骨折是由创伤引起的常见的骨骼疾病,现已成为重大的公共健康事件与社会经济负担[1]。尽管治疗方法不断改进,但仍有10%的骨折患者表现出骨折愈合延迟乃至不愈合的情况,最终导致患者生活质量显著下降[2,3]。骨折愈合过程复杂,大致可分为急性充血水肿期、急性炎症反应期、肉芽组织期、骨痂形成期与骨痂重构期5个时期[4]。整个过程精密有序,多种细胞与细胞因子参与其中。破骨细胞作为骨吸收的关键细胞,不仅参与骨折早期破碎骨组织的清理,还参与骨折愈合后期的骨痂形成与骨痂重塑[5,6]。临床研究显示,破骨细胞减少或功能障碍可导致骨硬化症或致密性成骨不全[7,8]。患有这类疾病的病人易出现骨损伤,存在骨折延迟愈合乃至不愈合的情况[7]。然而,目前临床上尚缺乏治疗破骨细胞缺陷所致骨折愈合障碍的药物。
破骨细胞是一种负责骨吸收的组织驻留巨噬细胞,起源于造血干细胞来源的单核细胞/巨噬细胞系[9]。许多转录因子和细胞因子在破骨细胞形成和成熟过程中起着不可或缺的作用,如巨噬细胞集落刺激因子(macrophage colony-stimulating factor,M-CSF,又叫CSF-1)、巨噬细胞集落刺激因子受体(colony- stimulating factor 1 receptor,CSF1R,又叫C-FMS)以及核因子κB活化因子受体配体(receptor activator of nuclear factor-κB ligand,RANKL)[10,11]。C-FMS是一种III型受体酪氨酸激酶(receptor tyrosine kinase,RTK),其与集落刺激因子M-CSF结合后可刺激核因子κB活化因子受体(receptor activator of nuclear factor-κB,RANK)的表达[12]。RANK是一种I型跨膜蛋白,属于肿瘤坏死因子(tumor necrosis factor,TNF)受体超家族成员,它高度表达在破骨细胞祖细胞和成熟破骨细胞的细胞膜上,而RANKL则为骨细胞中高表达的TNF相关活化诱导细胞因子,在破骨细胞上RANK与其配体RANKL结合后激活TNF受体相关因子(TNF receptor-associated factors,TRAFs)家族,通过一系列的信号级联传导途径调节破骨细胞的生成、激活和存活[13,14]。研究显示,CSF-1R缺陷小鼠(Mus musculus)和斑马鱼(Danio rerio)会出现功能性破骨细胞数量的减少[15⇓~17]。更为重要的是,对几个临床家系的研究发现,CSF-1R双等位基因突变会导致严重的脑畸形和骨硬化症[18]。破骨细胞缺陷型斑马鱼为研究破骨细胞缺陷型骨折愈合的过程和治疗提供了合适的研究材料。
本研究首先使用斑马鱼离体鳞片培养体系筛选到可以显著提高破骨细胞活性的化合物尿囊素(allantoin,ALL)。ALL是动植物体内的尿酸盐的代谢产物[23],也是美国食品药物管理局(the U.S. Food and Drug Administration,FDA)获批的药物,可以通过刺激成纤维细胞生长来提高动物创口愈合活性,因此可用作预防疤痕产生[24]。目前尚无报道显示尿囊素可影响破骨细胞与成骨细胞。fmsj4e1突变体斑马鱼是在C-FMS第一激酶结构域上产生突变,从而其丧失酪氨酸激酶功能出现破骨细胞缺陷的表型。为了研究ALL对破骨细胞缺陷型骨折愈合的作用,本研究使用fmsj4e1骨折活体模型验证了ALL对于骨折修复过程中破骨细胞活性和骨折愈合的影响。结果表明ALL可通过增强rankl和抑制骨保护素(osteoclastogenesis inhibitory factor,opg)在fmsj4e1突变体中的表达来增加破骨细胞活性,并且在使用28天后fmsj4e1显示出骨折愈合恢复的趋势。本研究有望为破骨细胞缺陷导致的骨折不愈合或延迟愈合的患者提供新的治疗策略。
1 材料与方法
1.1 材料
本研究所使用的斑马鱼品系包括AB野生型(wildtype,WT)及fmsj4e1突变体[25],由华南理工大学医学院发育生物学与再生医学团队培育。
1.2 斑马鱼的饲养与胚胎孵育
所有的斑马鱼实验都在华南理工大学动物伦理委员会(批准代码:20211067)的指导下进行。根据Westerfield书中所述,斑马鱼胚胎在28.5℃温度下,在添加美兰与苯基硫脲培养液里培养;20天至4个月大的斑马鱼饲养在温度28.5℃、光照14 h/黑暗10 h的流动水环境中[26]。
1.3 斑马鱼尾鳍骨折
约3个月大小的WT和fmsj4e1突变体斑马鱼用0.08%三卡因麻醉,在显微镜下用镊子按压斑马鱼尾鳍的鳞质鳍条进行骨折。在每条鱼的尾鳍背侧及腹侧的第二条鳞质鳍条进行骨折,操作完后将骨折的斑马鱼放入鱼缸中培养。使用显微镜(德国Zeiss公司)拍照记录骨折愈合过程中斑马鱼尾鳍骨折点各个时期的状态。其中骨折后0~3 dpi (day post injure)的斑马鱼处于急性充血水肿和急性炎症反应期[27],存在出血以及局部组织充血肿胀的情况,因此本研究使用4 dpi作为骨折创面修复观察的起始时间点。
1.4 抗酒石酸酸性磷酸酶染色
用4%多聚甲醛(美国Macklin公司)4℃过夜固定斑马鱼的尾鳍。固定好的样品需经双蒸水清洗3遍,每遍10 min,然后放入混合的抗酒石酸酸性磷酸酶(tartrate-resistant acid phosphatase, TRAP)染液中,在37℃孵育45 min。抗酒石酸酸性磷酸酶染液的配比参照TRAP/ALP双重染色试剂盒(日本WAKO公司)说明书配置[28]。9 mL酸性磷酸酶底物液A、1 mL酒石酸溶液与100 μL酸性磷酸酶底物液B均匀混合,现配现用。染色完成后,样品用PBST冲洗3次,每次5 min。染色后的尾鳍可以储存在-20℃、70%甘油中或直接在蔡司显微镜下观察。
1.5 鳞片培养及药物筛选
用0.08%三卡因麻醉受精4个月的WT斑马鱼。使用显微镊在解剖显微镜下从斑马鱼背侧收集鳞片,然后在PBS液中漂洗3次,每次5 min。接着将鳞片放入含有DMEM培养基(10%FBS)和DMSO或者小分子化合物(筛选浓度:100 μmol/L)的48孔板(美国Sigma-Aldrich公司)中培养。48孔板置于28℃恒温箱中离体培养24 h。然后去除培养基,用PBST冲洗鳞片3次,每次5 min。最后,用4%的PFA在室温下固定鳞片1 h,固定完后用去离子水冲洗3次,每次5 min。随后,每孔加入300 μL的TRAP染色液,置于37℃恒温培养箱中染色45 min。去除染色液,使用PBST冲洗后在显微镜(德国Zeiss公司)下拍照计数TRAP阳性信号。
1.6 药物处理骨折的斑马鱼
如上文1.3所述对WT和fmsj41突变体斑马鱼进行骨折,然后将骨折后的WT和fmsj4e1突变体斑马鱼随机放入实验组和对照组中。实验组设计为终浓度为40 μmol/L的尿囊素(美国Selleck公司)溶液,对照组为等体积的DMSO(德国Sigma 公司)溶液。在系统外饲养,一天喂一次,隔天换一次培养液。
1.7 原位杂交与免疫荧光染色
1.8 总RNA的提取与实时荧光定量PCR
将4个月大小的WT与fmsj4e1突变体随机分入实验组与对照组,药物处理7天后,用0.08%三卡因将其麻醉,拔取斑马鱼的鳞片。用TRIzol(美国Ambion公司)提取斑马鱼鳞片总RNA,然后按照试剂盒(美国Invitrogen公司)说明书将其反转录成cDNA。本研究经历3次生物学重复,每次样品设置3个复孔,每个引物10 pmol,采用FastStart Universal SYBR Green Master(瑞士Roche公司)在 Light Cycler Nano(瑞士Roche公司)仪器上进行实时荧光定量PCR反应。以管家基因ef-1α为内参,使用ΔΔCt阈值法将相对倍数变化标准化。引物由Primer 5设计,引物序列信息见表1。
表1 本研究所用的引物信息
Table 1
| 基因名称 | 引物序列(5′→3′) |
|---|---|
| col1a | F:CTGGAAACCGTGGTGAATCT |
| R:GACCAGGATGTCCACGAAGT | |
| rankl | F:TAGTGTGGCGATTCTGTTGC |
| R:ATTGGAAGGTGAGCTGATGG | |
| opg | F:GGCGTCTGAAGAAACCTCTG |
| R:GCAGGATTGGGATGCAGTAT | |
| acp5b(trap) | F:TGTCATCGTGGTTGGTCACT |
| R:CTCAACACCAGCTCCACTGA | |
| ef-1α | F:TACTTCTCAGGCTGACTGTG |
| R:ATCTTCTTGATGTATGCGCT |
F:上游引物;R:下游引物。
1.9 统计学分析
采用ImageJ测量骨折处的骨折创面面积和TRAP阳性信号面积。每个样本的骨折创面面积与骨折处TRAP染色面积的数值取2个骨折点的测量数据的均值。使用GraphPad Prism 8.3软件进行统计数据的分析,两组数据间的差异统计学意义通过双尾t检验(Student t test)来计算,而多组间的数据比较则采用单因素方差分析(one-way ANOVA)来统计。在所有图表中,误差线反映的是mean±SEM,P<0.05 表示差异具有统计学意义。
2 结果与分析
2.1 建立fmsj4e1斑马鱼破骨细胞缺陷骨折模型
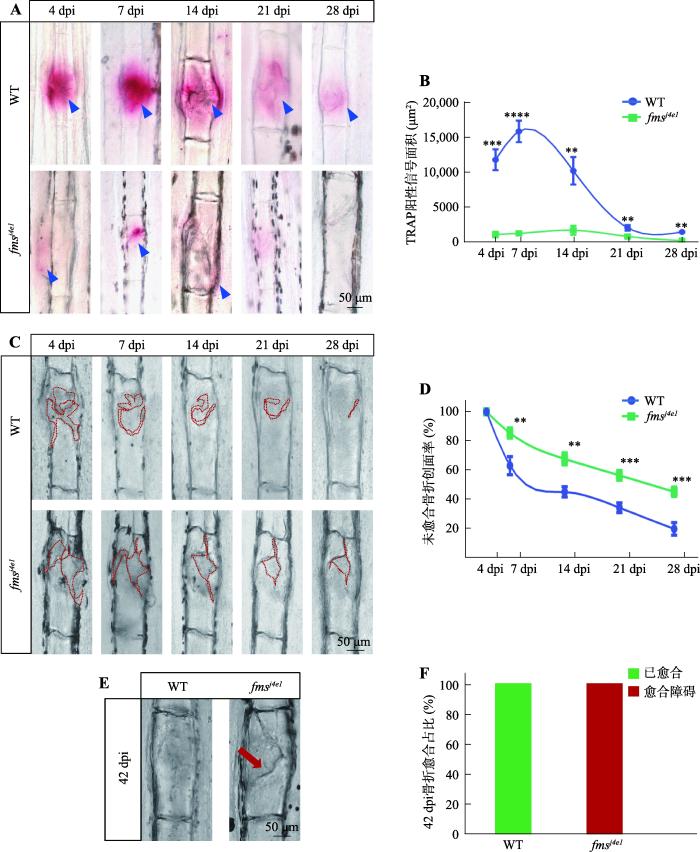
为了阐明破骨细胞在骨折恢复过程中的作用规律,本研究用镊子挤压WT斑马鱼尾鳍,模拟骨折过程。然后通过TRAP染色检测骨折后4 dpi、7 dpi、14 dpi、21 dpi、28 dpi骨折处破骨细胞酸性磷酸酶5a/b(acid phosphatase 5a/b,Acp5a/b)的酶活性变化。结果发现在正常的骨折愈合过程中,TRAP染色阳性的破骨细胞活性在骨折愈合7 dpi达到高峰,并在骨折愈合末期21 dpi~28 dpi降低(图1,A和B)。前期研究显示,Fms缺陷斑马鱼中破骨细胞出现成熟障碍[17]。为了验证破骨细胞的TRAP活性是否在破骨细胞缺陷型骨折修复中受到影响,本研究检测了fmsj4e1突变体中骨折愈合不同阶段的骨折处破骨细胞活性。结果发现与WT相比,fmsj4e1突变体骨折处的TRAP阳性的信号在4 dpi~28 dpi的各个时期中均较野生型显著减少(图1,A和B)。
图1
图1
fmsj4e1突变体破骨细胞缺陷型骨折模型的建立
A:WT与fmsj4e1突变体尾鳍骨折后各个时期骨折处TRAP染色情况。蓝色三角形指示TRAP阳性信号。B:图A中WT斑马鱼与fmsj4e1突变体尾鳍骨折处TRAP阳性信号面积统计图。样本量:4 dpi组WT和fmsj4e1分别为10、9;7 dpi组分别为15、13;14 dpi组分别为11、14;21 dpi组分别为14、16;28 dpi组分别为10、10。C:WT斑马鱼与fmsj4e1突变体同一骨折处不同时期的愈合情况。D:图C中WT斑马鱼与fmsj4e1突变体尾鳍骨折后各个时期骨折未愈合创面率(%)统计图。统计方式为x dpi时骨折的未愈合创面率%= x dpi 骨折创面面积(图C中红色虚线面积)/4 dpi 骨折创面面积;样本量:WT为7条,fmsj4e1突变体为9条。E:42 dpi时WT斑马鱼与fmsj4e1突变体骨折的尾鳍的愈合情况。红色箭头所指为不愈合情况。F:图E的统计图。WT和fmsj4e1样本量分别为10、8。图B、D中的检验方法为t检验;* P<0.05,** P<0.01,*** P<0.001,**** P<0.0001。
Fig. 1
Establishing an osteoclast-deficient fracture model on fmsj4e1
2.2 药物筛选发现ALL可增加离体培养的鳞片破骨细胞活性
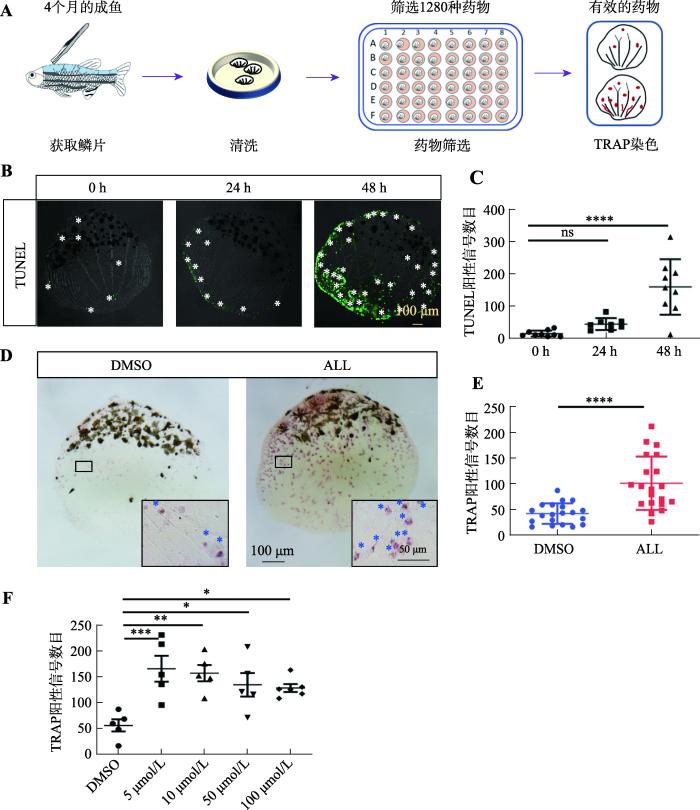
为了寻找可以促进破骨细胞缺陷型骨折愈合的新药物,首先需要筛选到可以提高破骨细胞活性的候选化合物。之前研究表明,斑马鱼鳞片作为骨组织,可以在28℃、5%CO2培养箱内进行72 h的离体培养[31]。因此,本研究拟利用鳞片培养系统作为初步筛选方法来获得提高破骨细胞活性的化合物。为了明确适宜的药物筛选条件,通过TUNEL(terminal deoxynucleotidyl transferase dUTP nick end labeling)检测分析在离体培养不同时长后鳞片上的细胞凋亡情况。结果显示,离体培养24 h鳞片上细胞的凋亡信号较少,并且与0 h相比无统计学差异,而鳞片在离体培养48 h时凋亡的信号显著增加(图2,B和C)。因此,本研究在培养体系中加入来自FDA成药库(含1280种FDA批准成药)的药物,以100 μmol/L的作为药物的初始筛选浓度来培养野生型鳞片,并在培养24 h后检测鳞片上破骨细胞的TRAP活性(图2A )。通过初步筛选,在FDA成药库中发现可以显著增加鳞片上破骨细胞TRAP活性的ALL(图2,D和E),并呈现浓度梯度依赖性(图2F)。
图2
图2
ALL可提高离体培养的鳞片上破骨细胞的TRAP活性
A:通过鳞片离体培养体系筛选提高破骨细胞活性药物的模式图。B:鳞片在离体培养不同时长时TUNEL染色的情况。白色星号指示TUNEL阳性信号。C:图B中0 h、24 h和48 h时鳞片上TUNEL阳性信号统计图。D:ALL与DMSO离体培养24 h后鳞片的TRAP染色结果。黑框内为20×的鳞片局部细节图,蓝色星号指示TRAP阳性信号。E:图D中ALL组与DMSO组鳞片上的TRAP阳性信号数目统计图。F:不同浓度ALL与DMSO离体培养鳞片24 h后,鳞片上TRAP阳性信号数目统计图。图C、F的检验方式为单因素方差分析;图E的检验方式为t检验;* P<0.05,** P<0.01,*** P<0.001,**** P<0.0001。
Fig. 2
ALL could increase TRAP activity of osteoclasts on ex-vivo culturing scales
2.3 ALL可提高活体fmsj4e1斑马鱼骨折处破骨细胞活性并促进骨折愈合
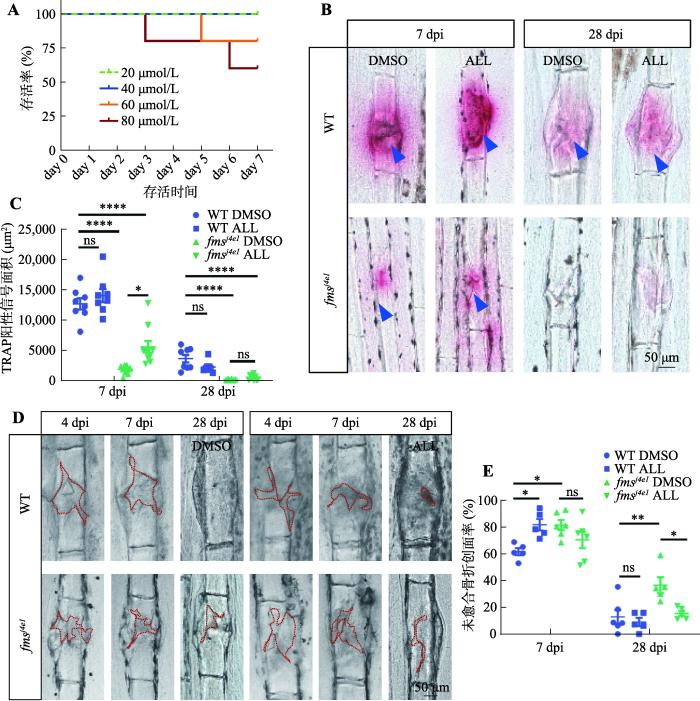
本研究通过离体培养体系筛选发现ALL可以提高体外破骨细胞活性,但尚不清楚ALL是否能在活体中同样增强破骨细胞缺陷型骨折愈合中破骨细胞的活性。通过设置药物梯度(20 μmol/L、40 μmol/L、60 μmol/L以及80 μmol/L)对成年斑马鱼进行浸泡给药处理,发现ALL最大不致死剂量为40 μmol/L (图3A),因此本研究使用40 μmol/L 的ALL和DMSO对骨折后的fmsj4e1和WT斑马鱼进行浸泡给药,然后在7 dpi(骨折愈合中破骨细胞作用的高峰时期)时观察骨折处破骨细胞活性的变化。结果显示,fmsj4e1突变体ALL处理组尾鳍骨折处的TRAP阳性信号与DMSO对照组相比有显著增加,但WT斑马鱼骨折处的TRAP阳性信号在ALL处理组中与对照组中无统计学差异(图3,B和C)。同样对28 dpi时各组的斑马鱼尾鳍进行TRAP染色,结果显示,28 dpi的 fmsj4e1突变体ALL处理组骨折处TRAP阳性信号与DMSO对照组无明显差异(图3,B和C)。这表明ALL能在骨折修复早期提高fmsj4e1突变体骨折处的破骨细胞活性。
图3
图3
ALL对fmsj4e1斑马鱼骨折处破骨细胞活性和骨折愈合的影响
A:不同浓度ALL处理下斑马鱼的生存曲线。用20 μmol/L、40 μmol/L、60 μmol/L和80 μmol/L的ALL处理fmsj4e1,每组5条,观察药物处理7天时间内不同浓度组间斑马鱼存活情况,并绘制生存曲线。B:WT和 fmsj4e1在ALL与DMSO处理下不同时期骨折处的TRAP染色情况。蓝色三角形指示TRAP阳性信号。C:图B中各组7 dpi 以及28 dpi 时TRAP阳性信号面积统计图。D:WT斑马鱼和 fmsj4e1在ALL与DMSO处理下不同时期骨折处创面愈合情况。红色虚线标记骨折处创面。E:图D中各组未愈合骨折创面率统计图。图C、E的检验方法为单因素方差分析;* P<0.05,** P<0.01,*** P<0.001,**** P<0.0001。
Fig. 3
The effect of allantoin on osteoclast activity and fracture healing in fmsj4e1 mutants
为了进一步研究ALL对fmsj4e1和WT斑马鱼骨折修复的影响,本研究通过测量骨折后创面的未愈合创面率记录了WT与fmsj4e1突变体在ALL组的骨折创面愈合情况。结果显示,7 dpi时ALL处理组中WT斑马鱼的骨折未愈合创面率与DMSO对照组的相比更大,fmsj4e1突变体的骨折未愈合创面率在ALL组与对照组间无明显差异;而在28 dpi时,WT斑马鱼ALL组与DMSO组的骨折未愈合创面率无统计学差异,而在fmsj4e1突变体中ALL组的骨折未愈合创面率较对照组小(图3,D和E)。这提示长期使用ALL能够促进fmsj4e1突变体骨折的愈合。同时尽管ALL在短期表现出不利于野生型斑马鱼的骨折愈合,但其长期使用不影响野生型斑马鱼的骨折创面修复。
2.4 ALL不影响fmsj4e1破骨细胞的生成
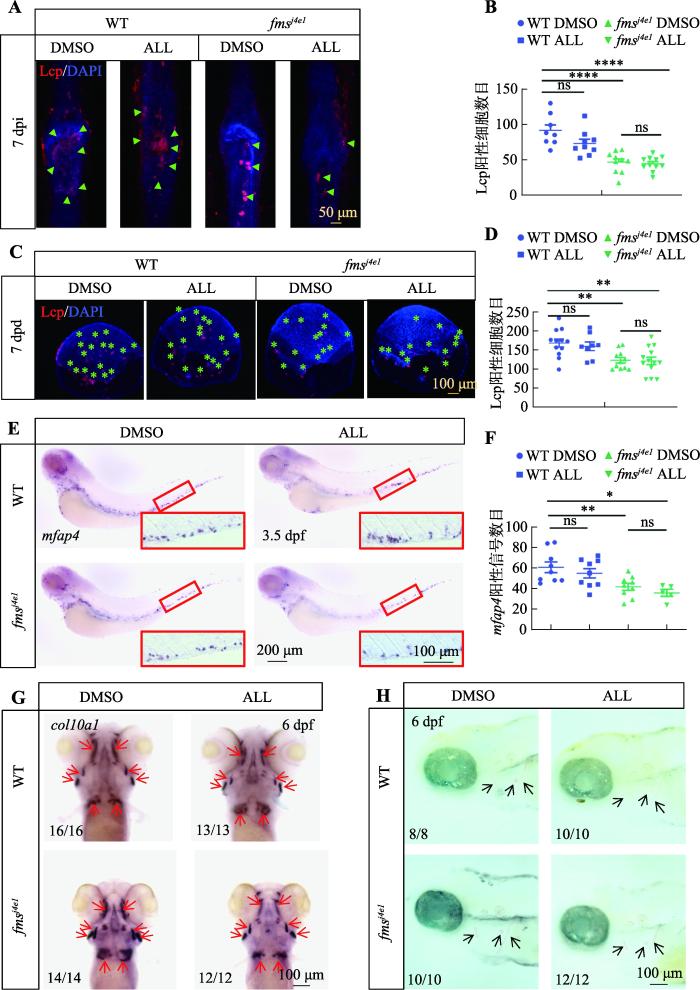
本研究发现ALL可提高fmsj4e1斑马鱼破骨细胞活性,为了进一步明确ALL对fmsj4e1斑马鱼破骨细胞数量是否有影响,本研究在ALL给药7天后(7 days post drug, 7 dpd),通过免疫染色方法检测破骨细胞标记物L-plastin(Lcp,标记不成熟以及成熟的破骨细胞)来检测破骨细胞数量[32,33]。结果显示,经ALL处理的野生型及fmsj4e1中尾鳍及鳞片上破骨细胞数量无明显改变(图4,A~D)。这提示ALL可能不影响fmsj4e1斑马鱼破骨细胞的生成。由于破骨细胞起源于巨噬细胞谱系,本研究还通过原位杂交检测ALL处理组与DMSO组中受精后3.5 天(day post fertilization, dpf)的WT与fmsj4e1胚胎上巨噬细胞标记物mfap4的信号数目[34],从而评估ALL对破骨细胞来源的影响。结果显示,WT与fmsj4e1胚胎ALL组mfap4阳性信号与DMSO组间无明显差异(图4,E和F),表明ALL不影响巨噬细胞的生成。
图4
图4
ALL不影响fmsj4e1突变体破骨细胞的生成
A:WT和fmsj4e1在ALL与DMSO处理下7 dpi 时,骨折处抗Lcp抗体和DAPI(4',6-diamidino-2-phenylindole)双染色。绿色三角形所示为Lcp阳性细胞。B:图A中各组骨折处Lcp阳性细胞数目的统计图。C:WT和fmsj4e1在药物处理7天(7 days post drug,7 dpd)时鳞片上Lcp抗体和DAPI双染色结果。绿色星号指示Lcp阳性细胞。D:图C中各组7 dpd 鳞片上Lcp阳性细胞数目统计图。E:WT和fmsj4e1胚胎在ALL与DMSO处理下,3.5 dpf时mfap4原位杂交结果。红色方框内为20×尾部造血组织细节图。F:图E中各组mfap4阳性信号统计图。G:WT和fmsj4e1胚胎在ALL与DMSO处理至6 dpf时col101a整体原位杂交。红色箭头所指为col101a阳性信号。H:WT和fmsj4e1胚胎在ALL与DMSO处理下6 dpf时茜素红染色。黑色箭头所指为茜素红染色阳性信号。图B、D、F的检验方法为单因素方差分析;* P<0.05,** P<0.01,*** P<0.001,**** P<0.0001。
Fig. 4
ALL does not affect the production of osteoclast in fmsj4e1 mutants
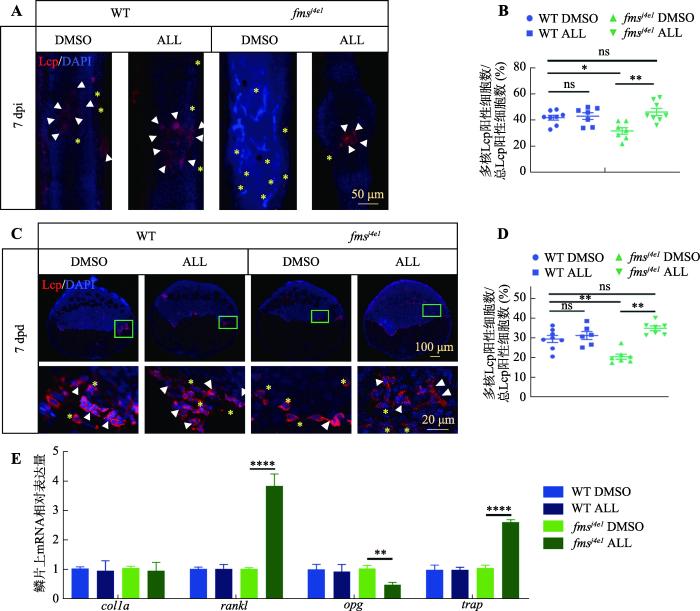
2.5 ALL促进fmsj4e1突变体破骨细胞成熟
基于ALL在不改变破骨细胞数量的前提下可以提高破骨细胞活性,本研究猜测ALL可能也促进破骨细胞成熟。因此,本研究利用Lcp与DAPI (4′,6-diamidino-2-phenylindole)共染来区分多核破骨细胞和单核破骨细胞,并计算ALL处理7天后WT与fmsj4e1斑马鱼多核破骨细胞比例的变化。结果显示ALL处理后,fmsj4e1斑马鱼鳞片及骨折处多核破骨细胞比例显著上升(图5,A~D),提示ALL可促进破骨细胞成熟。为了进一步明确ALL对破骨细胞活性的激活作用机制,本研究使用ALL对WT与fmsj4e1进行浸泡给药 7天后,通过qPCR检测了影响破骨细胞成熟和功能的关键因素的表达,如:rankl、opg和acp5b(TRAP)[36]。结果显示,在ALL处理的fmsj4e1突变体的鳞片中,rankl和trap的表达都明显升高,而破骨细胞生成抑制因子opg的表达则下降(图5E)。这些结果表明,ALL可以通过增强rankl和抑制opg在fmsj4e1突变体中的表达来促进破骨细胞成熟,提高破骨细胞活性。
图5
图5
ALL促进fmsj4e1突变体破骨细胞的成熟
A:各组7 dpi 时骨折处,抗Lcp抗体和DAPI双染色结果。白色三角形指示多核Lcp阳性细胞,黄色星号指示单核Lcp阳性细胞。B:图A中各组中骨折处多核Lcp阳性细胞数/总Lcp阳性细胞数%的统计图。C:WT和 fmsj4e1在ALL与DMSO处理下7 dpd时,鳞片抗Lcp抗体和DAPI双染色。下方图为40×的鳞片局部放大图。D:图C中各组鳞片上多核Lcp阳性细胞数/总Lcp阳性细胞数%的统计图。E:WT斑马鱼和fmsj4e1在ALL与DMSO处理7天时鳞片上的各组间colla、rankl、opg、trap相对表达量统计图。图B、D、E的检验方法为单因素方差分析;* P<0.05,** P<0.01,*** P<0.001,**** P<0.0001。
Fig. 5
ALL promotes the maturation of osteoclast in fmsj4e1 mutants
3 讨论
破骨细胞缺陷在临床上常伴有复发性骨折,且这类骨折在修复过程中多呈愈合困难或者不愈合。但临床上治疗此类骨折,多采取外科手术治疗,没有针对性治疗药物。本研究建立了一个fms突变体斑马鱼破骨细胞缺陷骨折模型。随后通过离体鳞片培养体系进行药物筛选与活体模型验证,发现可以激活破骨细胞活性的候选化合物。通过该方法,本研究筛选发现ALL可以促进fms突变体的骨折愈合。由于ALL是FDA批准的具有安全毒理学的药物,它对骨折愈合的新作用可以在哺乳动物模型及临床前试验中进一步开发。
临床研究表明,先天性破骨细胞缺乏导致骨硬化症,常伴有复发性骨折,且往往伴有愈合障碍[37,38]。破骨细胞活性下降可由破骨细胞形成或功能缺陷引起[39]。本研究,建立了 Fms缺乏(异常成熟破骨细胞)骨折模型。通过分析该模型的骨折愈合过程,发现破骨细胞在骨折愈合的不同阶段(4 dpi ~ 28 dpi)都起着重要作用,这与临床研究是一致的。破骨细胞形成或成熟的缺陷均可导致早期愈合异常,最终导致延迟愈合或者骨折不愈合。骨折愈合效果与功能性破骨细胞数量呈正相关。因此,本研究为重现临床破骨细胞缺乏性骨折不愈合症状提供了一种潜在的动物研究模型。但是,本研究还存在一定的局限性。例如,本研究分析骨折愈合过程是通过组织学方法结合Image J软件分析进行的。在以往的小鼠研究中micro-CT是观测骨折愈合更为有力的方法,然而普通micro-CT的分辨率不足以分析斑马鱼尾鳍损伤的愈合情况。未来高分辨的micro-CT或者荧光染色进行3D成像将是更好的选择。
通常情况下,药物发现需要首选体外细胞筛选,之后经体内治疗验证来确定其作用[40,41]。但是,由于体外细胞培养脱离了原有的生活环境,由此获得的药物有效率较低。斑马鱼鳞片作为一种骨组织可以离体培养,它们不仅含有多种骨细胞成分,而且容易获得和维持,是筛选提高破骨细胞活性药物的良好替代方法[20,40]。通过这种筛选方法可以提高药物筛选的准确效率。此外,通过对破骨细胞缺乏型骨折斑马鱼模型的验证,本研究发现ALL能促进fmsj4e1突变体破骨细胞成熟缺陷的骨折创面愈合。令人不解的是,ALL处理可以提高WT离体培养鳞片中破骨细胞的TRAP活性,但对WT体内骨折部位破骨细胞的TRAP活性没有明显增强。这可能是由于两个原因:一是药物在体外和体内培养组织中的吸收和代谢不同;另一种是在体内骨折愈合过程中,WT中破骨细胞已经被招募和激活,药物治疗并没有增加更多的额外活性。
ALL是FDA批准的药物,其药理作用和药物代谢途径较为明确。ALL是嘌呤降解产生的尿酸盐代谢物,存在于植物和动物中[23]。在人体中,由于缺乏功能性尿酸酶,尿酸也可以通过氧化转化为ALL,提示ALL是氧化应激的一个指标[42]。ALL通过刺激成纤维细胞生长,作为一种预防瘢痕的产品被广泛应用于临床[43,44]。ALL对骨折修复过程中破骨细胞的作用机制尚不清楚。本研究的数据表明,ALL通过增加rankl和抑制opg表达来增强fmsj4e1(破骨细胞成熟缺陷突变体)中破骨细胞的活性。因此,本研究推测ALL通过增加破骨细胞的活性和成熟来促进骨折愈合。然而,ALL调控opg和rankl表达的分子机制还有待进一步研究。
参考文献
A systematic review of hip fracture incidence and probability of fracture worldwide
The country-specific risk of hip fracture and the 10-year probability of a major osteoporotic fracture were determined on a worldwide basis from a systematic review of literature. There was a greater than 10-fold variation in hip fracture risk and fracture probability between countries.The present study aimed to update the available information base available on the heterogeneity in the risk of hip fracture on a worldwide basis. An additional aim was to document variations in major fracture probability as determined from the available FRAX models.Studies on hip fracture risk were identified from 1950 to November 2011 by a Medline OVID search. Evaluable studies in each country were reviewed for quality and representativeness and a study (studies) chosen to represent that country. Age-specific incidence rates were age-standardised to the world population in 2010 in men, women and both sexes combined. The 10-year probability of a major osteoporotic fracture for a specific clinical scenario was computed in those countries for which a FRAX model was available.Following quality evaluation, age-standardised rates of hip fracture were available for 63 countries and 45 FRAX models available in 40 countries to determine fracture probability. There was a greater than 10-fold variation in hip fracture risk and fracture probability between countries.Worldwide, there are marked variations in hip fracture rates and in the 10-year probability of major osteoporotic fractures. The variation is sufficiently large that these cannot be explained by the often multiple sources of error in the ascertainment of cases or the catchment population. Understanding the reasons for this heterogeneity may lead to global strategies for the prevention of fractures.
Delayed union and nonunions: epidemiology, clinical issues, and financial aspects
Cellular biology of fracture healing
DOI:10.1002/jor.24170
PMID:30370699
[本文引用: 1]

The biology of bone healing is a rapidly developing science. Advances in transgenic and gene-targeted mice have enabled tissue and cell-specific investigations of skeletal regeneration. As an example, only recently has it been recognized that chondrocytes convert to osteoblasts during healing bone, and only several years prior, seminal publications reported definitively that the primary tissues contributing bone forming cells during regeneration were the periosteum and endosteum. While genetically modified animals offer incredible insights into the temporal and spatial importance of various gene products, the complexity and rapidity of healing-coupled with the heterogeneity of animal models-renders studies of regenerative biology challenging. Herein, cells that play a key role in bone healing will be reviewed and extracellular mediators regulating their behavior discussed. We will focus on recent studies that explore novel roles of inflammation in bone healing, and the origins and fates of various cells in the fracture environment. © 2018 Orthopaedic Research Society. Published by Wiley Periodicals, Inc. J Orthop Res.© 2018 Orthopaedic Research Society. Published by Wiley Periodicals, Inc.
Bone remodeling during fracture repair: the cellular picture
DOI:10.1016/j.semcdb.2008.07.004
PMID:18692584
[本文引用: 1]

Fracture healing is a complex event that involves the coordination of a variety of different processes. Repair is typically characterized by four overlapping stages: the initial inflammatory response, soft callus formation, hard callus formation, initial bony union and bone remodeling. However, repair can also be seen to represent a juxtaposition of two distinct forces: anabolism or tissue formation, and catabolism or remodeling. These anabolic/catabolic concepts are useful for understanding bone repair without giving the false impression of temporally distinct stages that operate independently. They are also relevant when considering intervention. In normal bone development, bone remodeling conventionally refers to the removal of calcified bone tissue by osteoclasts. However, in the context of bone repair there are two phases of tissue catabolism: the removal of the initial cartilaginous soft callus, followed by the eventual remodeling of the bony hard callus. In this review, we have attempted to examine catabolism/remodeling in fractures in a systematic fashion. The first section briefly summarizes the traditional four-stage view of fracture repair in a physiological manner. The second section highlights some of the limitations of using a temporal rather than process-driven model and summarizes the anabolic/catabolic paradigm of fracture repair. The third section examines the cellular participants in soft callus remodeling and in particular the role of the osteoclast in endochondral ossification. Finally, the fourth section examines the effects of delaying osteoclast-dependent hard callus remodeling and also poses questions regarding the crosstalk between anabolism and catabolism in the latter stages of fracture repair.
In-vivo imaging of the fracture healing in medaka revealed two types of osteoclasts before and after the callus formation by osteoblasts
DOI:10.1016/j.ydbio.2014.08.007 URL [本文引用: 1]
"Challenges in the management of fractures in osteopetrosis"! Review of literature and technical tips learned from long-term management of seven patients
DOI:10.1016/j.injury.2009.02.009
PMID:19576583
[本文引用: 2]

Osteopetrosis is a metabolic disorder with diminished bone resorption due to osteoclastic abnormality. It causes hard and brittle marble bone which fractures easily. Most of these fractures can be treated conservatively. Operative intervention when needed presents with unique technical challenges. While osteopetrotic hard bone may be penetrated with a drill bit; high friction and prolonged drilling can make the drill bit blunt. The heat generated can cause bone necrosis and break the drill bit. Besides this, brittleness of bones can cause intra-operative fractures. Due to the difficulties during the operation, the operative time may be prolonged thereby increasing the risk of post-operative infection. There is also a risk of delay in consolidation and non-union owning to impaired bone remodelling. We present an account of seven patients treated for various fracture related problems occurring throughout their life due to this disease. Difficulties encountered during their treatment prompted us to present some general management principles.
Total joint arthroplasty in patients with osteopetrosis:a report of 5 cases and review of the literature
Macrophages and osteoclasts stem from a bipotent progenitor downstream of a macrophage/ osteoclast/dendritic cell progenitor
DOI:10.1182/bloodadvances.2017008540 URL [本文引用: 1]
Expression of colony- stimulating factor-1 in vivo during the formation of osteoclasts
Colony-stimulating factor-1 (CSF-1), originally described as a growth factor for macrophages, is essential for the proliferation and differentiation of the cells of the osteoclast lineage. The cytokine is synthesized either as a secreted or a membrane-bound protein, which are encoded by four transcripts. The aim of the present study was to investigate the expression of CSF-1 in vivo at the mRNA level. Transcripts encoding CSF-1 were determined in total RNA from fetal murine metatarsals of different ages by a quantitative reverse-transcription polymerase chain reaction assay. Within the investigated period of time, the bone rudiments contain cells of the osteoclastic lineage representing well-defined differentiation stages. We found that only low levels of transcripts encoding CSF-1 could be detected in metatarsals from 15-day-old fetuses. Transcript levels increased slowly during the following days to reach a maximum in the rudiments from 18-day-old fetuses. After birth, in newborn animals, transcript levels were lowered again. While in rudiments from 15-day-old fetuses a considerable portion of the transcripts encoded the membrane-bound molecule, a transcript encoding the secreted form of the cytokine was the predominant species during the following days. These results suggest that the maintenance of proliferating and postmitotic osteoclast precursors requires low levels of CSF-1 only. Highest levels of locally synthesized CSF-1 are required, however, during the initial recruitment and activation of osteoclasts. After birth, levels of CSF-1 transcripts decrease again, suggesting that newly synthesized CSF-1 may be replaced by protein released from the mineralized matrix during resorption. In conclusion, the present data further strengthen the notion that CSF-1 produced locally acts in a paracrine fashion during the formation of osteoclasts.
Signaling pathways in osteoclast differentiation
DOI:10.4068/cmj.2016.52.1.12
PMID:26865996
[本文引用: 1]

Osteoclasts are multinucleated cells of hematopoietic origin that are responsible for the degradation of old bone matrix. Osteoclast differentiation and activity are controlled by two essential cytokines, macrophage colony-stimulating factor (M-CSF) and the receptor activator of nuclear factor-κB ligand (RANKL). M-CSF and RANKL bind to their respective receptors c-Fms and RANK to stimulate osteoclast differentiation through regulation of delicate signaling systems. Here, we summarize the critical or essential signaling pathways for osteoclast differentiation including M-CSF-c-Fms signaling, RANKL-RANK signaling, and costimulatory signaling for RANK.
The M-CSF receptor in osteoclasts and beyond
DOI:10.1038/s12276-020-0484-z
PMID:32801364
[本文引用: 1]

Colony-stimulating factor 1 receptor (CSF1R, also known as c-FMS) is a receptor tyrosine kinase. Macrophage colony-stimulating factor (M-CSF) and IL-34 are ligands of CSF1R. CSF1R-mediated signaling is crucial for the survival, function, proliferation, and differentiation of myeloid lineage cells, including osteoclasts, monocytes/macrophages, microglia, Langerhans cells in the skin, and Paneth cells in the intestine. CSF1R also plays an important role in oocytes and trophoblastic cells in the female reproductive tract and in the maintenance and maturation of neural progenitor cells. Given that CSF1R is expressed in a wide range of myeloid cells, altered CSF1R signaling is implicated in inflammatory, neoplastic, and neurodegenerative diseases. Inhibiting CSF1R signaling through an inhibitory anti-CSF1R antibody or small molecule inhibitors that target the kinase activity of CSF1R has thus been a promising therapeutic strategy for those diseases. In this review, we cover the recent progress in our understanding of the various roles of CSF1R in osteoclasts and other myeloid cells, highlighting the therapeutic applications of CSF1R inhibitors in disease conditions.
Receptor activator of NF-kappaB recruits multiple TRAF family adaptors and activates c-Jun N-terminal kinase
Receptor activator of NF-kappaB (RANK) is a recently cloned member of the tumor necrosis factor receptor (TNFR) superfamily, and its function has been implicated in osteoclast differentiation and dendritic cell survival. Many of the TNFR family receptors recruit various members of the TNF receptor-associated factor (TRAF) family for transduction of their signals to NF-kappaB and c-Jun N-terminal kinase. In this study, the involvement of TRAF family members and the activation of the JNK pathway in signal transduction by RANK were investigated. TRAF1, 2, 3, 5, and 6 were found to bind RANK in vitro. Association of RANK with each of these TRAF proteins was also detected in vivo. Expression of RANK in cultured cells also induced the activation of JNK, which was blocked by a dominant-negative form of JNK. Furthermore, by employing various C-terminal deletion mutants of RANK, the regions responsible for TRAF interaction and JNK activation were identified. TRAF5 was determined to bind to the C-terminal 11 amino acids and the other TRAF members to a region N-terminal to the TRAF5 binding site. The domain responsible for JNK activation was localized to the same region where TRAF1, 2, 3, and 6 bound, which suggests that these TRAF molecules might mediate the RANK-induced JNK activation.
Hematopoietic stem cell: self- renewal versus differentiation
DOI:10.1002/wsbm.v2:6 URL [本文引用: 1]
Targeted disruption of the mouse colony-stimulating factor 1 receptor gene results in osteopetrosis, mononuclear phagocyte deficiency, increased primitive progenitor cell frequencies, and reproductive defects
DOI:10.1182/blood.V99.1.111 URL [本文引用: 1]
Conditional deletion of the colony stimulating factor-1 receptor (c-fms proto- oncogene) in mice
DOI:10.1002/(ISSN)1526-968X URL [本文引用: 1]
The synergistic role of Pu.1 and Fms in zebrafish osteoclast-reducing osteopetrosis and possible therapeutic strategies
DOI:10.1016/j.jgg.2020.09.002
PMID:33184003
[本文引用: 2]

Osteoclasts are bone resorption cells of myeloid origin. Osteoclast defects can lead to osteopetrosis, a genetic disorder characterized by bone sclerosis for which there is no effective drug treatment. It is known that Pu.1 and Fms are key regulators in myelopoiesis, and their defects in mice can lead to reduced osteoclast numbers and consequent osteopetrosis. Yet how Pu.1 and Fms genetically interact in the development of osteoclasts and the pathogenesis of osteopetrosis is still unclear. Here, we characterized pu.1;fms double-deficient zebrafish, which exhibited a greater deficiency of functional osteoclasts and displayed more severe osteopetrotic symptoms than the pu.1 or fms single mutants, suggesting a synergistic function of Pu.1 and Fms in the regulation of osteoclast development. We further demonstrated that Pu.1 plays a dominant role in osteoclastogenesis, whereas Fms plays a dominant role in osteoclast maturation. Importantly, treatment with the drug retinoic acid significantly relieved the different degrees of osteopetrosis symptoms in these models by increasing the number of functional osteoclasts. Thus, we report the development of valuable animal models of osteopetrosis, and our results shed light on drug development for antiosteopetrosis therapy.Copyright © 2020 Institute of Genetics and Developmental Biology, Chinese Academy of Sciences, and Genetics Society of China. Published by Elsevier Ltd. All rights reserved.
Bi-allelic CSF1R mutations cause skeletal dysplasia of dysosteosclerosis- pyle disease spectrum and degenerative encephalopathy with brain malformation
DOI:10.1016/j.ajhg.2019.03.004 URL [本文引用: 1]
Small teleost fish provide new insights into human skeletal diseases
Zebrafish as an emerging model for osteoporosis: a primary testing platform for screening new osteo-active compounds
DOI:10.3389/fendo.2019.00006 URL [本文引用: 2]
A new zebrafish bone crush injury model
DOI:10.1242/bio.2012877
PMID:23213486
[本文引用: 1]

While mammals have a limited capacity to repair bone fractures, zebrafish can completely regenerate amputated bony fin rays. Fin regeneration in teleosts has been studied after partial amputation of the caudal fin, which is not ideal to model human bone fractures because it involves substantial tissue removal, rather than local tissue injury. In this work, we have established a bone crush injury model in zebrafish adult caudal fin, which consists of the precise crush of bony rays with no tissue amputation. Comparing these two injury models, we show that the initial stages of injury response are the same regarding the activation of wound healing molecular markers. However, in the crush assay the expression of the blastema marker msxb appears later than during regeneration after amputation. Following the same trend, bone cells deposition and expression of genes involved in skeletogenesis are also delayed. We further show that bone and blood vessel patterning is also affected. Moreover, analysis of osteopontin and Tenascin-C reveals that they are expressed at later stages in crushed tissue, suggesting that in this case bone repair is prolonged for longer than in the case of regeneration after amputation. Due to the nature of the trauma inflicted, the crush injury model seems more similar to fracture bone repair in mammals than bony ray amputation. Therefore, the new model that we present here may help to identify the key processes that regulate bone fracture and contribute to improve bone repair in humans.
Clinical pathologies of bone fracture modelled in zebrafish
The biochemistry of nitrogen mobilization: purine ring catabolism
DOI:10.1016/j.tplants.2011.03.012
PMID:21482173
[本文引用: 2]

The enzymatic route of purine ring catabolism has recently been completed by the discovery of several novel enzymes identified through comparative genome analyses. Here, we review these recent discoveries and present an overview of purine ring catabolism in plants. Xanthine is oxidized to urate in the cytosol, followed by three enzymatic steps taking place in the peroxisome and four reactions in the endoplasmic reticulum releasing the four ring nitrogen as ammonia. Although the main physiological function of purine degradation might lie in the remobilization of nitrogen resources, it has also emerged that catabolic intermediates, the ureides allantoin and allantoate, are likely to be involved in protecting plants against abiotic stress. Conserved alternative splicing mediating the peroxisomal as well as cytosolic localization of allantoin synthase potentially links purine ring catabolism to brassinosteroid signaling.Copyright © 2011 Elsevier Ltd. All rights reserved.
Profile of wound healing process induced by allantoin
DOI:S0102-86502010000500014
PMID:20877959
[本文引用: 1]

To evaluate and characterize the wound healing process profile induced by allantoin incorporated in soft lotion oil/water emulsion using the planimetric and histological methods.Female Wistar rats (n=60) were randomly assigned to 3 experimental groups: (C) control group-without treatment; (E) group treated with soft lotion O/W emulsion excipients; (EA) group treated with soft lotion O/W emulsion containing allantoin 5%. The emulsions either containing or not allantoin were topically administered for 14 days and the wound area was evaluated by planimetry and by qualitative and quantitative histological analysis of open wound model.The data which were obtained and analyzed innovate by demonstrating, qualitatively and quantitatively, by histological analysis, the profile of healing process induced by allantoin. The results suggest that the wound healing mechanism induced by allantoin occurs via the regulation of inflammatory response and stimulus to fibroblastic proliferation and extracellular matrix synthesis.This work show, for the first time, the histological wound healing profile induced by allantoin in rats and demonstrated that it is able to ameliorate and fasten the reestablishment of the normal skin.
An orthologue of the kit-related gene fms is required for development of neural crest-derived xanthophores and a subpopulation of adult melanocytes in the zebrafish, Danio rerio
DOI:10.1242/dev.127.14.3031
PMID:10862741
[本文引用: 1]

Developmental mechanisms underlying traits expressed in larval and adult vertebrates remain largely unknown. Pigment patterns of fishes provide an opportunity to identify genes and cell behaviors required for postembryonic morphogenesis and differentiation. In the zebrafish, Danio rerio, pigment patterns reflect the spatial arrangements of three classes of neural crest-derived pigment cells: black melanocytes, yellow xanthophores and silver iridophores. We show that the D. rerio pigment pattern mutant panther ablates xanthophores in embryos and adults and has defects in the development of the adult pattern of melanocyte stripes. We find that panther corresponds to an orthologue of the c-fms gene, which encodes a type III receptor tyrosine kinase and is the closest known homologue of the previously identified pigment pattern gene, kit. In mouse, fms is essential for the development of macrophage and osteoclast lineages and has not been implicated in neural crest or pigment cell development. In contrast, our analyses demonstrate that fms is expressed and required by D. rerio xanthophore precursors and that fms promotes the normal patterning of melanocyte death and migration during adult stripe formation. Finally, we show that fms is required for the appearance of a late developing, kit-independent subpopulation of adult melanocytes. These findings reveal an unexpected role for fms in pigment pattern development and demonstrate that parallel neural crest-derived pigment cell populations depend on the activities of two essentially paralogous genes, kit and fms.
The zebrafish book : a guide for the laboratory use of zebrafish (Danio rerio)
Osteocytes as main responders to low-intensity pulsed ultrasound treatment during fracture healing
DOI:10.1038/s41598-021-89672-9
PMID:33986415
[本文引用: 1]

Ultrasound stimulation is a type of mechanical stress, and low-intensity pulsed ultrasound (LIPUS) devices have been used clinically to promote fracture healing. However, it remains unclear which skeletal cells, in particular osteocytes or osteoblasts, primarily respond to LIPUS stimulation and how they contribute to fracture healing. To examine this, we utilized medaka, whose bone lacks osteocytes, and zebrafish, whose bone has osteocytes, as in vivo models. Fracture healing was accelerated by ultrasound stimulation in zebrafish, but not in medaka. To examine the molecular events induced by LIPUS stimulation in osteocytes, we performed RNA sequencing of a murine osteocytic cell line exposed to LIPUS. 179 genes reacted to LIPUS stimulation, and functional cluster analysis identified among them several molecular signatures related to immunity, secretion, and transcription. Notably, most of the isolated transcription-related genes were also modulated by LIPUS in vivo in zebrafish. However, expression levels of early growth response protein 1 and 2 (Egr1, 2), JunB, forkhead box Q1 (FoxQ1), and nuclear factor of activated T cells c1 (NFATc1) were not altered by LIPUS in medaka, suggesting that these genes are key transcriptional regulators of LIPUS-dependent fracture healing via osteocytes. We therefore show that bone-embedded osteocytes are necessary for LIPUS-induced promotion of fracture healing via transcriptional control of target genes, which presumably activates neighboring cells involved in fracture healing processes.
Expression pattern of sonic hedgehog signaling and calcitonin gene-related peptide in the socket healing process after tooth extraction
DOI:10.1016/j.bbrc.2015.09.139 URL [本文引用: 1]
Whole mount RNA in situ hybridization on zebrafish embryos: probe synthesis
Definitive hematopoietic stem/progenitor cells manifest distinct differentiation output in the zebrafish VDA and PBI.
DOI:10.1242/dev.029637
PMID:19168679
[本文引用: 1]

One unique feature of vertebrate definitive hematopoiesis is the ontogenic switching of hematopoietic stem cells from one anatomical compartment or niche to another. In mice, hematopoietic stem cells are believed to originate in the aorta-gonad-mesonephros (AGM), subsequently migrate to the fetal liver (FL) and finally colonize the bone marrow (BM). Yet, the differentiation potential of hematopoietic stem cells within early niches such as the AGM and FL remains incompletely defined. Here, we present in vivo analysis to delineate the differentiation potential of definitive hematopoietic stem/progenitor cells (HSPCs) in the zebrafish AGM and FL analogies, namely the ventral wall of dorsal aorta (VDA) and the posterior blood island (PBI), respectively. Cell fate mapping and analysis of zebrafish runx1(w84x) and vlad tepes (vlt(m651)) mutants revealed that HSPCs in the PBI gave rise to both erythroid and myeloid lineages. However, we surprisingly found that HSPCs in the VDA were not quiescent but were uniquely adapted to generate myeloid but not erythroid lineage cells. We further showed that such distinct differentiation output of HSPCs was, at least in part, ascribed to the different micro-environments present in these two niches. Our results highlight the importance of niche in shaping the differentiation output of developing HSPCs.
Osteoblast and osteoclast behavior in zebrafish cultured scales
DOI:10.1007/s00441-012-1436-2
PMID:22669163
[本文引用: 1]

Fish scale culture can be used as a model to test the effects of several molecules on bone metabolism by histological and biochemical methods, although solid cell biology data about the behavior of the scale cells in culture are needed if such a model is to be employed for pharmacological applications. In the present study, we cultured zebrafish scales at various temperatures and for various times and analyzed the behavior of the bone cells in terms of viability and activity. We demonstrated that the cultured scale cells maintained their usual distribution at 28°C until 72 h, after which time episquamal osteoblasts showed an obvious change in their cell organization followed by an increase in cell death. Osteoclast tartrate-resistant acid phosphatase and osteoblast alkaline phosphatase activities were maintained until 72 h but were reduced at 96 h as a consequence of the massive cell death. This scenario indicates that zebrafish scales cultured until 72 h can be considered as an innovative model of explanted organ culture to assay the ability of chemical compounds to modulate the metabolism of bone cells.
Fimbrin in podosomes of monocyte-derived osteoclasts
DOI:10.1002/(ISSN)1097-0169 URL [本文引用: 1]
The actin-bundling protein L-plastin: a critical regulator of immune cell function
Macrophage-specific gene functions in Spi1-directed innate immunity
DOI:10.1182/blood-2010-01-262873 URL [本文引用: 1]
Tracking gene expression during zebrafish osteoblast differentiation
DOI:10.1002/dvdy.21838 URL [本文引用: 1]
Rank/Rankl/opg: literature review
The discovery of the receptor activator of nuclear factor-kB (RANK)/RANK Ligand (RANKL)/osteoprotegerin (OPG) pathway contributed to the understanding of how bone formation and resorption were processed and regulated. RANKL and OPG are members of the tumor necrosis factor (TNF) and TNF receptor (TNFr) superfamilies, respectively, and binding to receptor activator of NF-kB (RANK) not only regulate osteoclast formation, activation and survival in normal bone modeling and remode-ling, but also in several other pathologic conditions characterized by increased bone turnover. There is accumulating evidence of the potential role of OPG and RANKL in other tissues. Looking beyond the RANK/RANKL/OPG axis, Wingless (Wnt) pathway emerged as the osteoblast differentiation way, and also as a bone mass regulator. Researchers have been discovering new molecules and cytokines interactions. Altogether, data suggest that RANK/RANKL/OPG system could be targeted as a new treatment strategy in bone conditions. FREEDOM is the more recently published clinical trial about a RANKL-specific recombinant fully human monoclonal antibody (denosumab). OPG is also a potential innovative therapeutic option to be investigated.
Fracture patterns in malignant osteopetrosis(Albers-Schönberg disease)
DOI:10.1007/BF00443478 URL [本文引用: 1]
Fracture callus in osteopetrosis
Osteopetrosis: genetics, treatment and new insights into osteoclast function
DOI:10.1038/nrendo.2013.137
PMID:23877423
[本文引用: 1]

Osteopetrosis is a genetic condition of increased bone mass, which is caused by defects in osteoclast formation and function. Both autosomal recessive and autosomal dominant forms exist, but this Review focuses on autosomal recessive osteopetrosis (ARO), also known as malignant infantile osteopetrosis. The genetic basis of this disease is now largely uncovered: mutations in TCIRG1, CLCN7, OSTM1, SNX10 and PLEKHM1 lead to osteoclast-rich ARO (in which osteoclasts are abundant but have severely impaired resorptive function), whereas mutations in TNFSF11 and TNFRSF11A lead to osteoclast-poor ARO. In osteoclast-rich ARO, impaired endosomal and lysosomal vesicle trafficking results in defective osteoclast ruffled-border formation and, hence, the inability to resorb bone and mineralized cartilage. ARO presents soon after birth and can be fatal if left untreated. However, the disease is heterogeneous in clinical presentation and often misdiagnosed. This article describes the genetics of ARO and discusses the diagnostic role of next-generation sequencing methods. The management of affected patients, including guidelines for the indication of haematopoietic stem cell transplantation (which can provide a cure for many types of ARO), are outlined. Finally, novel treatments, including preclinical data on in utero stem cell treatment, RANKL replacement therapy and denosumab therapy for hypercalcaemia are also discussed.
Cell and small animal models for phenotypic drug discovery
DOI:10.2147/DDDT URL [本文引用: 2]
Psoralen accelerates bone fracture healing by activating both osteoclasts and osteoblasts
DOI:10.1096/fj.201801797R
PMID:30702934
[本文引用: 1]

Bone fracture healing is a complex, dynamic process that involves various cell types, with osteoclasts and osteoblasts playing indispensable roles. In this study, we found that psoralen, the main active ingredient in Psoralea corylifolia L. fruit extract, enhanced bone fracture healing through activation of osteoclast and osteoblast activity via the ERK signaling pathway. In detail, psoralen promoted receptor activator of nuclear factor-κB ligand-induced osteoclastogenesis, mRNA expression of osteoclast-specific genes, and osteoclastic bone resorption in primary bone marrow-derived macrophages. Meanwhile, psoralen induced osteogenic differentiation by promoting the mRNA expression of the osteoblast differentiation markers alkaline phosphatase, runt-related transcription factor 2, osterix, and osteocalcin. At the molecular level, psoralen preferentially activated ERK1/2 but not JNK or p38 MAPKs. Further experiments revealed that psoralen-induced osteoclast and osteoblast differentiation was abrogated by a specific inhibitor of phosphorylated ERK. In addition, psoralen accelerated bone fracture healing in a rat tibial fracture model, and the numbers of osteoclasts and osteoblasts were increased in psoralen-treated fracture callus. Taken together, our findings indicate that psoralen accelerates bone fracture healing through activation of osteoclasts and osteoblasts via ERK signaling and has potential as a novel drug in the orthopedic clinic for the treatment of bone fractures.-Zhang, T., Han, W., Zhao, K., Yang, W., Lu, X., Jia, Y., Qin, A., Qian, Y. Psoralen accelerates bone fracture healing by activating both osteoclasts and osteoblasts.
Allantoin in human plasma, serum, and nasal-lining fluids as a biomarker of oxidative stress: avoiding artifacts and establishing real in vivo concentrations
DOI:10.1089/ars.2008.2364 URL [本文引用: 1]
In vivo wound healing effects of Symphytum officinale L. leaves extract in different topical formulations
The present work evaluates wound healing activity of leaves extracts of Symphytum officinale L. (comfrey) incorporated in three pharmaceutical formulations. Wound healing activity of comfrey was determined by qualitative and quantitative histological analysis of open wound in rat model, using allantoin as positive control. Three topical formulations, carbomer gel, glycero-alcoholic solution and O/W emulsion (soft lotion) were compared. The histological analysis of the healing process shows significant differences in treatment, particularly on its intensity and rate. The results indicate that emulsion containing both extracts, commercial and prepared, induced the largest and furthest repair of damaged tissue. This could be evidenced from day 3 to 28 by increase in collagen deposition from 40% to 240% and reduction on cellular inflammatory infiltrate from 3% to 46%. However, 8% prepared extract in emulsion presented the best efficacy. This work clearly demonstrates that comfrey leaves have a wound healing activity. The O/W emulsion showed to be the vehicle most effective to induce healing activity, particularly with extracts obtained from comfrey leaves collected in Minas Gerais state in Brazil. It shows the best efficacy to control the inflammatory process and to induce collagen deposition at 8% concentration.
Antinociceptive and anti-inflammatory effects of Memora nodosa and allantoin in mice
DOI:S0378-8741(16)30201-X
PMID:27079223
[本文引用: 1]

The leaves and stems bark of Memora nodosa (Silva Manso) Miers (Bignoniaceae) are used in Brazilian traditional medicine in the treatment of external ulcers and wounds; its roots are used to treat abdominal pain and scabies.Our aim was to evaluate the antinociceptive and anti-inflammatory activities of Memora nodosa roots ethanolic extract (EMN) and allantoin, a secondary metabolite isolated from this plant.The EMN and allantoin antinociceptive activity were evaluated in mice using both chemical and heat-induced pain models such as acetic acid-induced writhing, formalin and tail-flick tests. In the formalin test, a pre-treatment with naloxone was used to verify an involvement of opioid receptor in the antinociceptive effect of EMN and allantoin. Pre-treatment with glibenclemide was used to verity an involvement of ATP-sensitive K(+)channel in the allantoin antinociceptive effect. EMN and allantoin anti-inflammatory activity were assessed by carrageenan-induced paw edema and pleurisy tests.The treatment with EMN (250, 500 and 1000mg/kg, p.o.) inhibit the acetic acid and formalin (both phases)-induced nociception. However, just at doses 500 and 1000mg/kg increased the latency time in tail-flick test. These results suggest the involvement of both peripheral and central antinociceptive mechanisms. The treatment with allantoin (40, 60 and 80mg/kg p.o.) produced a dose-dependent antinociceptive effect in both phases of formalin-induced nociception test; allantoin (60mg/kg) was not able to increase the latency time in tail flick-test. The pre-treatment with naloxone completely reversed the EMN (1000mg/kg) and allantoin (60mg/kg) effect in the first phase of formalin test; and glibenclamide reversed the allantoin effect. The administration of EMN (250, 500 and 1000mg/kg) and allantoin (60mg/kg) showed significant anti-inflammatory activity in the whole carrageenan-induced paw edema. Furthermore, EMN and allantoin reduced the leukocytes migration and pleural exudate to the pleural cavity.EMN have significant antinociceptive and anti-inflammatory effects, which appear to be, at least in part, due to the presence of allantoin. However, allantoin is not responsible for the EMN central antinociceptive activity. Allantoin has peripheral antinociceptive activity that involves the opioid receptor and ATP-sensitive K(+)channels. Opioid receptors are also involved in the EMN antinociceptive activity. These findings support the use of Memora nodosa in popular medicine and demonstrate that this plant has therapeutic potential for the development of antinociceptive and anti-inflammatory phytomedicines.Copyright © 2016 Elsevier Ireland Ltd. All rights reserved.