遗传 ›› 2026, Vol. 48 ›› Issue (1): 26-45.doi: 10.16288/j.yczz.25-107
收稿日期:2025-04-16
修回日期:2025-06-26
出版日期:2025-12-31
发布日期:2025-07-02
通讯作者:
夏梽丹,博士,副教授,研究方向:分子营养学-微量元素代谢。E-mail: xiazhidan@zju.edu.cn作者简介:李欣然,硕士研究生,专业方向:分子营养学。E-mail: 22218858@zju.edu.cn
基金资助:
Xinran Li1,2( ), Yanhan Feng1,2, Zhidan Xia1,2(
), Yanhan Feng1,2, Zhidan Xia1,2( )
)
Received:2025-04-16
Revised:2025-06-26
Published:2025-12-31
Online:2025-07-02
Supported by:摘要:
肌肉减少症(sarcopenia)简称“肌少症”,是以骨骼肌质量与功能进行性丧失为特征的年龄相关性退行性疾病,可引发跌倒、失能及全因死亡率递增等严重临床结局,显著降低老年群体生存质量。随着我国步入老龄化社会,肌少症将逐渐成为多数人群面临的健康威胁。本文系统归纳了导致肌少症的发病机制,指出代谢失衡和细胞氧化应激是肌肉功能退化的重要诱因,通过分析生活方式、生理特征和遗传因素的协同作用,阐释了人群易感性和疾病进展的病因学基础;重点总结了多个基因在维持肌肉功能中的作用,结合人群队列和动物模型研究阐明相关基因突变与肌少症的风险联系,同时揭示表观遗传因素对肌肉代谢和衰老的调控机制;最后,综合探讨了药物治疗、营养支持及运动疗法的干预效果与临床转化瓶颈。本文旨在为肌少症的基础研究和临床防控优化提供重要的理论支持。
李欣然, 冯彦涵, 夏梽丹. 肌肉减少症的发病机制和风险因素[J]. 遗传, 2026, 48(1): 26-45.
Xinran Li, Yanhan Feng, Zhidan Xia. Pathogenesis and risk factors of sarcopenia[J]. Hereditas(Beijing), 2026, 48(1): 26-45.
图1
肌少症的发病机制 IGF-1:胰岛素样生长因子-1;IGFR:胰岛素样生长因子受体;Insulin:胰岛素;IR:胰岛素受体;PI3K:激活磷脂酰肌醇3-激酶;AKT:蛋白激酶B;mTOR:雷帕霉素靶标;FoxO-P:磷酸化叉头框蛋白O;FoxO:叉头框蛋白O;Bnip3:BCL2/腺病毒E1B 19 kDa相互作用蛋白3;LC3:微管相关蛋白1A/1B轻链3;TNF-α:肿瘤坏死因子-α;TNFR:肿瘤坏死因子受体;NF-κB:激活核因子-κB;MuRF1:肌肉环指蛋白1;Atrogin-1:肌肉萎缩F-box蛋白1;Ub:泛素;UPS:泛素-蛋白酶系统;IL-1β:白细胞介素-1β;IL-6:白细胞介素-6;ROS:活性氧;TGF-β:转化生长因子β;ZIP14:溶质载体家族39成员14;MyHC:肌球蛋白重链。"
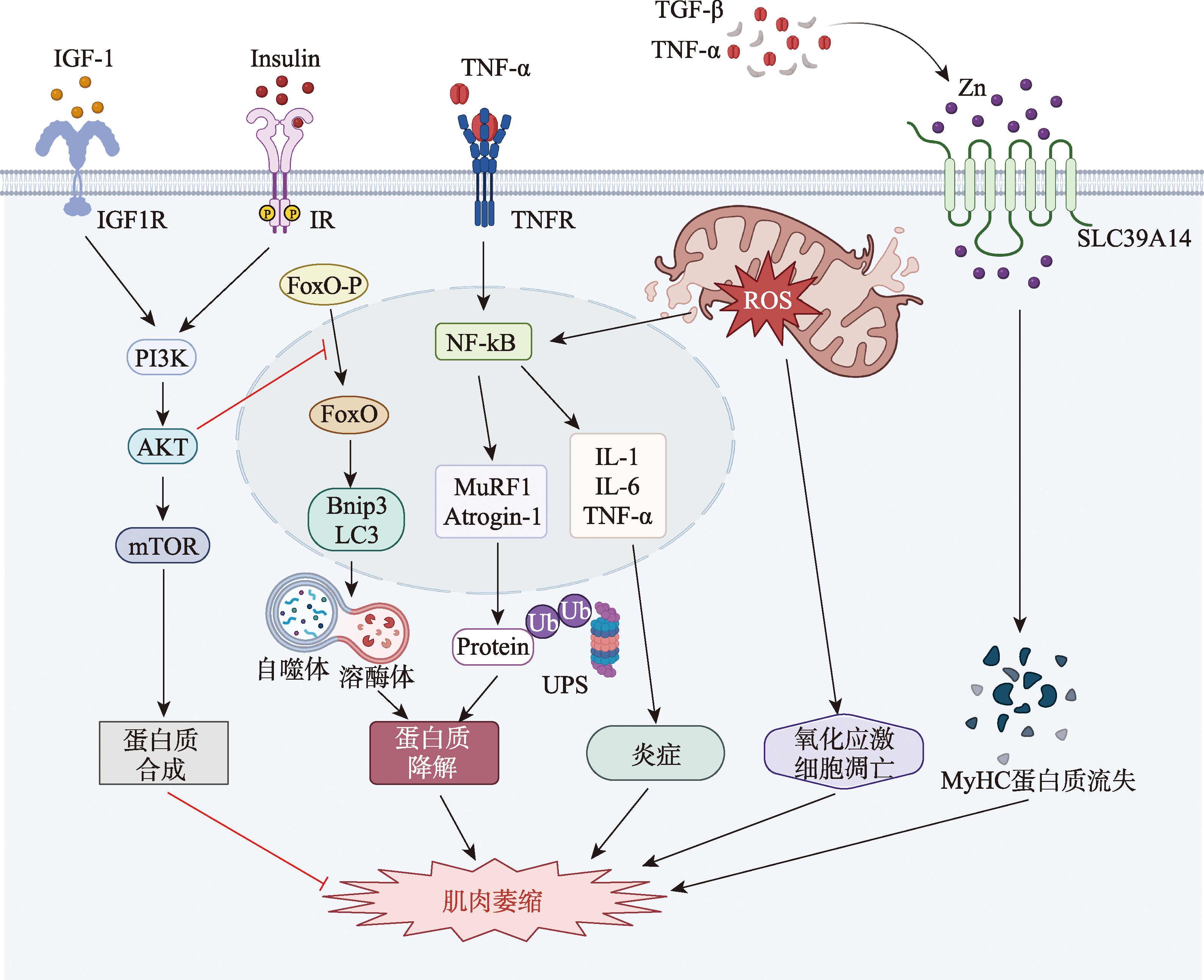
表1
肌少症患者基因突变位点汇总"
| 基因名称 | 突变位点 | 症状 | 意义 | 参考文献 |
|---|---|---|---|---|
| ACE | 内含子16的Alu重复序列 | D基因携带者ACE活性升高,更易患肌少症 | AT1受体阻滞剂氯沙坦治疗可恢复骨骼肌损伤后再生能力 | [ |
| ACTN3 | rs1815739、R577X | X基因携带者缺乏ACTN蛋白表达,更易患肌少症 | [ | |
| CAV | rs3807987、G14713A | A基因携带者更易患肌少症 | A等位基因是检测肌少症的早期标志物 | [ |
| MTHFR | rs1801131 T/G | G基因携带者更易患肌少症 | 有助于早发现肥胖群体中易患肌少症者 | [ |
| rs3737964、rs2066470、 rs4846048 | LBM改变 | [ | ||
| TRHR | rs7832552 C/T | C等位基因携带者LBM降低 | [ | |
| IRSI | rs2943656 A/G | A等位基因携带者LBM较低 | [ | |
| HSD17B11 | rs9991501 T/C | T等位基因携带者LBM较低 | [ | |
| VCAN | rs2287926 A/G | G等位基因携带者LBM较低 | [ | |
| ADAMTSL3 | rs4842924 T/C | T等位基因携带者LBM较低 | [ | |
| FTO | rs9936385 T/C | T等位基因携带者LBM较低 | [ |
| [1] |
Frontera WR, Ochala J. Skeletal muscle: a brief review of structure and function. Calcif Tissue Int, 2015, 96(3): 183-195.
pmid: 25294644 |
| [2] |
Mukund K, Subramaniam S. Skeletal muscle: a review of molecular structure and function, in health and disease. Wiley Interdiscip Rev Syst Biol Med, 2020, 12(1): e1462.
pmid: 31407867 |
| [3] | Rosenberg IH. Summary comments. Am J Clin Nutr, 1989, 50(5): 1231-1233. |
| [4] |
Kim JW, Kim R, Choi H, Lee SJ, Bae GU. Understanding of sarcopenia: from definition to therapeutic strategies. Arch Pharm Res, 2021, 44(9-10): 876-889.
pmid: 34537916 |
| [5] |
Breen L, Phillips SM. Skeletal muscle protein metabolism in the elderly: interventions to counteract the ‘anabolic resistance’ of ageing. Nutr Metab (Lond), 2011, 8(1): 68.
pmid: 21975196 |
| [6] | Li JL, Li J, Wang H. Age-associated proteostasis collapse. Hereditas(Beijing), 2022, 44(9): 733-744. |
| 黎嘉丽, 李瑾, 汪虎. 衰老相关的蛋白稳态失衡. 遗传, 2022, 44(9): 733-744. | |
| [7] |
Li H, Malhotra S, Kumar A. Nuclear factor-kappa B signaling in skeletal muscle atrophy. J Mol Med (Berl), 2008, 86(10): 1113-1126.
pmid: 18574572 |
| [8] |
Clavel S, Coldefy AS, Kurkdjian E, Salles J, Margaritis I, Derijard B. Atrophy-related ubiquitin ligases, atrogin-1 and MuRF1 are up-regulated in aged rat tibialis anterior muscle. Mech Ageing Dev, 2006, 127(10): 794-801.
pmid: 16949134 |
| [9] |
Husom AD, Peters EA, Kolling EA, Fugere NA, Thompson LV, Ferrington DA. Altered proteasome function and subunit composition in aged muscle. Arch Biochem Biophys, 2004, 421(1): 67-76.
pmid: 14678786 |
| [10] |
Bardag-Gorce F, Farout L, Veyrat-Durebex C, Briand Y, Briand M. Changes in 20S proteasome activity during ageing of the LOU rat. Mol Biol Rep, 1999, 26(1-2): 89-93.
pmid: 10363653 |
| [11] |
Marzetti E, Wohlgemuth SE, Lees HA, Chung HY, Giovannini S, Leeuwenburgh C. Age-related activation of mitochondrial caspase-independent apoptotic signaling in rat gastrocnemius muscle. Mech Ageing Dev, 2008, 129(9): 542-549.
pmid: 18579179 |
| [12] |
Rocchi A, He CC. Regulation of exercise-induced autophagy in skeletal muscle. Curr Pathobiol Rep, 2017, 5(2): 177-186.
pmid: 29057166 |
| [13] |
Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME. Akt promotes cell survival by phosphorylating and inhibiting a forkhead transcription factor. Cell, 1999, 96(6): 857-868.
pmid: 10102273 |
| [14] |
Wallace DC. A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: a dawn for evolutionary medicine. Annu Rev Genet, 2005, 39: 359-407.
pmid: 16285865 |
| [15] |
Jang YC, Lustgarten MS, Liu YH, Muller FL, Bhattacharya A, Liang HY, Salmon AB, Brooks SV, Larkin L, Hayworth CR, Richardson A, Van Remmen H. Increased superoxide in vivo accelerates age-associated muscle atrophy through mitochondrial dysfunction and neuromuscular junction degeneration. FASEB J, 2010, 24(5): 1376-1390.
pmid: 20040516 |
| [16] |
Peterson JM, Bakkar N, Guttridge DC. NF-κB signaling in skeletal muscle health and disease. Curr Top Dev Biol, 2011, 96: 85-119.
pmid: 21621068 |
| [17] |
He YY, Xie WQ, Li HZ, Jin HF, Zhang Y, Li YS. Cellular senescence in sarcopenia: possible mechanisms and therapeutic potential. Front Cell Dev Biol, 2022, 9: 793088.
pmid: 35083219 |
| [18] |
Moiseeva V, Cisneros A, Sica V, Deryagin O, Lai YW, Jung S, Andrés E, An J, Segalés J, Ortet L, Lukesova V, Volpe G, Benguria A, Dopazo A, Benitah SA, Urano Y, Del Sol A, Esteban MA, Ohkawa Y, Serrano AL, Perdiguero E, Muñoz-Cánoves P. Senescence atlas reveals an aged-like inflamed niche that blunts muscle regeneration. Nature, 2023, 613(7942): 169-178.
pmid: 36544018 |
| [19] |
Guo Q, Luo Q, Song GB. Control of muscle satellite cell function by specific exercise-induced cytokines and their applications in muscle maintenance. J Cachexia Sarcopenia Muscle, 2024, 15(2): 466-476.
pmid: 38375571 |
| [20] |
Tarantino U, Cariati I, Marini M, D’Arcangelo G, Tancredi V, Primavera M, Iundusi R, Gasbarra E, Scimeca M. Effects of simulated microgravity on muscle stem cells activity. Cell Physiol Biochem, 2020, 54(4): 736-747.
pmid: 32749090 |
| [21] |
Belizário JE, Fontes-Oliveira CC, Borges JP, Kashiabara JA, Vannier E. Skeletal muscle wasting and renewal: a pivotal role of myokine IL-6. Springerplus, 2016, 5: 619.
pmid: 27330885 |
| [22] |
Haddad F, Zaldivar F, Cooper DM, Adams GR. IL-6-induced skeletal muscle atrophy. J Appl Physiol, 2005, 98(3): 911-917.
pmid: 15542570 |
| [23] |
DeRuisseau KC, Park YM, DeRuisseau LR, Cowley PM, Fazen CH, Doyle RP. Aging-related changes in the iron status of skeletal muscle. Exp Gerontol, 2013, 48(11): 1294-1302.
pmid: 23994517 |
| [24] |
Perna S, Peroni G, Faliva MA, Bartolo A, Naso M, Miccono A, Rondanelli M. Sarcopenia and sarcopenic obesity in comparison: prevalence, metabolic profile, and key differences. A cross-sectional study in italian hospitalized elderly. Aging Clin Exp Res, 2017, 29(6): 1249-1258.
pmid: 28233283 |
| [25] |
Ru Q, Li YS, Zhang X, Chen L, Wu YX, Min JX, Wang FD. Iron homeostasis and ferroptosis in muscle diseases and disorders: mechanisms and therapeutic prospects. Bone Res, 2025, 13(1): 27.
pmid: 40000618 |
| [26] |
Huang Y, Wu BL, Shen DZ, Chen JL, Yu ZH, Chen C. Ferroptosis in a sarcopenia model of senescence accelerated mouse prone 8 (SAMP8). Int J Biol Sci, 2021, 17(1): 151-162.
pmid: 33390840 |
| [27] |
Ikeda Y, Imao M, Satoh A, Watanabe H, Hamano H, Horinouchi Y, Izawa-Ishizawa Y, Kihira Y, Miyamoto L, Ishizawa K, Tsuchiya K, Tamaki T. Iron-induced skeletal muscle atrophy involves an akt-forkhead box O3-E3 ubiquitin ligase-dependent pathway. J Trace Elem Med Biol, 2016, 35: 66-76.
pmid: 27049128 |
| [28] |
Ding HR, Chen SJ, Pan XH, Dai XS, Pan GH, Li Z, Mai XD, Tian Y, Zhang SS, Liu BD, Cao GC, Yao ZC, Yao XP, Gao L, Yang L, Chen XY, Sun J, Chen H, Han ML, Yin YL, Xu GH, Li HJ, Wu WD, Chen Z, Lin JC, Xiang LP, Hu J, Lu Y, Zhu X, Xie LW. Transferrin receptor 1 ablation in satellite cells impedes skeletal muscle regeneration through activation of ferroptosis. J Cachexia Sarcopenia Muscle, 2021, 12(3): 746-768.
pmid: 33955709 |
| [29] |
Park SJ, Park J, Won CW, Lee HJ. The inverse association of sarcopenia and protein-source food and vegetable intakes in the korean elderly: the Korean frailty and aging cohort study. Nutrients, 2022, 14(7): 1375.
pmid: 35405986 |
| [30] |
Gariballa S, Alessa A. Association between nutritional blood-based biomarkers and clinical outcome in sarcopenia patients. Clin Nutr ESPEN, 2018, 25: 145-148.
pmid: 29779810 |
| [31] |
Herzig S, Shaw RJ. AMPK: guardian of metabolism and mitochondrial homeostasis. Nat Rev Mol Cell Biol, 2018, 19(2): 121-135.
pmid: 28974774 |
| [32] |
Reddy SS, Addi UR, Pullakhandam R, Reddy GB. Dietary zinc deficiency disrupts skeletal muscle proteostasis and mitochondrial biology in rats. Nutrition, 2022, 98: 111625.
pmid: 35439650 |
| [33] |
Aydemir TB, Troche C, Kim J, Kim MH, Teran OY, Leeuwenburgh C, Cousins RJ. Aging amplifies multiple phenotypic defects in mice with zinc transporter Zip14 (Slc39a14) deletion. Exp Gerontol, 2016, 85: 88-94.
pmid: 27647172 |
| [34] |
Wang G, Biswas AK, Ma WC, Kandpal M, Coker C, Grandgenett PM, Hollingsworth MA, Jain R, Tanji K, Lόpez-Pintado S, Borczuk A, Hebert D, Jenkitkasemwong S, Hojyo S, Davuluri RV, Knutson MD, Fukada T, Acharyya S. Metastatic cancers promote cachexia through ZIP14 upregulation in skeletal muscle. Nat Med, 2018, 24(6): 770-781.
pmid: 29875463 |
| [35] |
Huang Q, Wan JF, Nan WB, Li SQ, He BM, Peng ZY. Association between manganese exposure in heavy metals mixtures and the prevalence of sarcopenia in US adults from NHANES 2011-2018. J Hazard Mater, 2024, 464: 133005.
pmid: 37988867 |
| [36] |
Xia ZD, Tang BY, Li XP, Li XR, Jia YF, Jiang JW, Chen JY, Song JS, Liu SY, Min JX, Wang FD. A novel role for the longevity-associated protein SLC39A11 as a manganese transporter. Research (Wash D C), 2024, 7: 440.
pmid: 39114488 |
| [37] |
Gonzalez-Freire M, de Cabo R, Studenski SA, Ferrucci L. The neuromuscular junction: aging at the crossroad between nerves and muscle. Front Aging Neurosci, 2014, 6: 208.
pmid: 25157231 |
| [38] |
Gillies AR, Lieber RL. Structure and function of the skeletal muscle extracellular matrix. Muscle Nerve, 2011, 44(3): 318-331.
pmid: 21949456 |
| [39] |
Pavan P, Monti E, Bondí M, Fan CL, Stecco C, Narici M, Reggiani C, Marcucci L. Alterations of extracellular matrix mechanical properties contribute to age-related functional impairment of human skeletal muscles. Int J Mol Sci, 2020, 21(11): 3992.
pmid: 32498422 |
| [40] |
Hammers DW, Matheny RW, Sell C, Adamo ML, Walters TJ, Estep JS, Farrar RP. Impairment of IGF-I expression and anabolic signaling following ischemia/reperfusion in skeletal muscle of old mice. Exp Gerontol, 2011, 46(4): 265-272.
pmid: 21094246 |
| [41] |
Qiu WD, Cai AP, Li LW, Feng YQ. Trend in prevalence, associated risk factors, and longitudinal outcomes of sarcopenia in China: a national cohort study. J Intern Med, 2024, 296(2): 156-167.
pmid: 38801732 |
| [42] |
Marzban Abbas Abadi M, Hosseinzade D, Khalilizad M. Prevalence of, and factors associated with, sarcopenia in Iran: a systematic review and meta-analysis. Front Nutr, 2025, 11: 1457768.
pmid: 39839289 |
| [43] |
Mazocco L, Gonzalez MC, Barbosa-Silva TG, Chagas P. Sarcopenia in Brazilian rural and urban elderly women: is there any difference? Nutrition, 2019, 58: 120-124.
pmid: 30391690 |
| [44] |
Xie WQ, Xiao GL, Fan YB, He M, Lv S, Li YS. Sarcopenic obesity: research advances in pathogenesis and diagnostic criteria. Aging Clin Exp Res, 2021, 33(2): 247-252.
pmid: 31845200 |
| [45] |
Lexell J, Taylor CC, Sjöström M. What is the cause of the ageing atrophy? total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15- to 83-year-old men. J Neurol Sci, 1988, 84(2-3): 275-294.
pmid: 3379447 |
| [46] |
Liu CR, Wong PY, Chung YL, Chow SKH, Cheung WH, Law SW, Chan JCN, Wong RMY. Deciphering the “obesity paradox” in the elderly: a systematic review and meta-analysis of sarcopenic obesity. Obes Rev, 2023, 24(2): e13534.
pmid: 36443946 |
| [47] |
Pourmotabbed A, Ghaedi E, Babaei A, Mohammadi H, Khazaie H, Jalili C, Symonds ME, Moradi S, Miraghajani M. Sleep duration and sarcopenia risk: a systematic review and dose-response meta-analysis. Sleep Breath, 2020, 24(4): 1267-1278.
pmid: 31832982 |
| [48] |
Steffl M, Bohannon RW, Petr M, Kohlikova E, Holmerova I. Relation between cigarette smoking and sarcopenia: meta-analysis. Physiol Res, 2015, 64(3): 419-426.
pmid: 25536323 |
| [49] |
Belavý DL, Miokovic T, Armbrecht G, Richardson CA, Rittweger J, Felsenberg D. Differential atrophy of the lower-limb musculature during prolonged bed-rest. Eur J Appl Physiol, 2009, 107(4): 489-499.
pmid: 19680682 |
| [50] |
Preedy VR, Adachi J, Ueno Y, Ahmed S, Mantle D, Mullatti N, Rajendram R, Peters TJ. Alcoholic skeletal muscle myopathy: definitions, features, contribution of neuropathy, impact and diagnosis. Eur J Neurol, 2001, 8(6): 677-687.
pmid: 11784353 |
| [51] |
Gao QQ, Hu KY, Yan CJ, Zhao B, Mei F, Chen F, Zhao L, Shang Y, Ma YX, Ma B. Associated factors of sarcopenia in community-dwelling older adults: a systematic review and meta-analysis. Nutrients, 2021, 13(12): 4291.
pmid: 34959843 |
| [52] |
Jogiat UM, Bédard ELR, Sasewich H, Turner SR, Eurich DT, Filafilo H, Baracos V. Sarcopenia reduces overall survival in unresectable oesophageal cancer: a systematic review and meta-analysis. J Cachexia Sarcopenia Muscle, 2022, 13(6): 2630-2636.
pmid: 36151845 |
| [53] |
Wathanavasin W, Banjongjit A, Avihingsanon Y, Praditpornsilpa K, Tungsanga K, Eiam-Ong S, Susantitaphong P. Prevalence of sarcopenia and its impact on cardiovascular events and mortality among dialysis patients: a systematic review and meta-analysis. Nutrients, 2022, 14(19): 4077.
pmid: 36235729 |
| [54] |
Ge HJ, Yang SL, Su WY, Guan WM, Dong SH, Chang WJ, Jia HY, Jiang S, Qin D, Ma GF. The relationship between sarcopenia and mental health status in Chinese older adults: the mediating role of activities of daily living. BMC Geriatr, 2025, 25(1): 64.
pmid: 39881231 |
| [55] |
Villani ER, Salerno A, Triolo F, Franza L, Vaccari G, Manni B, Vaccina AR, Bergamini L, Menon V, Zaccherini D, Fabbo A. Probable sarcopenia and depressive symptoms in community-dwelling older adults: exploring the role of frailty and comorbidities. Aging Clin Exp Res, 2025, 37(1): 104.
pmid: 40133568 |
| [56] |
Chen SJ, Zhang PX, Duan HM, Wang J, Qiu YYY, Cui ZB, Yin YL, Wan D, Xie LW. Gut microbiota in muscular atrophy development, progression, and treatment: new therapeutic targets and opportunities. Innovation (Camb), 2023, 4(5): 100479.
pmid: 37539440 |
| [57] |
Chen SJ, Huang LJ, Liu BD, Duan HM, Li Z, Liu YF, Li H, Fu X, Lin JC, Xu YL, Liu L, Wan D, Yin YL, Xie LW. Dynamic changes in butyrate levels regulate satellite cell homeostasis by preventing spontaneous activation during aging. Sci China Life Sci, 2024, 67(4): 745-764.
pmid: 38157106 |
| [58] |
Powers SK, Morton AB, Hyatt H, Hinkley MJ. The renin-angiotensin system and skeletal muscle. Exerc Sport Sci Rev, 2018, 46(4): 205-214.
pmid: 30001274 |
| [59] |
Afsar B, Afsar RE, Caliskan Y, Lentine KL, Edwards JC. Renin angiotensin system-induced muscle wasting: putative mechanisms and implications for clinicians. Mol Cell Biochem, 2025, 480(4): 1935-1949.
pmid: 38811433 |
| [60] |
Williams AG, Day SH, Folland JP, Gohlke P, Dhamrait S, Montgomery HE. Circulating angiotensin converting enzyme activity is correlated with muscle strength. Med Sci Sports Exerc, 2005, 37(6): 944-948.
pmid: 15947718 |
| [61] |
Kang HJ, Kim CH, Park DS, Choi SY, Lee DH, Nam HS, Hur JG, Woo JH. The impacts of ACE activity according to ACE I/D polymorphisms on muscular functions of people aged 65. Ann Rehabil Med, 2012, 36(4): 433-446.
pmid: 22977768 |
| [62] |
McCauley T, Mastana SS, Folland JP. ACE I/D and ACTN3 R/X polymorphisms and muscle function and muscularity of older caucasian men. Eur J Appl Physiol, 2010, 109(2): 269-277.
pmid: 20069311 |
| [63] |
North KN, Yang N, Wattanasirichaigoon D, Mills M, Easteal S, Beggs AH. A common nonsense mutation results in alpha-actinin-3 deficiency in the general population. Nat Genet, 1999, 21(4): 353-354.
pmid: 10192379 |
| [64] |
Vincent B, Windelinckx A, Nielens H, Ramaekers M, Van Leemputte M, Hespel P, Thomis MA. Protective role of alpha-actinin-3 in the response to an acute eccentric exercise bout. J Appl Physiol, 2010, 109(2): 564-573.
pmid: 20507967 |
| [65] |
Cho J, Lee I, Kang H. ACTN3 gene and susceptibility to sarcopenia and osteoporotic status in older Korean adults. Biomed Res Int, 2017, 2017: 4239648.
pmid: 28626757 |
| [66] |
Kiuchi Y, Makizako H, Nakai Y, Taniguchi Y, Tomioka K, Sato N, Wada A, Doi T, Kiyama R, Takenaka T. Associations of alpha-actinin-3 genotype with thigh muscle volume and physical performance in older adults with sarcopenia or pre-sarcopenia. Exp Gerontol, 2021, 154: 111525.
pmid: 34425205 |
| [67] |
Seto JT, Roeszler KN, Meehan LR, Wood HD, Tiong C, Bek L, Lee SF, Shah M, Quinlan KGR, Gregorevic P, Houweling PJ, North KN. ACTN3 genotype influences skeletal muscle mass regulation and response to dexamethasone. Sci Adv, 2021, 7(27): eabg0088.
pmid: 34215586 |
| [68] |
Garton FC, Houweling PJ, Vukcevic D, Meehan LR, Lee FXZ, Lek M, Roeszler KN, Hogarth MW, Tiong CF, Zannino D, Yang N, Leslie S, Gregorevic P, Head SI, Seto JT, North KN. The effect of ACTN3 gene doping on skeletal muscle performance. Am J Hum Genet, 2018, 102(5): 845-857.
pmid: 29706347 |
| [69] |
Lin CH, Lin CC, Tsai CW, Chang WS, Yang MD, Bau DT. A novel caveolin-1 biomarker for clinical outcome of sarcopenia. In Vivo, 2014, 28(3): 383-389.
pmid: 24815842 |
| [70] |
González Coraspe JA, Weis J, Anderson ME, Münchberg U, Lorenz K, Buchkremer S, Carr S, Zahedi RP, Brauers E, Michels H, Sunada Y, Lochmüller H, Campbell KP, Freier E, Hathazi D, Roos A. Biochemical and pathological changes result from mutated caveolin-3 in muscle. Skelet Muscle, 2018, 8(1): 28.
pmid: 30153853 |
| [71] |
Parker S, Peterkin HS, Baylis HA. Muscular dystrophy associated mutations in caveolin-1 induce neurotransmission and locomotion defects in caenorhabditis elegans. Invert Neurosci, 2007, 7(3): 157-164.
pmid: 17629760 |
| [72] |
Veeranki S, Tyagi SC. Defective homocysteine metabolism: potential implications for skeletal muscle malfunction. Int J Mol Sci, 2013, 14(7): 15074-15091.
pmid: 23873298 |
| [73] |
Moll S, Varga EA. Homocysteine and MTHFR mutations. Circulation, 2015, 132(1): e6-e9.
pmid: 26149435 |
| [74] |
Reilly R, McNulty H, Pentieva K, Strain JJ, Ward M. MTHFR 677TT genotype and disease risk: is there a modulating role for B-vitamins? Proc Nutr Soc, 2014, 73(1): 47-56.
pmid: 24131523 |
| [75] |
Khanal P, Williams AG, He LX, Stebbings GK, Onambele-Pearson GL, Thomis M, Degens H, Morse CI. Sarcopenia, obesity, and sarcopenic obesity: relationship with skeletal muscle phenotypes and single nucleotide polymorphisms. J Clin Med, 2021, 10(21): 4933.
pmid: 34768452 |
| [76] |
Liu XG, Zhao LJ, Liu YJ, Xiong DH, Recker RR, Deng HW. The MTHFR gene polymorphism is associated with lean body mass but not fat body mass. Hum Genet, 2008, 123(2): 189-196.
pmid: 18180959 |
| [77] |
Wiedemann A, Chery C, Coelho D, Flayac J, Gueguen N, Desquiret-Dumas V, Feillet F, Lavigne C, Neau JP, Fowler B, Baumgartner MR, Reynier P, Guéant JL, Oussalah A. Mutations in MTHFR and POLG impaired activity of the mitochondrial respiratory chain in 46-year-old twins with spastic paraparesis. J Hum Genet, 2020, 65(2): 91-98.
pmid: 31645654 |
| [78] |
Alnaji HA, Abduljaleel AK, Al-Saadi T, Almulla AF. Genetic variability of the MTHFR rs1801133 gene polymorphism and role of zinc level in obese patients. Arch Physiol Biochem, 2025, 14: 1-8.
pmid: 39951118 |
| [79] |
Liu XG, Tan LJ, Lei SF, Liu YJ, Shen H, Wang L, Yan H, Guo YF, Xiong DH, Chen XD, Pan F, Yang TL, Zhang YP, Guo Y, Tang NL, Zhu XZ, Deng HY, Levy S, Recker RR, Papasian CJ, Deng HW. Genome-wide association and replication studies identified TRHR as an important gene for lean body mass. Am J Hum Genet, 2009, 84(3): 418-423.
pmid: 19268274 |
| [80] |
Riedl I, Osler ME, Benziane B, Chibalin AV, Zierath JR. Association of the ACTN3 R577X polymorphism with glucose tolerance and gene expression of sarcomeric proteins in human skeletal muscle. Physiol Rep, 2015, 3(3): e12314.
pmid: 25780092 |
| [81] |
Cabello-Verrugio C, Morales MG, Rivera JC, Cabrera D, Simon F. Renin-angiotensin system: an old player with novel functions in skeletal muscle. Med Res Rev, 2015, 35(3): 437-463.
pmid: 25764065 |
| [82] |
Pereira A, Costa AM, Izquierdo M, Silva AJ, Bastos E, Marques MC. ACE I/D and ACTN3 R/X polymorphisms as potential factors in modulating exercise-related phenotypes in older women in response to a muscle power training stimuli. Age (Dordr), 2013, 35(5): 1949-1959.
pmid: 22855367 |
| [83] |
Zhao Q, Jing Y, Jiang XY, Zhang X, Liu FF, Huang HY, Zhang ZH, Wang HJ, Sun SH, Ma S, Zhang WQ, Yu Y, Fu XB, Zhao GG, Qu J, Wang S, Liu GH. SIRT5 safeguards against primate skeletal muscle ageing via desuccinylation of TBK1. Nat Metab, 2025, 7(3): 556-573.
pmid: 40087407 |
| [84] |
Muller FL, Song W, Jang YC, Liu YH, Sabia M, Richardson A, Van Remmen H. Denervation-induced skeletal muscle atrophy is associated with increased mitochondrial ROS production. Am J Physiol Regul Integr Comp Physiol, 2007, 293(3): R1159-R1168.
pmid: 17584954 |
| [85] |
Ahn B, Smith N, Saunders D, Ranjit R, Kneis P, Towner RA, Van Remmen H. Using MRI to measure in vivo free radical production and perfusion dynamics in a mouse model of elevated oxidative stress and neurogenic atrophy. Redox Biol, 2019, 26: 101308.
pmid: 31470261 |
| [86] |
Wray DW, Nishiyama SK, Monnet A, Wary C, Duteil SS, Carlier PG, Richardson RS. Antioxidants and aging: NMR-based evidence of improved skeletal muscle perfusion and energetics. Am J Physiol Heart Circ Physiol, 2009, 297(5): H1870-H1875.
pmid: 19767527 |
| [87] |
Ko F, Yu QL, Xue QL, Yao WL, Brayton C, Yang HL, Fedarko N, Walston J. Inflammation and mortality in a frail mouse model. Age (Dordr), 2012, 34(3): 705-715.
pmid: 21633802 |
| [88] |
Akki A, Yang HL, Gupta A, Chacko VP, Yano T, Leppo MK, Steenbergen C, Walston J, Weiss RG. Skeletal muscle ATP kinetics are impaired in frail mice. Age (Dordr), 2014, 36(1): 21-30.
pmid: 23695949 |
| [89] |
Zillikens MC, Demissie S, Hsu YH, Yerges-Armstrong LM, Chou WC, Stolk L, Livshits G, Broer L, Johnson T, Koller DL, Kutalik Z, Luan JA, Malkin I, Ried JS, Smith AV, Thorleifsson G, Vandenput L, Zhao JH, Zhang WH, Aghdassi A, Åkesson K, Amin N, Baier LJ, Barroso I, Bennett DA, Bertram L, Biffar R, Bochud M, Boehnke M, Borecki IB, Buchman AS, Byberg L, Campbell H, Obanda NC, Cauley JA, Cawthon PM, Cederberg H, Chen Z, Cho NH, Choi HJ, Claussnitzer M, Collins F, Cummings SR, De Jager PL, Demuth I, Dhonukshe- Rutten RAM, Diatchenko L, Eiriksdottir G, Enneman AW, Erdos M, Eriksson JG, Eriksson J, Estrada K, Evans DS, Feitosa MF, Fu M, Garcia M, Gieger C, Girke T, Glazer NL, Grallert H, Grewal J, Han BG, Hanson RL, Hayward C, Hofman A, Hoffman EP, Homuth G, Hsueh WC, Hubal MJ, Hubbard A, Huffman KM, Husted LB, Illig T, Ingelsson E, Ittermann T, Jansson JO, Jordan JM, Jula A, Karlsson M, Khaw KT, Kilpeläinen TO, Klopp N, Kloth JSL, Koistinen HA, Kraus WE, Kritchevsky S, Kuulasmaa T, Kuusisto J, Laakso M, Lahti J, Lang T, Langdahl BL, Launer LJ, Lee JY, Lerch MM, Lewis JR, Lind L, Lindgren C, Liu YM, Liu T, Liu YF, Ljunggren Ö, Lorentzon M, Luben RN, Maixner W, McGuigan FE, Medina-Gomez C, Meitinger T, Melhus H, Mellström D, Melov S, Michaëlsson K, Mitchell BD, Morris AP, Mosekilde L, Newman A, Nielson CM, O'Connell JR, Oostra BA, Orwoll ES, Palotie A, Parker SCJ, Peacock M, Perola M, Peters A, Polasek O, Prince RL, Räikkönen K, Ralston SH, Ripatti S, Robbins JA, Rotter JI, Rudan I, Salomaa V, Satterfield S, Schadt EE, Schipf S, Scott L, Sehmi J, Shen J, Shin CS, Sigurdsson G, Smith S, Soranzo N, Stančáková A, Steinhagen-Thiessen E, Streeten EA, Styrkarsdottir U, Swart KMA, Tan ST, Tarnopolsky MA, Thompson P, Thomson CA, Thorsteinsdottir U, Tikkanen E, Tranah GJ, Tuomilehto J, van Schoor NM, Verma A, Vollenweider P, Völzke H, Wactawski-Wende J, Walker M, Weedon MN, Welch R, Wichmann HE, Widen E, Williams FMK, Wilson JF, Wright NC, Xie WJ, Yu L, Zhou YH, Chambers JC, Döring A, van Duijn CM, Econs MJ, Gudnason V, Kooner JS, Psaty BM, Spector TD, Stefansson K, Rivadeneira F, Uitterlinden AG, Wareham NJ, Ossowski V, Waterworth D, Loos RJF, Karasik D, Harris TB, Ohlsson C, Kiel DP. Large meta-analysis of genome- wide association studies identifies five loci for lean body mass. Nat Commun, 2017, 8(1): 80.
pmid: 28724990 |
| [90] |
Yedigaryan L, Gatti M, Marini V, Maraldi T, Sampaolesi M. Shared and divergent epigenetic mechanisms in cachexia and sarcopenia. Cells, 2022, 11(15): 2293.
pmid: 35892590 |
| [91] |
Bigot A, Duddy WJ, Ouandaogo ZG, Negroni E, Mariot V, Ghimbovschi S, Harmon B, Wielgosik A, Loiseau C, Devaney J, Dumonceaux J, Butler-Browne G, Mouly V, Duguez S. Age-associated methylation suppresses SPRY1, leading to a failure of Re-quiescence and loss of the reserve stem cell pool in elderly muscle. Cell Rep, 2015, 13(6): 1172-1182.
pmid: 26526994 |
| [92] |
Fan HT, Zhang R, Tesfaye D, Tholen E, Looft C, Hölker M, Schellander K, Cinar MU. Sulforaphane causes a major epigenetic repression of myostatin in porcine satellite cells. Epigenetics, 2012, 7(12): 1379-1390.
pmid: 23092945 |
| [93] |
Shvedunova M, Akhtar A. Modulation of cellular processes by histone and non-histone protein acetylation. Nat Rev Mol Cell Biol, 2022, 23(5): 329-349.
pmid: 35042977 |
| [94] |
Yoshihara T, Machida S, Tsuzuki T, Kakigi R, Chang SW, Sugiura T, Naito H. Age-related changes in histone modification in rat gastrocnemius muscle. Exp Gerontol, 2019, 125: 110658.
pmid: 31302168 |
| [95] |
Ryder DJ, Judge SM, Beharry AW, Farnsworth CL, Silva JC, Judge AR. Identification of the acetylation and ubiquitin-modified proteome during the progression of skeletal muscle atrophy. PLoS One, 2015, 10(8): e0136247.
pmid: 26302492 |
| [96] |
Walsh ME, Bhattacharya A, Sataranatarajan K, Qaisar R, Sloane L, Rahman MM, Kinter M, Van Remmen H. The histone deacetylase inhibitor butyrate improves metabolism and reduces muscle atrophy during aging. Aging Cell, 2015, 14(6): 957-970.
pmid: 26290460 |
| [97] |
Stouth DW, Manta A, Ljubicic V. Protein arginine methyltransferase expression, localization, and activity during disuse-induced skeletal muscle plasticity. Am J Physiol Cell Physiol, 2018, 314(2): C177-C190.
pmid: 29092819 |
| [98] |
Stouth DW, vanLieshout TL, Mikhail AI, Ng SY, Raziee R, Edgett BA, Vasam G, Webb EK, Gilotra KS, Markou M, Pineda HC, Bettencourt-Mora BG, Noor H, Moll Z, Bittner ME, Gurd BJ, Menzies KJ, Ljubicic V. CARM1 drives mitophagy and autophagy flux during fasting- induced skeletal muscle atrophy. Autophagy, 2023, 20(6): 1247-1269.
pmid: 38018843 |
| [99] |
Gerosa L, Malvandi AM, Gomarasca M, Verdelli C, Sansoni V, Faraldi M, Ziemann E, Olivieri F, Banfi G, Lombardi G. Murine myoblasts exposed to SYUIQ-5 acquire senescence phenotype and differentiate into sarcopenic-like myotubes, an in vitro study. J Gerontol A Biol Sci Med Sci, 2024, 79(4): glae022.
pmid: 38267369 |
| [100] |
Mattiroli F, Penengo L. Histone ubiquitination: an integrative signaling platform in genome stability. Trends Genet, 2021, 37(6): 566-581.
pmid: 33485674 |
| [101] |
Crist CG, Montarras D, Buckingham M. Muscle satellite cells are primed for myogenesis but maintain quiescence with sequestration of Myf5 mRNA targeted by microRNA-31 in mRNP granules. Cell Stem Cell, 2012, 11(1): 118-126.
pmid: 22770245 |
| [102] |
Cheung TH, Quach NL, Charville GW, Liu L, Park L, Edalati A, Yoo B, Hoang P, Rando TA. Maintenance of muscle stem-cell quiescence by microRNA-489. Nature, 2012, 482(7386): 524-528.
pmid: 22358842 |
| [103] |
Quattrocelli M, Sampaolesi M. The mesmiRizing complexity of microRNAs for striated muscle tissue engineering. Adv Drug Deliv Rev, 2015, 88: 37-52.
pmid: 25912658 |
| [104] |
Guo RC, Wu ZM, Liu A, Li QW, Han TY, Shen CL. Hypoxic preconditioning-engineered bone marrow mesenchymal stem cell-derived exosomes promote muscle satellite cell activation and skeletal muscle regeneration via the miR-210-3p/KLF7 mechanism. Int Immunopharmacol, 2024, 142(Pt B): 113143.
pmid: 39306891 |
| [105] |
Zheng Y, Liu T, Li Q, Li J. Integrated analysis of long non-coding RNAs (lncRNAs) and mRNA expression profiles identifies lncRNA PRKG1-AS1 playing important roles in skeletal muscle aging. Aging (Albany NY), 2021, 13(11): 15044-15060.
pmid: 34051073 |
| [106] |
Jin JJ, Du MM, Wang J, Guo YB, Zhang JL, Zuo H, Hou YQ, Wang SS, Lv W, Bai W, Wang J, Zhan XZ, Peng YX, Tong Q, Chai J, Xu ZY, Zuo B. Conservative analysis of synaptopodin-2 intron sense-overlapping lncRNA reveals its novel function in promoting muscle atrophy. J Cachexia Sarcopenia Muscle, 2022, 13(4): 2017-2030.
pmid: 35592920 |
| [107] |
Zhu MH, Lian C, Chen G, Zou P, Qin BG. CircRNA FUT10 regulates the regenerative potential of aged skeletal muscle stem cells by targeting HOXA9. Aging (Albany NY), 2021, 13(13): 17428-17441.
pmid: 34257163 |
| [108] |
Lu XM, Liang BS, Li SJ, Chen Z, Chang WK. Modulation of HOXA9 after skeletal muscle denervation and reinnervation. Am J Physiol Cell Physiol, 2020, 318(6): C1154-C1165.
pmid: 32233950 |
| [109] |
Ørtenblad N, Westerblad H, Nielsen J. Muscle glycogen stores and fatigue. J Physiol, 2013, 591(18): 4405-4413.
pmid: 23652590 |
| [110] |
Reeves ND, Narici MV, Maganaris CN. Myotendinous plasticity to ageing and resistance exercise in humans. Exp Physiol, 2006, 91(3): 483-498.
pmid: 16469817 |
| [111] |
Studenski S, Perera S, Patel K, Rosano C, Faulkner K, Inzitari M, Brach J, Chandler J, Cawthon P, Connor EB, Nevitt M, Visser M, Kritchevsky S, Badinelli S, Harris T, Newman AB, Cauley J, Ferrucci L, Guralnik J. Gait speed and survival in older adults. JAMA, 2011, 305(1): 50-58.
pmid: 21205966 |
| [112] |
Lieffers JR, Bathe OF, Fassbender K, Winget M, Baracos VE. Sarcopenia is associated with postoperative infection and delayed recovery from colorectal cancer resection surgery. Br J Cancer, 2012, 107(6): 931-936.
pmid: 22871883 |
| [113] |
Krell RW, Kaul DR, Martin AR, Englesbe MJ, Sonnenday CJ, Cai SJ, Malani PN. Association between sarcopenia and the risk of serious infection among adults undergoing liver transplantation. Liver Transpl, 2013, 19(12):1396-1402.
pmid: 24151041 |
| [114] |
Landi F, Marzetti E, Liperoti R, Pahor M, Russo A, Martone AM, Colloca G, Capoluongo E, Bernabei R. Nonsteroidal anti-inflammatory drug (NSAID) use and sarcopenia in older people: results from the ilSIRENTE study. J Am Med Dir Assoc, 2013, 14(8): 626. e9-13.
pmid: 23747142 |
| [115] |
Hardee JP, Lynch GS. Current pharmacotherapies for sarcopenia. Expert Opin Pharmacother, 2019, 20(13): 1645-1657.
pmid: 31120352 |
| [116] |
Lee SJ. Targeting the myostatin signaling pathway to treat muscle loss and metabolic dysfunction. J Clin Invest, 2021, 131(9): e148372.
pmid: 33938454 |
| [117] |
Burks TN, Andres-Mateos E, Marx R, Mejias R, Van Erp C, Simmers JL, Walston JD, Ward CW, Cohn RD. Losartan restores skeletal muscle remodeling and protects against disuse atrophy in sarcopenia. Sci Transl Med, 2011, 3(82): 82ra37.
pmid: 21562229 |
| [118] |
Nieuwenhuizen WF, Weenen H, Rigby P, Hetherington MM. Older adults and patients in need of nutritional support: review of current treatment options and factors influencing nutritional intake. Clin Nutr, 2010, 29(2): 160-169.
pmid: 19828215 |
| [119] |
Otsuka R, Kato Y, Nishita Y, Tange C, Tomida M, Nakamoto M, Imai T, Ando F, Shimokata H. Age-related changes in energy intake and weight in community- dwelling middle-aged and elderly Japanese. J Nutr Health Aging, 2016, 20(4): 383-390.
pmid: 26999237 |
| [120] |
Robinson SM, Reginster JY, Rizzoli R, Shaw SC, Kanis JA, Bautmans I, Bischoff-Ferrari H, Bruyère O, Cesari M, Dawson-Hughes B, Fielding RA, Kaufman JM, Landi F, Malafarina V, Rolland Y, van Loon LJ, Vellas B, Visser M, Cooper C, ESCEO working group. Does nutrition play a role in the prevention and management of sarcopenia? Clin Nutr, 2018, 37(4): 1121-1132.
pmid: 28927897 |
| [121] |
Halfon M, Phan O, Teta D. Vitamin D: a review on its effects on muscle strength, the risk of fall, and frailty. Biomed Res Int, 2015, 2015: 953241.
pmid: 26000306 |
| [122] |
Visser M, Deeg DJH, Lips P, Longitudinal Aging Study Amsterdam. Low vitamin D and high parathyroid hormone levels as determinants of loss of muscle strength and muscle mass (sarcopenia): the longitudinal aging study amsterdam. J Clin Endocrinol Metab, 2003, 88(12): 5766-5772.
pmid: 14671166 |
| [123] |
Nakamura S, Sato Y, Kobayashi T, Kaneko Y, Ito E, Soma T, Okada H, Miyamoto K, Oya A, Matsumoto M, Nakamura M, Kanaji A, Miyamoto T. Vitamin D protects against immobilization-induced muscle atrophy via neural crest-derived cells in mice. Sci Rep, 2020, 10(1): 12242.
pmid: 32699341 |
| [124] |
Lauretani F, Semba RD, Bandinelli S, Dayhoff- Brannigan M, Lauretani F, Corsi AM, Guralnik JM, Ferrucci L. Carotenoids as protection against disability in older persons. Rejuvenation Res, 2008, 11(3): 557-563.
pmid: 18593275 |
| [125] |
Kaiser M, Bandinelli S, Lunenfeld B. Frailty and the role of nutrition in older people. A review of the current literature. Acta Biomed, 2010, 81 Suppl 1: 37-45.
pmid: 20518190 |
| [126] |
Visser M, Pluijm SMF, Stel VS, Bosscher RJ, Deeg DJH, Longitudinal Aging Study Amsterdam. Physical activity as a determinant of change in mobility performance: the longitudinal aging study Amsterdam. J Am Geriatr Soc, 2002, 50(11): 1774-1781.
pmid: 12410894 |
| [127] |
Hoseini R, Hoseini Z, Kamangar A. Myogenic differentiation markers in muscle tissue after aerobic training. Heliyon, 2025, 11(2): e41888.
pmid: 39897925 |
| [128] |
Fiatarone MA, Marks EC, Ryan ND, Meredith CN, Lipsitz LA, Evans WJ. High-intensity strength training in nonagenarians. Effects on skeletal muscle. JAMA, 1990, 263(22): 3029-3034.
pmid: 2342214 |
| [129] |
Peterson MD, Rhea MR, Sen A, Gordon PM. Resistance exercise for muscular strength in older adults: a meta-analysis. Ageing Res Rev, 2010, 9(3): 226-237.
pmid: 20385254 |
| [130] |
Petermann-Rocha F, Balntzi V, Gray SR, Lara J, Ho FK, Pell JP, Celis-Morales C. Global prevalence of sarcopenia and severe sarcopenia: a systematic review and meta-analysis. J Cachexia Sarcopenia Muscle, 2022, 13(1): 86-99.
pmid: 34816624 |
| [1] | 孙朝冉, 吴旭东. 组蛋白变体H2A.Z的转录调控功能与动态作用机制[J]. 遗传, 2024, 46(4): 279-289. |
| [2] | 杨韵霏, 沈义栋. 脉络丛及其与衰老相关疾病的关系[J]. 遗传, 2024, 46(2): 109-125. |
| [3] | 张翌, 吴志英. 伴皮质下梗死和白质脑病的常染色体显性遗传性脑动脉病的发病机制及治疗研究进展[J]. 遗传, 2023, 45(7): 568-579. |
| [4] | 何山, 赵健, 宋晓峰. N6-甲基腺苷修饰对女性生殖系统功能的影响[J]. 遗传, 2023, 45(6): 472-487. |
| [5] | 商晓康, 张思萌, 倪军军. 组织蛋白酶B参与脑衰老及阿尔兹海默症发生发展研究进展[J]. 遗传, 2023, 45(3): 212-220. |
| [6] | 张茜, 王子豪, 田烨. 跨组织线粒体应激信号交流调控机体衰老研究进展[J]. 遗传, 2023, 45(3): 187-197. |
| [7] | 黎嘉丽, 李瑾, 汪虎. 衰老相关的蛋白稳态失衡[J]. 遗传, 2022, 44(9): 733-744. |
| [8] | 赵岩, 王晨鑫, 杨天明, 李春爽, 张丽宏, 杜冬妮, 王若曦, 王静, 魏民, 巴雪青. DNA氧化损伤8-羟鸟嘌呤与肿瘤的发生发展[J]. 遗传, 2022, 44(6): 466-477. |
| [9] | 蒋卓远, 查艳, 石小峰, 张永彪. 神经嵴细胞和神经嵴病及其致病机制的研究进展[J]. 遗传, 2022, 44(2): 117-133. |
| [10] | 何江平, 陈捷凯. 转座元件、表观遗传调控与细胞命运决定[J]. 遗传, 2021, 43(9): 822-834. |
| [11] | 袁洁, 蔡时青. 衰老过程中行为和认知功能退化的调控机制研究[J]. 遗传, 2021, 43(6): 545-570. |
| [12] | 刘紫妍, 高艾. 炎性衰老在血液系统疾病中的研究进展[J]. 遗传, 2021, 43(12): 1132-1141. |
| [13] | 刘学文, 吴红梅, 白瑛, 曾群, 曹泽民, 吴秀山, 唐旻. 钾离子通道蛋白Shaker对果蝇心脏衰老的保护作用[J]. 遗传, 2021, 43(1): 94-99. |
| [14] | 刘传明,丁利军,李佳音,戴建武,孙海翔. 衰老导致卵巢功能低下研究进展[J]. 遗传, 2019, 41(9): 816-826. |
| [15] | 潘云枫, 王演怡, 陈静雯, 范怡梅. 线粒体代谢介导的表观遗传改变与衰老研究[J]. 遗传, 2019, 41(10): 893-904. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||

www.chinagene.cn
备案号:京ICP备09063187号-4
总访问:,今日访问:,当前在线:
