratching to improve pregnancy rate in couples with unexplained subfertility: a randomized controlled trial. J Obstet Gynaecol Res, 2013, 39(3): 680-684.
pmid: 23106834 |
| [54] |
Gnainsky Y, Granot I, Aldo PB, Barash A, Or Y, Schechtman E, Mor G, Dekel N. Local injury of the endometrium induces an inflammatory response that promotes successful implantation. Fertil Steril, 2010, 94(6): 2030-2036.
pmid: 20338560 |
| [55] |
Shrestha N, Holland OJ, Kent NL, Perkins AV, McAinch AJ, Cuffe JSM, Hryciw DH. Maternal high linoleic acid alters placental fatty acid composition. Nutrients, 2020, 12(8): 2183.
pmid: 32717842 |
| [56] |
Basak S, Das MK, Duttaroy AK. Fatty acid-induced angiogenesis in first trimester placental trophoblast cells: possible roles of cellular fatty acid-binding proteins. Life Sci, 2013, 93(21): 755-762.
pmid: 24095946 |
| [57] |
Demiral Keleş I, Ülgen E, Erkan MB, Çelik SE, Aydın Y, Önem AN, Kandemir H, Arslanoğlu T, Apak MR, Sezerman U, Yeh J, Buyru F, Baştu E. Comparison of endometrial prostanoid profiles in three infertile subgroups: the missing part of receptivity? Fertil Steril, 2020, 113(3): 670-678.e1.
pmid: 32061358 |
| [58] |
Achache H, Tsafrir A, Prus D, Reich R, Revel A. Defective endometrial prostaglandin synthesis identified in patients with repeated implantation failure undergoing in vitro fertilization. Fertil Steril, 2010, 94(4): 1271-1278.
pmid: 19815191 |
| [59] |
Li WJ, Lu JW, Zhang CY, Wang WS, Ying H, Myatt L, Sun K. PGE2 vs PGF2α in human parturition. Placenta, 2021, 104: 208-219.
pmid: 33429118 |
| [60] |
Kaczynski P, Bauersachs S, Goryszewska E, Baryla M, Waclawik A. Synergistic action of estradiol and PGE2 on endometrial transcriptome in vivo resembles pregnancy effects better than estradiol alone. Biol Reprod, 2021, 104(4): 818-834.
pmid: 33354726 |
| [61] |
Kaczynski P, Goryszewska E, Baryla M, Waclawik A. Prostaglandin F2α stimulates angiogenesis at the embryo-maternal interface during early pregnancy in the pig. Theriogenology, 2020, 142: 169-176.
pmid: 31600637 |
| [62] |
Baryla M, Kaczynski P, Goryszewska E, Riley SC, Waclawik A. Prostaglandin F2α stimulates adhesion, migration, invasion and proliferation of the human trophoblast cell line HTR-8/SVneo. Placenta, 2019, 77: 19-29.
pmid: 30827352 |
| [63] |
Peng L, Ye Y, Mullikin H, Lin L, Kuhn C, Rahmeh M, Mahner S, Jeschke U, von Schönfeldt V. Expression of trophoblast derived prostaglandin E2 receptor 2 (EP2) is reduced in patients with recurrent miscarriage and EP2 regulates cell proliferation and expression of inflammatory cytokines. J Reprod Immunol, 2020, 142: 103210.
pmid: 33011635 |
| [64] |
Ye Y, Peng L, Chelariu-Raicu A, Kuhn C, Dong X, Jeschke U, von Schönfeldt V. Prostaglandin E2 receptor 3 promotes M1 macrophages polarization in unexplained recurrent pregnancy loss. Biol Reprod, 2022, 106(5): 910-918.
pmid: 35134851 |
| [65] |
Bhale AS, Meilhac O, d’Hellencourt CL, Vijayalakshmi MA, Venkataraman K. Cholesterol transport and beyond: illuminating the versatile functions of HDL apolipoproteins through structural insights and functional implications. BioFactors, 2024, 50(5): 922-956.
pmid: 38661230 |
| [66] |
Oriá RB, de Almeida JZ, Moreira CN, Guerrant RL, Figueiredo JR. Apolipoprotein E effects on mammalian ovarian steroidogenesis and human fertility. Trends Endocrinol Metab, 2020, 31(11): 872-883.
pmid: 32684408 |
| [67] |
Zhou JH, Mo H, Feng Q, Li L, La JH. ApoC3 is expressed in oocytes and increased expression is associated with PCOS progression. J Ovarian Res, 2023, 16(1): 188.
pmid: 37689737 |
| [68] |
Ulloque-Badaracco JR, Al-kassab-Córdova A, Hernandez- Bustamante EA, Alarcon-Braga EA, Huayta-Cortez M, Carballo-Tello XL, Seminario-Amez RA, Herrera- Añazco P, Benites-Zapata VA. Association of apolipoproteins and lipoprotein(a) with metabolic syndrome: a systematic review and meta-analysis. Lipids Health Dis, 2023, 22(1): 98.
pmid: 37420190 |
| [69] |
Liu Q, Wu L, Wang LL, Chen K, Wu YT, Xia JH, Wang YJ. Associations between maternal mid-pregnancy apolipoprotein A-1, apolipoprotein B, apolipoprotein B/apolipoprotein A-1 ratio and preterm birth. Clin Chim Acta, 2022, 536: 12-17.
pmid: 36113556 |
| [70] |
Gao MM, Yang C, Wang XW, Guo MM, Yang L, Gao SS, Zhang X, Ruan GY, Li XP, Tian WH, Lu GT, Dong XY, Ma SS, Li WQ, Wang YH, Zhu HB, He JM, Yang HY, Liu G, Xian XD. ApoC2 deficiency elicits severe hypertriglyceridemia and spontaneous atherosclerosis: A rodent model rescued from neonatal death. Metabolism, 2020, 109: 154296.
pmid: 32562799 |
| [71] |
Gaudet D, Brisson D, Tremblay K, Alexander VJ, Singleton W, Hughes SG, Geary RS, Baker BF, Graham MJ, Crooke RM, Witztum JL. Targeting APOC3 in the familial chylomicronemia syndrome. N Engl J Med, 2014, 371(23): 2200-2206.
pmid: 25470695 |
| [72] |
Fuior EV, Gafencu AV. Apolipoprotein C1: its pleiotropic effects in lipid metabolism and beyond. Int J Mol Sci, 2019, 20(23): 5939.
pmid: 31779116 |
| [73] |
Zerbinatti CV, Mayer LP, Audet RG, Dyer CA. Apolipoprotein E is a putative autocrine regulator of the rat ovarian theca cell compartment. Biol Reprod, 2001, 64(4): 1080-1089.
pmid: 11259253 |
| [74] |
Yamazaki Y, Zhao N, Caulfield TR, Liu CC, Bu GJ. Apolipoprotein E and Alzheimer disease: pathobiology and targeting strategies. Nat Rev Neurol, 2019, 15(9): 501-518.
pmid: 31367008 |
| [75] |
Corbo RM, Ulizzi L, Scacchi R, Martínez-Labarga C, De Stefano GF. Apolipoprotein E polymorphism and fertility: a study in pre-industrial populations. Mol Hum Reprod, 2004, 10(8): 617-620.
pmid: 15220465 |
| [76] |
Trumble BC, Charifson M, Kraft T, Garcia AR, Cummings DK, Hooper P, Lea AJ, Eid Rodriguez D, Koebele SV, Buetow K, Beheim B, Minocher R, Gutierrez M, Thomas GS, Gatz M, Stieglitz J, Finch CE, Kaplan H, Gurven M. Apolipoprotein-ε4 is associated with higher fecundity in a natural fertility population. Sci Adv, 2023, 9(32): eade9797.
pmid: 37556539 |
| [77] |
Jasienska G, Ellison PT, Galbarczyk A, Jasienski M, Kalemba-Drozdz M, Kapiszewska M, Nenko I, Thune I, Ziomkiewicz A. Apolipoprotein E (ApoE) polymorphism is related to differences in potential fertility in women: a case of antagonistic pleiotropy? Proc Biol Sci, 2015, 282(1803): 20142395.
pmid: 25673673 |
| [78] |
Jiang HH, Si MF, Tian T, Shi HF, Huang N, Chi HB, Yang R, Long XY, Qiao J. Adiposity and lipid metabolism indicators mediate the adverse effect of glucose metabolism indicators on oogenesis and embryogenesis in PCOS women undergoing IVF/ICSI cycles. Eur J Med Res, 2023, 28(1): 216.
pmid: 37400924 |
| [79] |
Nagler L, Eißmann C, Wasenitz M, Bahlmann F, Naimi AA. The association between maternal obesity and fetomaternal outcomes in twin pregnancies. PLoS One, 2024, 19(7): e0306877.
pmid: 38985749 |
| [80] |
Stang J, Huffman LG. Position of the academy of nutrition and dietetics: obesity, reproduction, and pregnancy outcomes. J Acad Nutr Diet, 2016, 116(4): 677-691.
pmid: 27017177 |
| [81] |
Mączka K, Stasiak O, Przybysz P, Grymowicz M, Smolarczyk R. The impact of the endocrine and immunological function of adipose tissue on reproduction in women with obesity. Int J Mol Sci, 2024, 25(17): 9391.
pmid: 39273337 |
| [82] |
Toufaily C, Vargas A, Lemire M, Lafond J, Rassart E, Barbeau B. MFSD2a, the syncytin-2 receptor, is important for trophoblast fusion. Placenta, 2013, 34(1): 85-88.
pmid: 23177091 |
| [83] |
Aisike G, Kuerbanjiang M, Muheyati D, Zaibibuli K, Lv MX, Han J. Correlation analysis of obesity phenotypes with leptin and adiponectin. Sci Rep, 2023, 13(1): 17718.
pmid: 37853077 |
| [84] |
Serazin V, Duval F, Wainer R, Ravel C, Vialard F, Molina-Gomes D, Dieudonne MN, Dos Santos E. Are leptin and adiponectin involved in recurrent pregnancy loss? J Obstet Gynaecol Res, 2018, 44(6): 1015-1022.
pmid: 29536593 |
| [85] |
Cheng LX, Shi H, Jin Y, Li XX, Pan JS, Lai YM, Lin Y, Jin Y, Roy G, Zhao A, Li FH. Adiponectin deficiency leads to female subfertility and ovarian dysfunctions in mice. Endocrinology, 2016, 157(12): 4875-4887.
pmid: 27700136 |
| [86] |
Sarankhuu BE, Jeon HJ, Jeong DU, Park SR, Kim TH, Lee SK, Han AR, Yu SL, Kang J. Adiponectin receptor 1 regulates endometrial receptivity via the adenosine monophosphate‑activated protein kinase/E-cadherin pathway. Mol Med Rep, 2024, 30(4): 184.
pmid: 39155876 |
| [87] |
Qiao LP, Wattez JS, Lee S, Guo ZY, Schaack J, Hay WW Jr, Zita MM, Parast M, Shao JH. Knockout maternal adiponectin increases fetal growth in mice: potential role for trophoblast IGFBP-1. Diabetologia, 2016, 59(11): 2417-2425.
pmid: 27495989 |
| [88] |
Jiang JJ, Cai XL, Pan YS, Du XY, Zhu HP, Yang XH, Zheng DQ, Gaisano H, Wei TM, He Y. Relationship of obesity to adipose tissue insulin resistance. BMJ Open Diab Res Care, 2020, 8(1): e000741.
pmid: 32245824 |
| [89] |
De Boer AA, Monk JM, Liddle DM, Hutchinson AL, Power KA, Ma DWL, Robinson LE. Fish-oil-derived n-3 polyunsaturated fatty acids reduce NLRP3 inflammasome activity and obesity-related inflammatory cross- talk between adipocytes and CD11b+ macrophages. J Nutr Biochem, 2016, 34: 61-72.
pmid: 27208584 |
| [90] |
Yu CX, Liu SJ, Chen LQ, Shen J, Niu YM, Wang TY, Zhang WQ, Fu L. Effect of exercise and butyrate supplementation on microbiota composition and lipid metabolism. J Endocrinol, 2019, 243(2): 125-135.
pmid: 31454784 |
| [91] |
Wu XZ, Chen Z, Wu Y, Chen YF, Jia JP, Shen NQ, Chiba H, Hui SP. Flazin as a lipid droplet regulator against lipid disorders. Nutrients, 2022, 14(7): 1501.
pmid: 35406114 |
| [92] |
Baek SC, Nam KH, Yi SA, Jo MS, Lee KH, Lee YH, Lee J, Kim KH. Anti-adipogenic effect of β-carboline alkaloids from garlic (Allium sativum). Foods, 2019, 8(12): 673.
pmid: 31842405 |
| [93] |
Dibwe DF, Oba S, Takeishi N, Sakurai T, Tsukui T, Chiba H, Hui SP. Food-derived β-carboline alkaloids ameliorate lipid droplet accumulation in human hepatocytes. Pharmaceuticals (Basel), 2022, 15(5): 578.
pmid: 35631404 |
| [94] |
Gao Y, Zhang W, Zeng LQ, Bai H, Li J, Zhou J, Zhou GY, Fang CW, Wang F, Qin XJ. Exercise and dietary intervention ameliorate high-fat diet-induced NAFLD and liver aging by inducing lipophagy. Redox Biol, 2020, 36: 101635.
pmid: 32863214 |
| [95] |
Gitsi E, Livadas S, Argyrakopoulou G. Nutritional and exercise interventions to improve conception in women suffering from obesity and distinct nosological entities. Front Endocrinol (Lausanne), 2024, 15: 1426542.
pmid: 39006367 |
| [96] | Li L, Yang XY, Fang GL, Li PF, Feng LS. Effects of exercise on FGF-21 pathway functions in hepatic lipid metabolism. Med Sci Sports Exerc, 2017, 49(5S): 436. |
| [97] |
Yang CF, Wang C, Ye M, Jin CL, He WM, Wang F, McKeehan WL, Luo YD. Control of lipid metabolism by adipocyte FGFR1-mediated adipohepatic communication during hepatic stress. Nutr Metab (Lond), 2012, 9(1): 94.
pmid: 23106963 |
| [98] |
Cheang KI, Nestler JE. Should insulin-sensitizing drugs be used in the treatment of polycystic ovary syndrome? Reprod Biomed Online, 2004, 8(4): 440-447.
pmid: 15149568 |
| [99] |
Dewi M, Martianto D, Andarwulan N, Kazimierczak R, Średnicka-Tober D. Plant sterol-enriched palm oil intervention to improve lipid profile and inflammation status in hyperlipidemic individuals. Nutrients, 2024, 16(19): 3370.
pmid: 39408337 |
| [100] |
Davis HR, Veltri EP. Zetia: inhibition of Niemann-Pick C1 Like 1 (NPC1L1) to reduce intestinal cholesterol absorption and treat hyperlipidemia. J Atheroscler Thromb, 2007, 14(3): 99-108.
pmid: 17587760 |
| [101] |
Shepherd J. Mechanism of action of bile acid sequestrants and other lipid-lowering drugs. Cardiology, 1989, 76(Suppl 1): 65-74.
pmid: 2713876 |
| [102] |
Kulkarni S, Watts MM, Kostapanos M. Statins. BMJ, 2024, 384: e072584.
pmid: 38267068 |
| [103] | Shigemitsu A, Naruse K, Akasaka J, Tsunemi T, Yamada Y, Sado T, Kobayashi H. Lipid oxidative stress and deposition markers for feto-maternal interaction in pregnancy. J Reprod Immunol, 2016, 115: 35. |
| [104] |
Zejnullahu VA, Zejnullahu VA, Kosumi E. The role of oxidative stress in patients with recurrent pregnancy loss: a review. Reprod Health, 2021, 18(1): 207.
pmid: 34656123 |
| [105] |
Igosheva N, Abramov AY, Poston L, Eckert JJ, Fleming TP, Duchen MR, McConnell J. Maternal diet-induced obesity alters mitochondrial activity and redox status in mouse oocytes and zygotes. PLoS One, 2010, 5(4): e10074.
pmid: 20404917 |
| [106] |
Boggess KA, Oury TD, Kay HH, Crapo JD. Extracellular superoxide dismutase localization and activity within the human placenta. Placenta, 1998, 19(5-6): 417-422.
pmid: 9699963 |
| [107] | Bhatti TA. PIH3--role of antioxidants in recurrent pregnancy losses, low birth weight, and gestational duration. Value Health, 2013, 16(7): A709. |
| [108] |
Busso D, David A, Penailillo R, Echeverría G, Rigotti A, Kovalskys I, Gómez G, Cortés Sanabria LY, Yépez García MC, Pareja RG, Herrera-Cuenca M, Fisberg M, On Behalf Of The Elans Study Group. Intake of vitamin E and C in women of reproductive age: results from the latin american study of nutrition and health (ELANS). Nutrients, 2021, 13(6): 1954.
pmid: 34200192 |
| [109] |
Akarsu S, Gode F, Isik AZ, Dikmen ZG, Tekindal MA. The association between coenzyme Q10 concentrations in follicular fluid with embryo morphokinetics and pregnancy rate in assisted reproductive techniques. J Assist Reprod Genet, 2017, 34(5): 599-605.
pmid: 28185121 |
| [1] | Haoqiang Zhao, Xiaofei Wang, Shaopei Gao. Progress on the functional role of oleosin gene family in plants [J]. Hereditas(Beijing), 2022, 44(12): 1128-1140. |
| [2] | Zhiquan Tang, Li Shi, Jing Xiong. Progress of solute carrier SLC family in nonalcoholic fatty liver disease [J]. Hereditas(Beijing), 2022, 44(10): 881-898. |
| [3] | Wanzi Jiang, Liwen Zhang, Caihong He, Meihua Ruan, Yong Ji, Jianrong Yu, Hongwen Zhou. Progress on familial hypercholesterolemia [J]. Hereditas(Beijing), 2021, 43(11): 1011-1022. |
| [4] | Enquan Zhang, Weicong Cai, Guiling Li, Jian Li, Jingwen Liu. Analysis of microRNA expression profile in Emiliania huxleyi in response to virus infection [J]. Hereditas(Beijing), 2021, 43(11): 1088-1100. |
| [5] | Wanlong Huang,Xiuxiu Zhang,Ai Li,Xiangyang Miao. Identification of differentially expressed genes between subcutaneous and intramuscular adipose tissue of Large White pig using RNA-seq [J]. Hereditas(Beijing), 2017, 39(6): 501-511. |
| [6] | Jia Zheng, Xinhua Xiao, Qian Zhang, Miao Yu, Jianping Xu, Zhixin Wang, Yijing Liu, Mingmin Li. PPARγ links maternal malnutrition and abnormal glucose and lipid metabolism in the offspring of mice [J]. HEREDITAS(Beijing), 2015, 37(1): 70-76. |
| [7] | Li Wang, Yanmei Xu, Zhujun Cheng, Zhaoping Xiong, Libin Deng. The progress of genetics of cholesterol metabolism disorder [J]. HEREDITAS(Beijing), 2014, 36(9): 857-863. |
| [8] | Meiting Li, Linlin Cao, Yang Yang. The role of epigenetic modification in glucose and lipid metabolism [J]. HEREDITAS, 2014, 36(3): 200-207. |
| [9] | TANG Xiao-Li DENG Li-Bin LIN Jia-Ri ZHANG Wei-Long LIU Shuang-Mei WEI Yi MEI Pu-Ming WANG Yan LIANG Shang-Dong. Sterol regulatory element binding protein 1 and its target gene networks [J]. HEREDITAS, 2013, 35(5): 607-615. |
| [10] | XIE Yu-Xiao GAO Shi-Zheng ZHAO Su-Mei. Function of comparative gene identification-58 (CGI-58) on lipid metabolism in animals [J]. HEREDITAS, 2013, 35(5): 595-598. |
| [11] | DAI Yan-Fang, SUN Li-Yuan, ZHANG Xin-Bei, WANG Lu-Ya. Research progression of LDLR mutations in Chinese Familial hyper- cholesterolemia [J]. HEREDITAS, 2011, 33(1): 1-8. |
| [12] | JIAO Xu, TUN Fen-Fang, SU Feng, LI Qiang-Wei. Research progress on the MACPF/CDC family of pore-forming toxins [J]. HEREDITAS, 2010, 32(11): 1126-1132. |
| [13] | TANG Qing-Qiu, JIANG Jing-Yan, YANG Chu-Fen, JU Xiao-Ting, DONG Xiao-Yang. Research and development of Lipin family [J]. HEREDITAS, 2010, 32(10): 981-993. |
| [14] | XU Ying-Jie, WANG Lv-Ya. Research progress on the association between genetic variations in lipid metabolism and premature coronary artery disease [J]. HEREDITAS, 2008, 30(6): 671-676. |
| [15] | NI Yu, GUO Yan-Jun. Progress in the study on genes encoding enzymes involved in bio-synthesis of very long chain fatty acids and cuticular wax in plants [J]. HEREDITAS, 2008, 30(5): 561-567. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||