遗传 ›› 2023, Vol. 45 ›› Issue (11): 1018-1027.doi: 10.16288/j.yczz.23-236
收稿日期:2023-09-11
修回日期:2023-10-29
出版日期:2023-11-20
发布日期:2023-11-03
通讯作者:
谢建平
E-mail:1977015429@qq.com;georgex@swu.edu.cn
作者简介:向莎莎,硕士研究生,专业方向:分枝杆菌遗传学调控和分子机制。E-mail: 基金资助:
Shasha Xiang( ), Jianping Xie(
), Jianping Xie( )
)
Received:2023-09-11
Revised:2023-10-29
Published:2023-11-20
Online:2023-11-03
Contact:
Jianping Xie
E-mail:1977015429@qq.com;georgex@swu.edu.cn
Supported by:摘要:
错配修复(mismatch repair, MMR)是生物体DNA复制后的一种常见修复系统,对于维持基因组稳定性至关重要,其关键步骤由MutS和MutL蛋白家族的成员执行,尽管这种修复途径十分重要,但在许多古菌和放线菌基因组中并不存在MutS或MutL的同源蛋白。这类细菌(例如分枝杆菌等)采用另一种非典型的MMR途径,由核酸内切酶EndoMS/NucS发挥关键作用,与典型MMR蛋白(MutS/MutL)相比没有结构同源性。EndoMS/NucS介导的非典型错配修复在分枝杆菌DNA修复、突变和同源重组以及抗生素耐药等方面发挥重要作用。本文通过对比典型MMR途径和非典型MMR途径,深入阐述了分枝杆菌EndoMS/NucS介导的非典型MMR途径及其最新进展,以期为分枝杆菌错配修复分子机制带来新见解以及对分枝杆菌抗生素治疗提供研究线索。
向莎莎, 谢建平. 分枝杆菌非典型错配修复及其在抗生素耐药中的研究进展[J]. 遗传, 2023, 45(11): 1018-1027.
Shasha Xiang, Jianping Xie. Progress on the non-canonical mismatch repair in Mycobacterium and its role in antibiotic resistance[J]. Hereditas(Beijing), 2023, 45(11): 1018-1027.
图2
结核分枝杆菌的非典型MMR途径模型 1:复制过程中Dna E1聚合酶进行碱基选择,并通过其PHP结构域进行校对活动(3′-5′核酸外切酶)。2:NucS对产生的错配进行识别和修复。3:NucS与含有错配的ds DNA结合,通过与滑动夹相互作用激发其活性。4.:NucS在错配附近切割两条链,形成双链断裂。最后,DSB和错配可以通过HR途径或其他DSB修复机制进行修复。DnaE1聚合酶(α亚基,蓝色)、滑动夹(β亚基,粉色)和NucS二聚体(绿色)。Rv1547:DNA聚合酶III,一种多链酶,负责复制合成,该DNA聚合酶还具有3′-5′外切核酸酶活性。Rv1321:NucS,一种DNA修复内切核酸酶。"
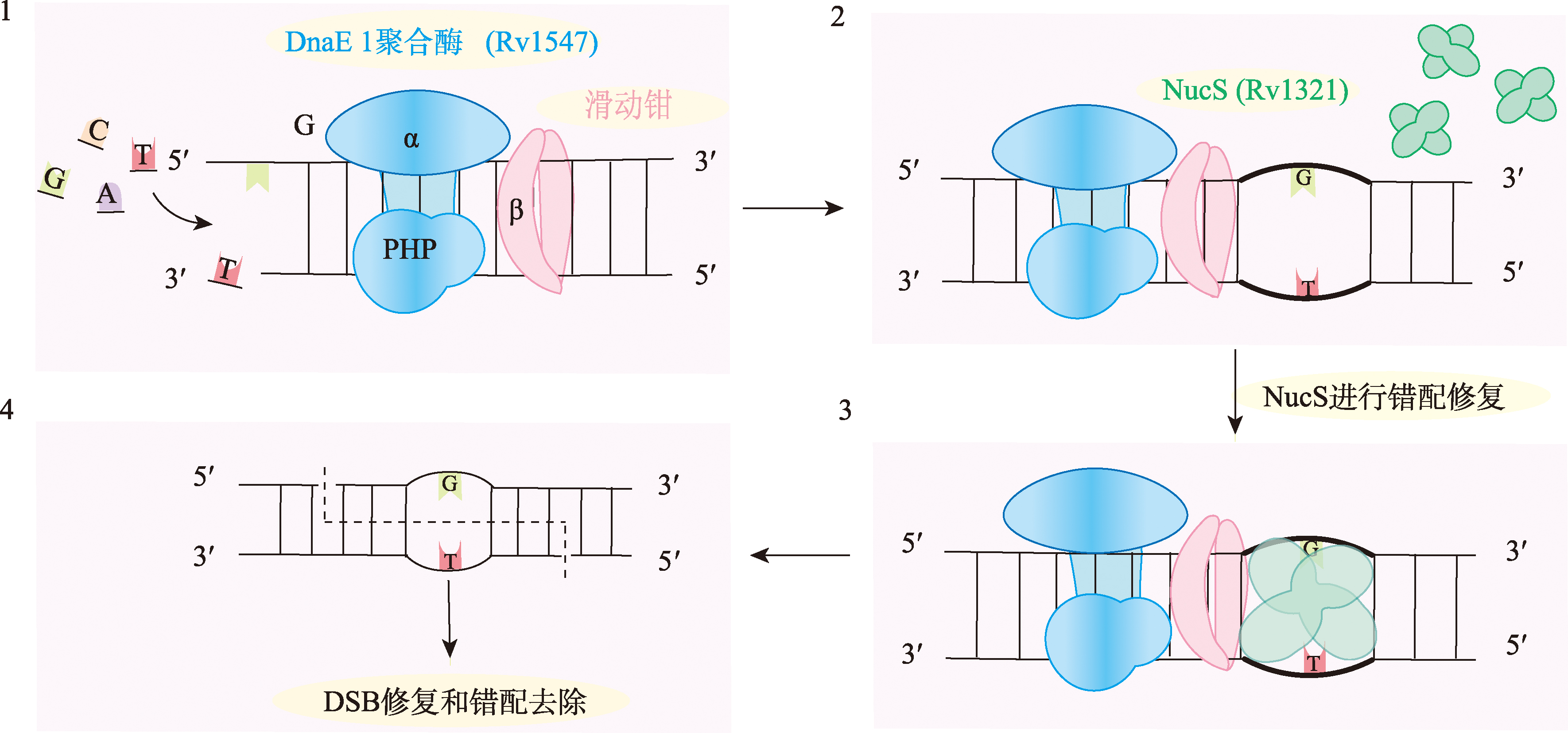
表1
典型错配修复和非典型错配修复的关键成分及功能总结"
| 修复类型 | 生物种类 | 关键成分 | 功能 |
|---|---|---|---|
| 细菌典型错配修复 | 大肠杆菌E. coli | MutS | 识别错配DNA |
| MutL | 下游介质 | ||
| MutH | 内切酶 | ||
| UvrD | 解旋酶 | ||
| Exo1/ExoX | 3′→5′核酸外切酶 | ||
| ExoVII/RecJ | 5′→3′核酸外切酶 | ||
| DNA聚合酶III | 聚合酶 | ||
| 甲基化酶Dam酶 | DNA甲基化酶 | ||
| SSB结合蛋白 | 稳定DNA的单链区域,增强DNA聚合酶的活性 | ||
| DNA连接酶 | 连接酶 | ||
| 真核生物典型错配修复 | 真核生物 | MutSα/MutSβ | 识别错配DNA |
| MutLα | 下游介质;核酸内切酶 | ||
| Exo1 | 外切酶 | ||
| DNA聚合酶δ | 聚合酶 | ||
| PCNA/RFC | 激活MutLα活性 | ||
| DNA连接酶 | 连接酶 | ||
| 非典型错配修复 | 分枝杆菌 | NucS | 识别错配碱基,核酸内切酶 |
| DnaE1聚合酶 | DNA聚合酶 |
| [1] |
Chatterjee N, Walker GC. Mechanisms of DNA damage, repair, and mutagenesis. Environ Mol Mutagen, 2017, 58(5): 235-263.
doi: 10.1002/em.22087 pmid: 28485537 |
| [2] |
Olave MC, Graham RP. Mismatch repair deficiency: the what, how and why it is important. Genes Chromosomes Cancer, 2022, 61(6): 314-321.
doi: 10.1002/gcc.v61.6 |
| [3] |
Lenhart JS, Pillon MC, Guarné A, Biteen JS, Simmons LA. Mismatch repair in Gram-positive bacteria. Res Microbiol, 2016, 167(1): 4-12.
doi: 10.1016/j.resmic.2015.08.006 pmid: 26343983 |
| [4] |
Dos Vultos T, Mestre O, Tonjum T, Gicquel B. DNA repair in Mycobacterium tuberculosis revisited. FEMS Microbiol Rev, 2009, 33(3): 471-487.
doi: 10.1111/j.1574-6976.2009.00170.x pmid: 19385996 |
| [5] |
Fishel R. Mismatch repair. J Biol Chem, 2015, 290(44): 26395-26403.
doi: 10.1074/jbc.R115.660142 pmid: 26354434 |
| [6] |
Hsieh P, Yamane K. DNA mismatch repair: molecular mechanism, cancer, and ageing. Mech Ageing Dev, 2008, 129(7-8): 391-407.
doi: 10.1016/j.mad.2008.02.012 pmid: 18406444 |
| [7] |
Putnam CD. Evolution of the methyl directed mismatch repair system in Escherichia coli. DNA Repair (Amst), 2016, 38: 32-41.
doi: 10.1016/j.dnarep.2015.11.016 |
| [8] |
Barras F, Marinus MG. The great GATC: DNA methylation in E. coli. Trends Genet, 1989, 5(5): 139-43.
pmid: 2667217 |
| [9] |
Hu CK, Zhao YQ, Sun HY, Yang YX.Synergism of Dam, MutH, and MutS in methylation-directed mismatch repair in Escherichia coli. Mutat Res, 2017, 795: 31-33.
doi: 10.1016/j.mrfmmm.2016.12.002 |
| [10] |
Groothuizen FS, Sixma TK. The conserved molecular machinery in DNA mismatch repair enzyme structures. DNA Repair (Amst), 2016, 38: 14-23.
doi: 10.1016/j.dnarep.2015.11.012 |
| [11] |
Junop MS, Yang W, Funchain P, Clendenin W, Miller JH. In vitro and in vivo studies of MutS, MutL and MutH mutants: correlation of mismatch repair and DNA recombination. DNA Repair (Amst), 2003, 2(4): 387-405.
doi: 10.1016/S1568-7864(02)00245-8 |
| [12] |
Jeong C, Cho WK, Song KM, Cook C, Yoon TY, Ban C, Fishel R, Lee JB. MutS switches between two fundamentally distinct clamps during mismatch repair. Nat Struct Mol Biol, 2011, 18(3): 379-385.
doi: 10.1038/nsmb.2009 pmid: 21278758 |
| [13] |
Au KG, Welsh K, Modrich P. Initiation of methyl-directed mismatch repair. J Biol Chem, 1992, 267(17): 12142-12148.
pmid: 1601880 |
| [14] |
Yamaguchi M, Dao V, Modrich P. MutS and MutL activate DNA helicase II in a mismatch-dependent manner. J Biol Chem, 1998, 273(15): 9197-9201.
doi: 10.1074/jbc.273.15.9197 pmid: 9535910 |
| [15] |
Grilley M, Griffith J, Modrich P. Bidirectional excision in methyl-directed mismatch repair. J Biol Chem, 1993, 268(16): 11830-11837.
pmid: 8505311 |
| [16] |
Modrich P, Lahue R. Mismatch repair in replication fidelity, genetic recombination, and cancer biology. Annu Rev Biochem, 1996, 65: 101-133.
pmid: 8811176 |
| [17] |
Hsieh P. Molecular mechanisms of DNA mismatch repair. Mutat Res, 2001, 486(2): 71-87.
doi: 10.1016/S0921-8777(01)00088-X |
| [18] |
Liu DK, Keijzers G, Rasmussen LJ. DNA mismatch repair and its many roles in eukaryotic cells. Mutat Res Rev Mutat Res, 2017, 773: 174-187.
doi: 10.1016/j.mrrev.2017.07.001 pmid: 28927527 |
| [19] |
Schroering AG, Edelbrock MA, Richards TJ, Williams KJ. The cell cycle and DNA mismatch repair. Exp Cell Res, 2007, 313(2): 292-304.
pmid: 17157834 |
| [20] |
Iyer RR, Pluciennik A, Burdett V, Modrich PL. DNA mismatch repair: functions and mechanisms. Chem Rev, 2006, 106(2): 302-323.
pmid: 16464007 |
| [21] |
McCulloch SD, Gu LY, Li GM. Bi-directional processing of DNA loops by mismatch repair-dependent and -independent pathways in human cells. J Biol Chem, 2003, 278(6): 3891-3896.
doi: 10.1074/jbc.M210687200 pmid: 12458199 |
| [22] |
Manhart CM, Alani E. Roles for mismatch repair family proteins in promoting meiotic crossing over. DNA Repair (Amst), 2016, 38: 84-93.
doi: 10.1016/j.dnarep.2015.11.024 |
| [23] |
Plotz G, Raedle J, Brieger A, Trojan J, Zeuzem S. hMutSalpha forms an ATP-dependent complex with hMutLalpha and hMutLbeta on DNA. Nucleic Acids Res, 2002, 30(3): 711-718.
pmid: 11809883 |
| [24] |
Kadyrov FA, Dzantiev L, Constantin N, Modrich P. Endonucleolytic function of MutLalpha in human mismatch repair. Cell, 2006, 126(2): 297-308.
pmid: 16873062 |
| [25] |
Tran PT, Erdeniz N, Symington LS, Liskay RM. EXO1-A multi-tasking eukaryotic nuclease. DNA Repair (Amst), 2004, 3(12): 1549-1559.
doi: 10.1016/j.dnarep.2004.05.015 |
| [26] |
Nielsen FC, Jäger AC, Lützen A, Bundgaard JR, Rasmussen LJ. Characterization of human exonuclease 1 in complex with mismatch repair proteins, subcellular localization and association with PCNA. Oncogene, 2004, 23(7): 1457-1468.
pmid: 14676842 |
| [27] |
Longley MJ, Pierce AJ, Modrich P. DNA polymerase delta is required for human mismatch repair in vitro. J Biol Chem, 1997, 272(16): 10917-10921.
doi: 10.1074/jbc.272.16.10917 pmid: 9099749 |
| [28] |
Ban C, Junop M, Yang W. Transformation of MutL by ATP binding and hydrolysis: a switch in DNA mismatch repair. Cell, 1999, 97(1): 85-97.
pmid: 10199405 |
| [29] |
Mizrahi V, Andersen SJ. DNA repair in Mycobacterium tuberculosis. What have we learnt from the genome sequence?. Mol Microbiol, 1998, 29(6): 1331-1339.
pmid: 9781872 |
| [30] |
Ren B, Kühn J, Meslet-Cladiere L, Briffotaux J, Norais C, Lavigne R, Flament D, Ladenstein R, Myllykallio H. Structure and function of a novel endonuclease acting on branched DNA substrates. EMBO J, 2009, 28(16): 2479-2489.
doi: 10.1038/emboj.2009.192 pmid: 19609302 |
| [31] |
Castañeda-García A, Prieto AI, Rodríguez-Beltrán J, Alonso N, Cantillon D, Costas C, Pérez-Lago L, Zegeye ED, Herranz M, Plociński P, Tonjum T, García de Viedma D, Paget M, Waddell SJ, Rojas AM, Doherty AJ, Blázquez J. A non-canonical mismatch repair pathway in prokaryotes. Nat Commun, 2017, 8: 14246.
doi: 10.1038/ncomms14246 pmid: 28128207 |
| [32] |
Meslet-Cladiére L, Norais C, Kuhn J, Briffotaux J, Sloostra JW, Ferrari E, Hübscher U, Flament D, Myllykallio H. A novel proteomic approach identifies new interaction partners for proliferating cell nuclear antigen. J Mol Biol, 2007, 372(5): 1137-1148.
pmid: 17720188 |
| [33] |
Ishino S, Nishi Y, Oda S, Uemori T, Sagara T, Takatsu N, Yamagami T, Shirai T, Ishino Y. Identification of a mismatch-specific endonuclease in hyperthermophilic Archaea. Nucleic Acids Res, 2016, 44(7): 2977-2986.
doi: 10.1093/nar/gkw153 pmid: 27001046 |
| [34] |
Cebrián-Sastre E, Martín-Blecua I, Gullón S, Blázquez J, Castañeda-García A. Control of genome stability by EndoMS/NucS-mediated non-canonical mismatch repair. Cells, 2021, 10(6): 1314.
doi: 10.3390/cells10061314 |
| [35] |
Nakae S, Hijikata A, Tsuji T, Yonezawa K, Kouyama KI, Mayanagi K, Ishino S, Ishino Y, Shirai T. Structure of the EndoMS-DNA complex as mismatch restriction endonuclease. Structure, 2016, 24(11): 1960-1971.
doi: S0969-2126(16)30294-5 pmid: 27773688 |
| [36] |
Pingoud A, Fuxreiter M, Pingoud V, Wende W. Type II restriction endonucleases: structure and mechanism. Cell Mol Life Sci, 2005, 62(6): 685-707.
pmid: 15770420 |
| [37] |
Takemoto N, Numata I, Su'etsugu M, Miyoshi-Akiyama T. Bacterial EndoMS/NucS acts as a clamp-mediated mismatch endonuclease to prevent asymmetric accumulation of replication errors. Nucleic Acids Res, 2018, 46(12): 6152-6165.
doi: 10.1093/nar/gky481 pmid: 29878158 |
| [38] |
Kunkel TA, Bebenek K. DNA replication fidelity. Annu Rev Biochem, 2000, 69: 497-529.
pmid: 10966467 |
| [39] | Xie ZH. The fidelity mechanism of DNA synthesis. Hereditas(Beijing), 2012, 34(6): 679-686. |
| 谢兆辉. DNA合成的忠实性机制. 遗传, 2012, 34(6): 679-686. | |
| [40] |
Rock JM, Lang UF, Chase MR, Ford CB, Gerrick ER, Gawande R, Coscolla M, Gagneux S, Fortune SM, Lamers MH. DNA replication fidelity in Mycobacterium tuberculosis is mediated by an ancestral prokaryotic proofreader. Nat Genet, 2015, 47(6): 677-681.
doi: 10.1038/ng.3269 pmid: 25894501 |
| [41] |
Grabowski B, Kelman Z. Archeal DNA replication: eukaryal proteins in a bacterial context. Annu Rev Microbiol, 2003, 57: 487-516.
pmid: 14527289 |
| [42] |
Creze C, Ligabue A, Laurent S, Lestini R, Laptenok SP, Khun J, Vos MH, Czjzek M, Myllykallio H, Flament D. Modulation of the Pyrococcus abyssi NucS endonuclease activity by replication clamp at functional and structural levels. J Biol Chem, 2012, 287(19): 15648-15660.
doi: 10.1074/jbc.M112.346361 pmid: 22431731 |
| [43] |
Ishino S, Skouloubris S, Kudo H l'Hermitte-Stead C, Es-Sadik A, Lambry JC, Ishino Y, Myllykallio H. Activation of the mismatch-specific endonuclease EndoMS/NucS by the replication clamp is required for high fidelity DNA replication. Nucleic Acids Res, 2018, 46(12): 6206-6217.
doi: 10.1093/nar/gky460 pmid: 29846672 |
| [44] |
Castañeda-García A, Martín-Blecua I, Cebrián-Sastre E, Chiner-Oms A, Torres-Puente M, Comas I, Blázquez J. Specificity and mutagenesis bias of the mycobacterial alternative mismatch repair analyzed by mutation accumulation studies. Sci Adv, 2020, 6(7): eaay4453.
doi: 10.1126/sciadv.aay4453 |
| [45] |
Garibyan L, Huang T, Kim M, Wolff E, Nguyen A, Nguyen T, Diep A, Hu K, Iverson A, Yang H, Miller JH. Use of the rpoB gene to determine the specificity of base substitution mutations on the Escherichia coli chromosome. DNA Repair (Amst), 2003, 2(5): 593-608.
doi: 10.1016/S1568-7864(03)00024-7 |
| [46] |
Schofield MJ, Hsieh P. DNA mismatch repair: molecular mechanisms and biological function. Annu Rev Microbiol, 2003, 57: 579-608.
pmid: 14527292 |
| [47] |
Tham KC, Kanaar R, Lebbink JHG. Mismatch repair and homeologous recombination. DNA Repair (Amst), 2016, 38: 75-83.
doi: 10.1016/j.dnarep.2015.11.010 |
| [48] |
Tham KC, Hermans N, Winterwerp HHK, Cox MM, Wyman C, Kanaar R, Lebbink JHG. Mismatch repair inhibits homeologous recombination via coordinated directional unwinding of trapped DNA structures. Mol Cell, 2013, 51(3): 326-337.
doi: 10.1016/j.molcel.2013.07.008 |
| [49] |
Worth L Jr, Clark S, Radman M, Modrich P. Mismatch repair proteins MutS and MutL inhibit RecA-catalyzed strand transfer between diverged DNAs. Proc Natl Acad Sci USA, 1994, 91(8): 3238-3241.
pmid: 8159731 |
| [50] |
Spies M, Fishel R. Mismatch repair during homologous and homeologous recombination. Cold Spring Harb Perspect Biol, 2015, 7(3): a022657.
doi: 10.1101/cshperspect.a022657 |
| [51] |
Matic I, Radman M, Taddei F, Picard B, Doit C, Bingen E, Denamur E, Elion J. Highly variable mutation rates in commensal and pathogenic Escherichia coli. Science, 1997, 277(5333): 1833-1834.
doi: 10.1126/science.277.5333.1833 pmid: 9324769 |
| [52] |
Taddei F, Radman M, Maynard-Smith J, Toupance B, Gouyon PH, Godelle B. Role of mutator alleles in adaptive evolution. Nature, 1997, 387(6634): 700-702.
doi: 10.1038/42696 |
| [53] |
Gutierrez A, Laureti L, Crussard S, Abida H, Rodríguez- Rojas A, Blázquez J, Baharoglu Z, Mazel D, Darfeuille F, Vogel J, Matic I. β-Lactam antibiotics promote bacterial mutagenesis via an RpoS-mediated reduction in replication fidelity. Nat Commun, 2013, 4: 1610.
doi: 10.1038/ncomms2607 pmid: 23511474 |
| [54] |
Oliver A, Mena A. Bacterial hypermutation in cystic fibrosis, not only for antibiotic resistance. Clin Microbiol Infect, 2010, 16(7): 798-808.
doi: 10.1111/j.1469-0691.2010.03250.x |
| [55] |
Gagneux S. Ecology and evolution of Mycobacterium tuberculosis. Nat Rev Microbiol, 2018, 16(4): 202-213.
doi: 10.1038/nrmicro.2018.8 pmid: 29456241 |
| [56] |
Ford CB, Shah RR, Maeda MK, Gagneux S, Murray MB, Cohen T, Johnston JC, Gardy J, Lipsitch M, Fortune SM. Mycobacterium tuberculosis mutation rate estimates from different lineages predict substantial differences in the emergence of drug-resistant tuberculosis. Nat Genet, 2013, 45(7): 784-790.
doi: 10.1038/ng.2656 pmid: 23749189 |
| [57] |
Fressatti Cardoso R, Martín-Blecua I, Pietrowski Baldin V, Meneguello JE, Valverde JR, Blázquez J, Castañeda-García A. Noncanonical mismatch repair protein NucS modulates the emergence of antibiotic resistance in Mycobacterium abscessus. Microbiol Spectr, 2022, 10(6): e0222822.
doi: 10.1128/spectrum.02228-22 |
| [58] |
Daley CL, Iaccarino JM, Lange C, Cambau E, Wallace RJ Jr, Andrejak C, Böttger EC, Brozek J, Griffith DE, Guglielmetti L, Huitt GA, Knight SL, Leitman P, Marras TK, Olivier KN, Santin M, Stout JE, Tortoli E, van Ingen J, Wagner D, Winthrop KL. Treatment of nontuberculous mycobacterial pulmonary disease: an official ATS/ERS/ESCMID/IDSA clinical practice guideline. Eur Respir J, 2020, 56(1): 2000535.
doi: 10.1183/13993003.00535-2020 |
| [59] |
Boshoff HI, Myers TG, Copp BR, McNeil MR, Wilson MA, Barry CE 3rd. The transcriptional responses of Mycobacterium tuberculosis to inhibitors of metabolism: novel insights into drug mechanisms of action. J Biol Chem, 2004, 279(38): 40174-40184.
doi: 10.1074/jbc.M406796200 pmid: 15247240 |
| [1] | 张沥元, 黄芙静, 许峻旗, 龚真, 谢建平. 结核分枝杆菌酸抗性基因及其调控网络[J]. 遗传, 2018, 40(7): 546-560. |
| [2] | 刘洋, 王邦兴, 刘志永, 韩轶, 谭耀驹, 李昕洁, 刘健雄, 谭守勇, 张天宇. 非一线抗结核药物耐药机制及耐药性诊断研究进展[J]. 遗传, 2016, 38(10): 928-939. |
| [3] | 张玉娇, 李晓静, 米凯霞. 结核分枝杆菌耐氟喹诺酮类药物的分子机制研究进展[J]. 遗传, 2016, 38(10): 918-927. |
| [4] | 王婷, 焦伟伟, 申阿东. 结核分枝杆菌乙胺丁醇耐药机制的研究进展[J]. 遗传, 2016, 38(10): 910-917. |
| [5] | 樊祥宇, 何颖, 谢建平. 以分枝杆菌噬菌体为例探索生命科学研究型教学[J]. 遗传, 2014, 36(8): 842-846. |
| [6] | 杜庆林,樊祥宇,毛金校,谢建平. 分枝杆菌中基因敲除操作工具研究进展[J]. 遗传, 2012, 34(7): 857-862. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||

www.chinagene.cn
备案号:京ICP备09063187号-4
总访问:,今日访问:,当前在线:
