Hereditas(Beijing) ›› 2025, Vol. 47 ›› Issue (9): 967-978.doi: 10.16288/j.yczz.25-034
• Review • Previous Articles Next Articles
AARS1/2: dual functions in protein translation and metabolic- immune regulation
Zongwang Zhang1,2,3( ), Jingwei Xiong1,4(
), Jingwei Xiong1,4( )
)
- 1. Beijing Key Laboratory of Cardiometabolic Molecular Medicine, Institute of Molecular Medicine, College of Future Technology, Peking University, Beijing 100871, China
2. Center for Life Sciences, Shaoxing Institute, Zhejiang University, Shaoxing 321000, China
3. Zhejiang Provincial Key Laboratory of Cancer Molecular Cell Biology, Life Sciences Institute, Zhejiang University, Hangzhou 310058, China
4. School of Basic Medical Sciences and The Second Affiliated Hospital, Jiangxi Medical College, Nanchang University, Nanchang 330031, China
-
Received:2025-02-08Revised:2025-04-02Online:2025-04-03Published:2025-04-03 -
Contact:Jingwei Xiong E-mail:zhang_zw@zju.edu.cn;jingwei_xiong@pku.edu.cn -
Supported by:National Key R&D Program of China(2023YFA1800600);National Natural Science Foundation of China(32230032)
Cite this article
Zongwang Zhang, Jingwei Xiong. AARS1/2: dual functions in protein translation and metabolic- immune regulation[J]. Hereditas(Beijing), 2025, 47(9): 967-978.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
Table 1
Diseases caused by AARS1 mutations"
| 疾病名称 | 英文名 | 病变组织/器官/细胞 | AARS1表达模式 | 参考文献 |
|---|---|---|---|---|
| 成人发病的轴突球体和 色素胶质细胞白质脑病 | Adult-onset leukoencephalopathy with spheroids and pigmented glia | 轴突球体和色素胶质细胞 | 下调 | [ |
| 白质病变 | White matter disease | 髓鞘 | 下调 | [ |
| 复发性急性肝衰竭 | Recurrent acute liver failure | 成纤维细胞 | 下调 | [ |
| 腓骨肌萎缩症 | Charcot-marie-tooth disease | 神经细胞 | 下调 | [ |
| 硫化物发育不良症 | Trichothiodystrophy | 头发、指甲和皮肤 | 下调 | [ |
| 十二指肠癌 | Duodenal cancer | 十二指肠 | 上调 | [ |
| 结肠癌 | Colon adenocarcinoma | 结肠 | 上调 | [ |
Table 2
Diseases caused by AARS2 mutations"
| 疾病名称 | 英文名 | 病变组织/器官/细胞 | AARS2 表达模式 | 参考文献 |
|---|---|---|---|---|
| 结肠癌 | Colon adenocarcinoma | 结肠 | 上调 | [ |
| 成人发病的轴突球体和 色素胶质细胞白质脑病 | Adult-onset leukoencephalopathy with axonal spheroids and pigmented glia | 轴突球体和色素胶质细胞 | 下调 | [ |
| 脑白质病 | Leukodystrophy | 脑 | 下调 | [ |
| 肝细胞癌 | Hepatocellular carcinoma | 肝细胞 | 上调 | [ |
| 心肌病 | Cardiomyopathy | 心肌细胞 | 下调 | [ |
| 卵巢发育不全 | Ovarian insufficiency | 卵巢 | 下调 | [ |
| [1] |
Steiner RE, Ibba M. Regulation of tRNA-dependent translational quality control. IUBMB Life, 2019, 71(8): 1150-1157.
pmid: 31135095 |
| [2] |
Kunkel TA, Bebenek K. DNA replication fidelity. Annu Rev Biochem, 2000, 69: 497-529.
pmid: 10966467 |
| [3] |
Bouadloun,Donner D, Kurland CG. Codon-specific missense errors in vivo. EMBO J, 1983, 2(8): 1351-1356.
pmid: 10872330 |
| [4] |
Ognjenović J, Simonović M. Human aminoacyl-tRNA synthetases in diseases of the nervous system. RNA Biol, 2018, 15(4-5): 623-634.
pmid: 28534666 |
| [5] |
Meyer-Schuman R, Antonellis A. Emerging mechanisms of aminoacyl-tRNA synthetase mutations in recessive and dominant human disease. Hum Mol Genet, 2017, 26(R2): R114-R127.
pmid: 28633377 |
| [6] |
Sissler M, González-Serrano LE, Westhof E. Recent advances in mitochondrial aminoacyl-tRNA synthetases and disease. Trends Mol Med, 2017, 23(8): 693-708.
pmid: 28716624 |
| [7] |
Shimada N, Matsuzaki K, Suzuki T, Suzuki T, Watanabe K. Quality control of translation through the kinetic discrimination of tRNAs in the network of aminoacyl-tRNA synthetases. Nucleic Acids Res Suppl, 2022, (2): 79-80.
pmid: 12903114 |
| [8] |
Lu YW, Liang ZM, Guo HP, Fernandes T, Espinoza-Lewis RA, Wang TT, Li K, Li X, Singh GB, Wang Y, Cowan D, Mably JD, Philpott CC, Chen H, Wang DZ. PCBP1 regulates alternative splicing of AARS2 in congenital cardiomyopathy. bioRxiv, 2023, doi: 10.1101/2023.05.18.540420.
pmid: 37293078 |
| [9] |
Lakshmanan R, Adams ME, Lynch DS, Kinsella JA, Phadke R, Schott JM, Murphy E, Rohrer JD, Chataway J, Houlden H, Fox NC, Davagnanam I. Redefining the phenotype of ALSP and AARS2 mutation-related leukodystrophy. Neurol Genet, 2017, 3(2): e135.
pmid: 28243630 |
| [10] |
De Michele G, Maione L, Cocozza S, Tranfa M, Pane C, Galatolo D, De Rosa A, De Michele G, Saccà F, Filla A. Ataxia and hypogonadism: a review of the associated genes and syndromes. Cerebellum, 2024, 23(2): 688-701.
pmid: 36997834 |
| [11] |
Almuqbil MA, Vernon HJ, Ferguson M, Kline AD. PARS2-associated mitochondrial disease:a case report of a patient with prolonged survival and literature review. Mol Genet Metab Rep, 2020, 24: 100613.
pmid: 32514400 |
| [12] |
Dallabona C, Diodato D, Kevelam SH, Haack TB, Wong LJ, Salomons GS, Baruffini E, Melchionda L, Mariotti C, Strom TM, Meitinger T, Prokisch H, Chapman K, Colley A, Rocha H, Ounap K, Schiffmann R, Salsano E, Savoiardo M, Hamilton EM, Abbink TE, Wolf NI, Ferrero I, Lamperti C, Zeviani M, Vanderver A, Ghezzi D, van der Knaap MS. Novel (ovario) leukodystrophy related to AARS2 mutations. Neurology, 2014, 82(23): 2063-2071.
pmid: 24808023 |
| [13] |
Ravel JM, Dreumont N, Mosca P, Smith DEC, Mendes MI, Wiedemann A, Coelho D, Schmitt E, Rivière JB, Tran Mau-Them F, Thevenon J, Kuentz P, Polivka M, Fuchs SA, Kok G, Thauvin-Robinet C, Guéant JL, Salomons GS, Faivre L, Feillet F. A bi-allelic loss-of-function SARS1 variant in children with neurodevelopmental delay, deafness, cardiomyopathy, and decompensation during fever. Hum Mutat, 2021, 42(12): 1576-1583.
pmid: 34570399 |
| [14] |
Skupińska M, Belter A, Giel-Pietraszuk M, Rychlewski L, Barciszewski J. AARS--the etiological factor and the attractive target of many disorders. Postepy Biochem, 2009, 55(4): 373-384.
pmid: 20201350 |
| [15] |
Antonellis A, Green ED. The role of aminoacyl-tRNA synthetases in genetic diseases. Annu Rev Genomics Hum Genet, 2008, 9: 87-107.
pmid: 18767960 |
| [16] |
Kuo ME, Parish M, Jonatzke KE, Antonellis A. Comprehensive assessment of recessive, pathogenic AARS1 alleles in a humanized yeast model reveals loss-of-function and dominant-negative effects. bioRxiv, 2024, doi: 10.1101/2024.06.20.599900.
pmid: 38979321 |
| [17] |
Wu JY, Liu TT, Zhang BY, Liu C, Luan XH, Cao L. An AARS1 variant identified to cause adult-onset leukoencephalopathy with neuroaxonal spheroids and pigmented glia. Transl Neurodegener, 2023, 12(1): 19.
pmid: 37106376 |
| [18] |
Simons C, Griffin LB, Helman G, Golas G, Pizzino A, Bloom M, Murphy JL, Crawford J, Evans SH, Topper S, Whitehead MT, Schreiber JM, Chapman KA, Tifft C, Lu KB, Gamper H, Shigematsu M, Taft RJ, Antonellis A, Hou YM, Vanderver A. Loss-of-function alanyl-tRNA synthetase mutations cause an autosomal-recessive early-onset epileptic encephalopathy with persistent myelination defect. Am J Hum Genet, 2015, 96(4): 675-681.
pmid: 25817015 |
| [19] |
Nakayama T, Wu J, Galvin-Parton P, Weiss J, Andriola MR, Hill RS, Vaughan DJ, El-Quessny M, Barry BJ, Partlow JN, Barkovich AJ, Ling JQ, Mochida GH. Deficient activity of alanyl-tRNA synthetase underlies an autosomal recessive syndrome of progressive microcephaly, hypomyelination, and epileptic encephalopathy. Hum Mutat, 2017, 38(10): 1348-1354.
pmid: 28493438 |
| [20] |
Marten LM, Brinkert F, Smith DEC, Prokisch H, Hempel M, Santer R. Recurrent acute liver failure in alanyl-tRNA synthetase-1 (AARS1) deficiency. Mol Genet Metab Rep, 2020, 25: 100681.
pmid: 33294374 |
| [21] |
Helman G, Mendes MI, Nicita F, Darbelli L, Sherbini O, Moore T, Derksen A, Amy P, Carrozzo R, Torraco A, Catteruccia M, Aiello C, Goffrini P, Figuccia S, Smith DEC, Hadzsiev K, Hahn A, Biskup S, Brösse I, Kotzaeridou U, Gauck D, Grebe TA, Elmslie F, Stals K, Gupta R, Bertini E, Thiffault I, Taft RJ, Schiffmann R, Brandl U, Haack TB, Salomons GS, Simons C, Bernard G, van der Knaap MS, Vanderver A, Husain RA. Expanded phenotype of AARS1-related white matter disease. Genet Med, 2021, 23(12): 2352-2359.
pmid: 34446925 |
| [22] |
Botta E, Theil AF, Raams A, Caligiuri G, Giachetti S, Bione S, Accadia M, Lombardi A, Smith DEC, Mendes MI, Swagemakers SMA, van der Spek PJ, Salomons GS, Hoeijmakers JHJ, Yesodharan D, Nampoothiri S, Ogi T, Lehmann AR, Orioli D, Vermeulen W. Protein instability associated with AARS1 and MARS1 mutations causes trichothiodystrophy. Hum Mol Genet, 2021, 30(18): 1711-1720.
pmid: 33909043 |
| [23] |
Li LL, Jiang DX, Liu H, Guo CM, Zhao R, Zhang Q, Xu C, Qin ZY, Feng JW, Liu Y, Wang HX, Chen WJ, Zhang X, Li B, Bai L, Tian S, Tan SB, Yu ZX, Chen LL, Huang J, Zhao JY, Hou YY, Ding C. Comprehensive proteogenomic characterization of early duodenal cancer reveals the carcinogenesis tracks of different subtypes. Nat Commun, 2023, 14(1): 1751.
pmid: 36991000 |
| [24] |
Jin BB, Xie LQ, Zhan D, Zhou LP, Feng Z, He JY, Qin J, Zhao CJ, Luo LF, Li L. Nrf2 dictates the neuronal survival and differentiation of embryonic zebrafish harboring compromised alanyl-tRNA synthetase. Development, 2022, 149(17): dev200342.
pmid: 35929539 |
| [25] |
Dulski J, Koga S, Dickson DW, Wszolek ZK. Report of a family with adult-onset leukoencephalopathy with axonal spheroids and pigmented glia (ALSP) without mutations in CSF1R, AARS1 or AARS2. Mov Disord Clin Pract, 2023, 10(2): 307-312.
pmid: 36825047 |
| [26] |
Huang HS, Zhang Y, Yang MX, Lian BR, Guo R, Cao LM. Case report:early-onset charcot-marie-tooth 2N with reversible white matter lesions repeatedly mimicked stroke or encephalitis. Front Pediatr, 2022, 10: 935721.
pmid: 35911843 |
| [27] |
Lee AJ, Nam DE, Choi YJ, Nam SH, Choi BO, Chung KW. Alanyl-tRNA synthetase 1 (AARS1) gene mutation in a family with intermediate Charcot-Marie-Tooth neuropathy. Genes Genomics, 2020, 42(6): 663-672.
pmid: 32314272 |
| [28] |
Høyer H, Busk ØL, Esbensen QY, Røsby O, Hilmarsen HT, Russell MB, Nyman TA, Braathen GJ, Nilsen HL. Clinical characteristics and proteome modifications in two Charcot-Marie-Tooth families with the AARS1 Arg326Trp mutation. BMC Neurol, 2022, 22(1): 299.
pmid: 35971119 |
| [29] |
Setlere S, Jurcenko M, Gailite L, Rots D, Kenina V. Alanyl-tRNA synthetase 1 gene variants in hereditary neuropathy: genotype and phenotype overview. Neurol Genet, 2022, 8(5): e200019.
pmid: 36092982 |
| [30] |
Forrest ME, Meyer AP, Laureano Figueroa SM, Antonellis A. A missense, loss-of-function YARS1 variant in a patient with proximal-predominant motor neuropathy. Cold Spring Harb Mol Case Stud, 2022, 8(7): a006246.
pmid: 36307205 |
| [31] |
Sun LT, Wei N, Kuhle B, Blocquel D, Novick S, Matuszek Z, Zhou HH, He WW, Zhang JJ, Weber T, Horvath R, Latour P, Pan T, Schimmel P, Griffin PR, Yang XL. CMT2N-causing aminoacylation domain mutants enable Nrp1 interaction with AlaRS. Proc Natl Acad Sci USA, 2021, 118(13): e2012898118.
pmid: 33753480 |
| [32] |
Miressi F, Magdelaine C, Cintas P, Bourthoumieux S, Nizou A, Derouault P, Favreau F, Sturtz F, Faye PA, Lia AS. One multilocus genomic variation is responsible for a severe charcot-marie-tooth axonal form. Brain Sci, 2020, 10(12): 986.
pmid: 33333791 |
| [33] |
Ruan GT, Xie HL, Zhu LC, Ge YZ, Yan L, Liao C, Gong YZ, Shi HP. Immune ULBP1 is elevated in colon adenocarcinoma and predicts prognosis. Front Genet, 2022, 13: 762514.
pmid: 35211154 |
| [34] |
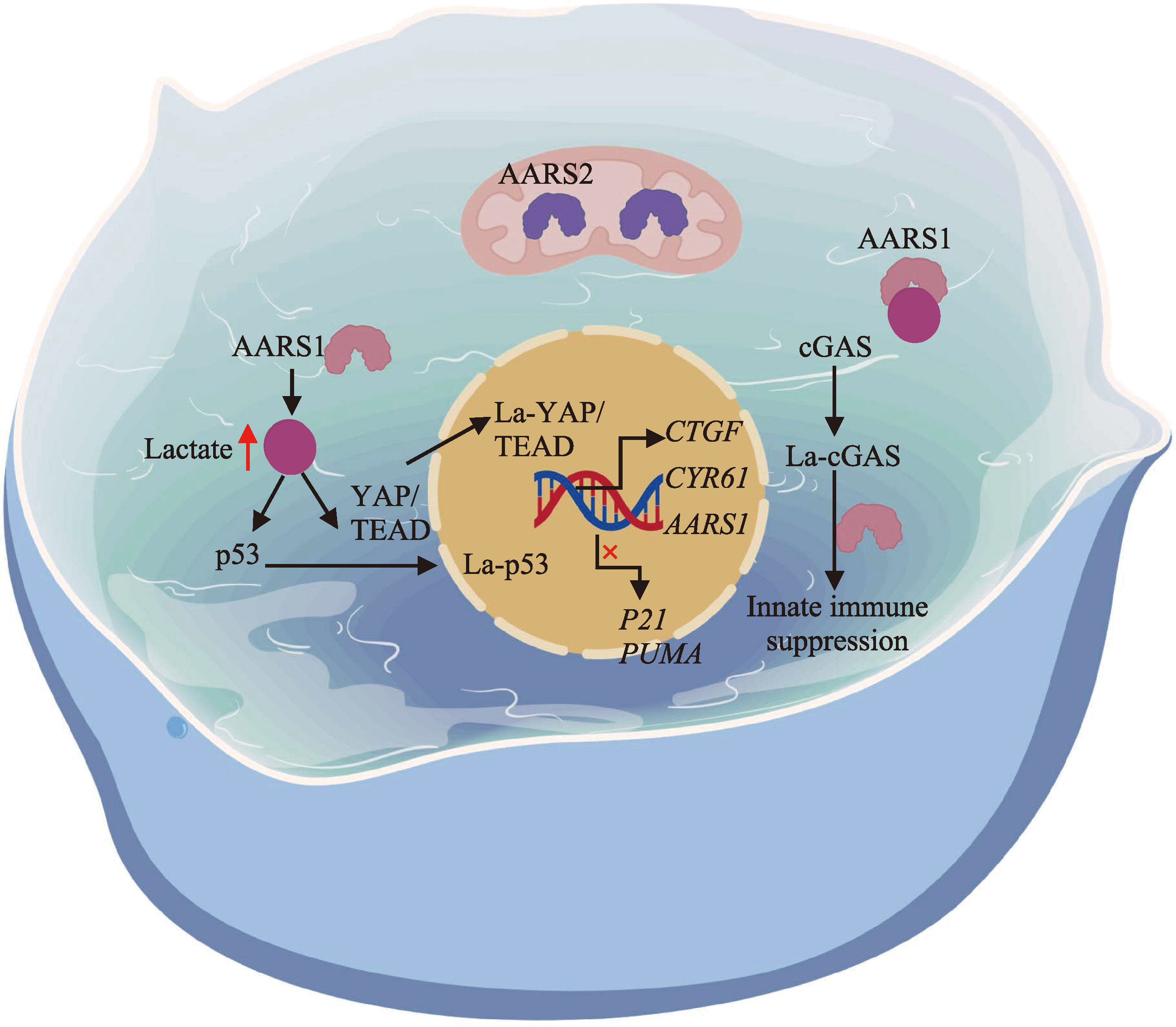
Zong Z, Xie F, Wang S, Wu XJ, Zhang ZY, Yang B, Zhou FF. Alanyl-tRNA synthetase, AARS1, is a lactate sensor and lactyltransferase that lactylates p53 and contributes to tumorigenesis. Cell, 2024, 187(10): 2375-2392.e33.
pmid: 38653238 |
| [35] |
Shiba K, Ripmaster T, Suzuki N, Nichols R, Plotz P, Noda T, Schimmel P. Human alanyl-tRNA synthetase: conservation in evolution of catalytic core and microhelix recognition. Biochemistry, 1995, 34(33): 10340-10349.
pmid: 7654687 |
| [36] |
Ju JY, Zhang H, Lin MB, Yan ZF, An LW, Cao ZF, Geng DD, Yue JW, Tang Y, Tian LY, Chen F, Han Y, Wang WJ, Zhao SM, Jiao S, Zhou ZC. The alanyl-tRNA synthetase AARS1 moonlights as a lactyltransferase to promote YAP signaling in gastric cancer. J Clin Invest, 2024, 134(10): e174587.
pmid: 38512451 |
| [37] |
Euro L, Konovalova S, Asin-Cayuela J, Tulinius M, Griffin H, Horvath R, Taylor RW, Chinnery PF, Schara U, Thorburn DR, Suomalainen A, Chihade J, Tyynismaa H. Structural modeling of tissue-specific mitochondrial alanyl-tRNA synthetase (AARS2) defects predicts differential effects on aminoacylation. Front Genet, 2015, 6: 21.
pmid: 25705216 |
| [38] |
Nielsen SK, Hansen F, Schrøder HD, Wibrand F, Gustafsson F, Mogensen J. Recessive inheritance of a rare variant in the nuclear mitochondrial gene for AARS2 in late-onset dilated cardiomyopathy. Circ Genom Precis Med, 2020, 13(5): 560-562.
pmid: 32938192 |
| [39] |
Zhang X, Li J, Zhang YY, Gao MN, Peng T, Tian T. AARS2-related leukodystrophy:a case report and literature review. Cerebellum, 2023, 22(1): 59-69.
pmid: 35084689 |
| [40] |
Parra SP, Heckers SH, Wilcox WR, McKnight CD, Jinnah HA. The emerging neurological spectrum of AARS2- associated disorders. Parkinsonism Relat Disord, 2021, 93: 50-54.
pmid: 34784527 |
| [41] |
Mao YZ, Zhang JJ, Zhou Q, He XD, Zheng ZF, Wei Y, Zhou KQ, Lin Y, Yu HW, Zhang HH, Zhou YN, Lin PC, Wu BX, Yuan YY, Zhao JY, Xu W, Zhao SM. Hypoxia induces mitochondrial protein lactylation to limit oxidative phosphorylation. Cell Res, 2024, 34(1): 13-30.
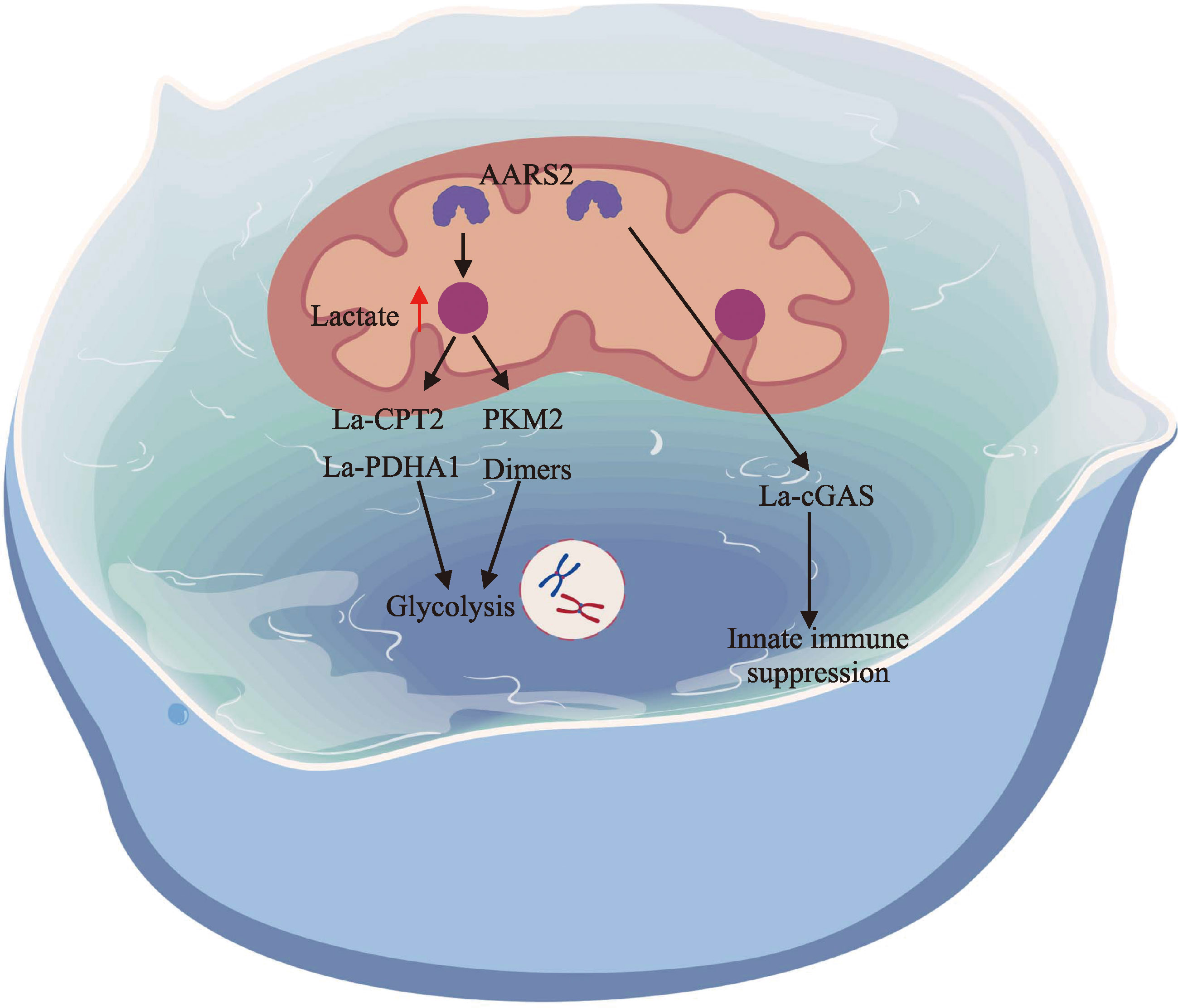
pmid: 38163844 |
| [42] |
Vasilescu C, Ojala TH, Brilhante V, Ojanen S, Hinterding HM, Palin E, Alastalo TP, Koskenvuo J, Hiippala A, Jokinen E, Jahnukainen T, Lohi J, Pihkala J, Tyni TA, Carroll CJ, Suomalainen A. Genetic basis of severe childhood-onset cardiomyopathies. J Am Coll Cardiol, 2018, 72(19): 2324-2338.
pmid: 30384889 |
| [43] |
Mazurova S, Magner M, Kucerova-Vidrova V, Vondrackova A, Stranecky V, Pristoupilova A, Zamecnik J, Hansikova H, Zeman J, Tesarova M, Honzik T. Thymidine kinase 2 and alanyl-tRNA synthetase 2 deficiencies cause lethal mitochondrial cardiomyopathy: case reports and review of the literature. Cardiol Young, 2017, 27(5): 936-944.
pmid: 27839525 |
| [44] |
Lv LL, Huang YQ, Li Q, Wu Y, Zheng L. A comprehensive prognostic model for colon adenocarcinoma depending on nuclear-mitochondrial-related genes. Technol Cancer Res Treat, 2024, 23: 15330338241258570.
pmid: 38832431 |
| [45] |
Chen ZL, Mei K, Xiao Y, Xiong Y, Long W, Wang Q, Zhong J, Di DM, Ge YX, Luo Y, Li ZY, Huang Y, Gu RJ, Wang B. Prognostic assessment of oxidative stress-related genes in colorectal cancer and new insights into tumor immunity. Oxid Med Cell Longev, 2022, 2022: 2518340.
pmid: 36299603 |
| [46] |
Zhu ZY, Hou QS, Wang BS, Li CH, Liu LG, Gong WP, Chai J, Guo HL. A novel mitochondria-related gene signature for controlling colon cancer cell mitochondrial respiration and proliferation. Hum Cell, 2022, 35(4): 1126-1139.
pmid: 35429326 |
| [47] |
Kinoshita M, Oyanagi K, Matsushima A, Kondo Y, Hirano S, Ishizawa K, Ishihara K, Terada S, Inoue T, Yazawa I, Washimi Y, Yamada M, Nakayama J, Mitsuyama Y, Ikeda SI, Sekijima Y. Adult-onset leukoencephalopathy with axonal spheroids and pigmented glia (ALSP): estimation of pathological lesion stage from brain images. J Neurol Sci, 2024, 461: 123027.
pmid: 38805875 |
| [48] |
Ferrer I. The primary microglial leukodystrophies: a review. Int J Mol Sci, 2022, 23(11): 6341.
pmid: 35683020 |
| [49] |
Fan Y, Han JM, Yang YY, Chen TZ. Novel mitochondrial alanyl-tRNA synthetase 2 (AARS2) heterozygous mutations in a Chinese patient with adult-onset leukoencephalopathy. BMC Neurol, 2022, 22(1): 214.
pmid: 35676634 |
| [50] |
Wang JY, Chen SF, Zhang HQ, Wang MY, Zhu JH, Zhang X. A homozygous mutation of alanyl-transfer RNA synthetase 2 in a patient of adult-onset leukodystrophy: a case report and literature review. Brain Behav, 2019, 9(7): e01313.
pmid: 31106991 |
| [51] |
Wang DQ, Yu M, Zhang W, Wang ZX, Yuan Y. AARS2 compound heterozygous variants in a case of adult-onset leukoencephalopathy with axonal spheroids and pigmented glia. J Neuropathol Exp Neurol, 2018, 77(11): 997-1000.
pmid: 30272204 |
| [52] |
Lynch DS, Zhang WJ, Lakshmanan R, Kinsella JA, Uzun GA, Karbay M, Tüfekçioglu Z, Hanagasi H, Burke G, Foulds N, Hammans SR, Bhattacharjee A, Wilson H, Adams M, Walker M, Nicoll JAR, Chataway J, Fox N, Davagnanam I, Phadke R, Houlden H. Analysis of mutations in AARS2 in a series of CSF1R-negative patients with adult-onset leukoencephalopathy with axonal spheroids and pigmented glia. JAMA Neurol, 2016, 73(12): 1433-1439.
pmid: 27749956 |
| [53] |
Green K, MacIver CL, Ebden S, Rees DA, Peall KJ. Pearls & oy-sters: AARS2 leukodystrophy-tremor and tribulations. Neurology, 2024, 102(8): e209296.
pmid: 38507676 |
| [54] |
Kazakova E, Téllez-Martínez JA, Flores-Lagunes L, Sosa-Ortiz AL, Carillo-Sánchez K, Molina-Garay C, González-Domínguez CA, Jimenez-Olivares M, Fernandez- Valverde F, Vargas-Cañas ES, Vázquez-Memije ME, Garcia-Latorre EA, Martínez-Duncker I, Alaez-Verson C. Uterus infantilis: a novel phenotype associated with AARS2 new genetic variants. A case report. Front Neurol, 2023, 14: 878446.
pmid: 37456626 |
| [55] |
Li YJ, Xu JM, Xu Y, Li CZ, Wu Y, Liu ZJ. Clinical, genetic, and molecular characteristics in a central-southern Chinese cohort of genetic leukodystrophies. Ann Clin Transl Neurol, 2023, 10(9): 1556-1568.
pmid: 37434390 |
| [56] |
Chakraborty AP, Mukherjee A, Bhattacharyya A, Bhattacharyya D, Ray BK, Biswas A. Gait apraxia with exaggerated upper limb movements as presentation of AARS2 related leukoencephalopathy. Tremor Other Hyperkinet Mov (N Y), 2022, 12: 24.
pmid: 35975211 |
| [57] |
Axelsen TM, Vammen TL, Bak M, Pourhadi N, Stenør CM, Grønborg S. Case report: 'AARS2 leukodystrophy'. Mol Genet Metab Rep, 2021, 28: 100782.
pmid: 34285876 |
| [58] |
De Cocker LJL, Castillo M. Distinctive diffusion- weighted imaging features in late-onset genetic leukoencephalopathies. Neuroradiology, 2021, 63(1): 153-156.
pmid: 32879996 |
| [59] |
Wang XG, Wang Q, Tang HF, Chen B, Dong X, Niu ST, Li SW, Shi YZ, Shan W, Zhang ZQ. Novel alanyl-tRNA synthetase 2 pathogenic variants in leukodystrophies. Front Neurol, 2019, 10: 1321.
pmid: 31920941 |
| [60] |
Tang Y, Qin Q, Xing Y, Guo DM, Di L, Jia JP. AARS2 leukoencephalopathy: a new variant of mitochondrial encephalomyopathy. Mol Genet Genomic Med, 2019, 7(4): e00582.
pmid: 30706699 |
| [61] |
Liu L, Gao J, Liu XD, Zhang F, Hu BW, Zhang HP, Wang ZH, Tang HW, Shi JH, Zhang SJ. AARS2 as a novel biomarker for prognosis and its molecular characterization in pan-cancer. Cancer Med, 2023, 12(23): 21531-21544.
pmid: 37990642 |
| [62] |
Sommerville EW, Zhou XL, OláhováM, Jenkins J, Euro L, Konovalova S, Hilander T, Pyle A, He LP, Habeebu S, Saunders C, Kelsey A, Morris AAM, McFarland R, Suomalainen A, Gorman GS, Wang ED, Thiffault I, Tyynismaa H, Taylor RW. Instability of the mitochondrial alanyl-tRNA synthetase underlies fatal infantile-onset cardiomyopathy. Hum Mol Genet, 2019, 28(2): 258-268.
pmid: 30285085 |
| [63] |
Kamps R, Szklarczyk R, Theunissen TE, Hellebrekers DMEI, Sallevelt SCEH, Boesten IB, de Koning B, van den Bosch BJ, Salomons GS, Simas-Mendes M, Verdijk R, Schoonderwoerd K, de Coo IFM, Vanoevelen JM, Smeets HJM. Genetic defects in mtDNA-encoded protein translation cause pediatric, mitochondrial cardiomyopathy with early-onset brain disease. Eur J Hum Genet, 2018, 26(4): 537-551.
pmid: 29440775 |
| [64] | Zhang ZW, Zheng LX, Chen Y, Chen YY, Hou JJ, Xiao CL, Zhu XJ, Zhao SM, Xiong JW. AARS2 ameliorates myocardial ischemia via fine-tuning PKM2-mediated metabolism. bioRxiv, 2024, doi: 10.1101/2024.06.04.597368. |
| [65] |
França MM, Mendonca BB. Genetics of ovarian insufficiency and defects of folliculogenesis. Best Pract Res Clin Endocrinol Metab, 2022, 36(1): 101594.
pmid: 34794894 |
| [66] |
Tiosano D, Mears JA, Buchner DA. Mitochondrial dysfunction in primary ovarian insufficiency. Endocrinology, 2019, 160(10): 2353-2366.
pmid: 31393557 |
| [67] |
Zhou YR, Chen BL, Li L, Pan H, Liu BH, Li TY, Wang RY, Ma X, Wang BB, Cao YX. Novel alanyl-tRNA synthetase 2 (AARS2) homozygous mutation in a consanguineous Chinese family with premature ovarian insufficiency. Fertil Steril, 2019, 112(3): 569-576.e562.
pmid: 31280959 |
| [68] |
Taglia I, Di Donato I, Bianchi S, Cerase A, Monti L, Marconi R, Orrico A, Rufa A, Federico A, Dotti MT. AARS2-related ovarioleukodystrophy: clinical and neuroimaging features of three new cases. Acta Neurol Scand, 2018, 138(4): 278-283.
pmid: 29749055 |
| [69] |
Lee JM, Yang HJ, Kwon JH, Kim WJ, Kim SY, Lee EM, Park JY, Weon YC, Park SH, Gwon BJ, Ryu JC, Lee ST, Kim HJ, Jeon B. Two Korean siblings with recently described ovarioleukodystrophy related to AARS2 mutations. Eur J Neurol, 2017, 24(4): e21-e22.
pmid: 28322004 |
| [70] |
Götz A, Tyynismaa H, Euro L, Ellonen P, Hyötyläinen T, Ojala T, Hämäläinen RH, Tommiska J, Raivio T, Oresic M, Karikoski R, Tammela O, Simola KOJ, Paetau A, Tyni T, Suomalainen A. Exome sequencing identifies mitochondrial alanyl-tRNA synthetase mutations in infantile mitochondrial cardiomyopathy. Am J Hum Genet, 2011, 88(5): 635-642.
pmid: 21549344 |
| [71] |
Méndez-Lucas A, Li XL, Hu JJ, Che L, Song XH, Jia JY, Wang JX, Xie CC, Driscoll PC, Tschaharganeh DF, Calvisi DF, Yuneva M, Chen X. Glucose catabolism in liver tumors induced by c-MYC can be sustained by various PKM1/PKM2 ratios and pyruvate kinase activities. Cancer Res, 2017, 77(16): 4355-4364.
pmid: 28630053 |
| [72] |
Wong N, Ojo D, Yan J, Tang DM. PKM2 contributes to cancer metabolism. Cancer Lett, 2015, 356(2 Pt A): 184-191.
pmid: 24508027 |
| [73] |
Li HY, Liu C, Li R, Zhou LL, Ran Y, Yang QQ, Huang HZ, Lu HS, Song H, Yang B, Ru H, Lin SX, Zhang L. AARS1 and AARS2 sense L-lactate to regulate cGAS as global lysine lactyltransferases. Nature, 2024, 634(8036): 1229-1237.
pmid: 39322678 |
| [1] | Wenhui Nan, Xunsi Qin, Rong Li. Lipid metabolism imbalance: potential pathological mechanism and new intervention ideas for recurrent miscarriage [J]. Hereditas(Beijing), 2025, 47(9): 979-991. |
| [2] | Mengting An, Guanlin Guo, Jie Wu, Wenjing Sun, Xueyuan Jia. Analysis of regulatory mechanisms of enhancers in gastric cancer with double minute chromosomes based on bioinformatics [J]. Hereditas(Beijing), 2025, 47(5): 558-572. |
| [3] | Can Liu, Weiwei Zhai, Xuemei Lu. Evolutionary ecology in tumor evolution: concept, application and innovation [J]. Hereditas(Beijing), 2025, 47(2): 228-236. |
| [4] | Zhang Yiwen, Huang Qin, Wu Yanyun, Sun Yue, Wei Yonglong. Progress on the role of LIN28A/B in tumor development and progression [J]. Hereditas(Beijing), 2024, 46(6): 452-465. |
| [5] | Yuan Shen, Jintao Li, Miao Yin, Qunying Lei. The roles of branched-chain amino acids metabolism in tumorigenesis and progression [J]. Hereditas(Beijing), 2024, 46(6): 438-451. |
| [6] | Mingjie Sun, Jiali Lu, Yue Pang. Lamprey——an excellent model for iron metabolism [J]. Hereditas(Beijing), 2024, 46(5): 387-397. |
| [7] | Hui Li, Guangming Wu. Progress on the relationship between tumor suppressor PDCD4 and diseases based on the analysis of structural characteristics [J]. Hereditas(Beijing), 2024, 46(4): 290-305. |
| [8] | Xu Yan, Ying Guo, Donglin Sun, Nan Wu, Yan Jin. Drug resistance mechanism of anti-angiogenesis therapy in tumor [J]. Hereditas(Beijing), 2024, 46(11): 911-919. |
| [9] | Xin Wen, Jin Mei, Meiyu Qian, Yidan Jiang, Juan Wang, Shibo Xu, Cuizhe Wang, Jun Zhang. Screening and analysis of GULP1 downstream target genes based on transcriptomic sequencing [J]. Hereditas(Beijing), 2024, 46(10): 860-870. |
| [10] | Yuxin Wan, Xinyu Zhu, Yu Zhao, Na Sun, Tiantongfei Jiang, Juan Xu. Computational dissection of the regulatory mechanisms of aberrant metabolism in remodeling the microenvironment of breast cancer [J]. Hereditas(Beijing), 2024, 46(10): 871-885. |
| [11] | Qingyu Sun, Yang Zhou, Lijuan Du, Mengke Zhang, Jiale Wang, Yuanyuan Ren, Fang Liu. Analysis between macrophage-related genes with prognosis and tumor microenvironment in non-small cell lung cancer [J]. Hereditas(Beijing), 2023, 45(8): 684-699. |
| [12] | Chenghao Yan, Weiyu Bai, Zhimeng Zhang, Junling Shen, Youjun Wang, Jianwei Sun. The roles and mechanism of STIM1 in tumorigenesis and metastasis [J]. Hereditas(Beijing), 2023, 45(5): 395-408. |
| [13] | Chunhui Ma, Haixu Hu, Lijuan Zhang, Yi Liu, Tianyi Liu. Establishment and verification of a digital PCR assay for the detection of CK19 expression in quantitative analysis of circulating tumor cell [J]. Hereditas(Beijing), 2023, 45(3): 250-260. |
| [14] | Mao Shuyu, Zhao Changrui, Liu Chang. The nuclear receptor REV-ERBα integrates circadian clock and energy metabolism [J]. Hereditas(Beijing), 2023, 45(2): 99-114. |
| [15] | Dong Chang, Xiangxiang Liu, Rui Liu, Jianwei Sun. The role and regulatory mechanism of FSCN1 in breast tumorigenesis and progression [J]. Hereditas(Beijing), 2023, 45(2): 115-127. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||