遗传 ›› 2020, Vol. 42 ›› Issue (1): 100-111.doi: 10.16288/j.yczz.19-258
王昕源1,2, 张雨1,2, 杨楠1, 程禾1,2( ), 孙玉洁1,2(
), 孙玉洁1,2( )
)
收稿日期:2019-10-29
修回日期:2019-12-27
出版日期:2020-01-20
发布日期:2020-01-07
通讯作者:
程禾,孙玉洁
E-mail:chenghe@njmu.edu.cn;yujiesun@njmu.edu.cn
作者简介:王昕源,硕士,专业方向:LINE-1的异常激活机制。E-mail: wangxinyuan2727@gmail.com
基金资助:
Xinyuan Wang1,2, Yu Zhang1,2, Nan Yang1, He Cheng1,2( ), Yujie Sun1,2(
), Yujie Sun1,2( )
)
Received:2019-10-29
Revised:2019-12-27
Online:2020-01-20
Published:2020-01-07
Contact:
Cheng He,Sun Yujie
E-mail:chenghe@njmu.edu.cn;yujiesun@njmu.edu.cn
Supported by:摘要:
药物诱导的长散在重复序列LINE-1异常激活可促进细胞基因组不稳定,而基因组不稳定是促进肿瘤发生发展和耐药表型形成的重要因素。因此,探索LINE-1异常激活的分子机制具有重要的理论和临床意义。DNA甲基化是调控基因表达的重要方式,已知DNA甲基转移酶家族成员DNMT3a不仅能通过促进基因启动子甲基化抑制基因表达,还可通过增强基因内部甲基化上调基因表达。本实验室前期研究发现,将乳腺癌细胞暴露于化疗药物可诱导LINE-1异常高表达,但LINE-1启动子甲基化水平并无显著改变。本研究进一步探讨了在化疗药物压力下DNMT3a是否可通过增强LINE-1基因内部甲基化水平促进LINE-1在乳腺癌细胞中的异常高表达。ChIP实验和甲基分析结果显示,用化疗药物紫杉醇(PTX)处理乳腺癌细胞,不仅可以诱导DNMT3a表达,而且可以促进DNMT3a与LINE-1基因内部区域的结合,提升其基因内部甲基化水平, 进而上调LINE-1的表达水平。利用表达载体增加细胞内DNMT3a的表达水平,可显著上调LINE-1基因内部的甲基化及基因的表达水平,而下调DNMT3a的表达可有效抑制LINE-1表达。上述研究结果表明,DNMT3a介导的基因非启动子区甲基化在药物诱导的LINE-1异常激活中发挥重要作用,为认识LINE-1在乳腺癌化疗耐药性形成过程中异常激活的机制提供了新思路。
王昕源, 张雨, 杨楠, 程禾, 孙玉洁. DNMT3a通过提升基因内部甲基化介导紫杉醇诱导的LINE-1异常表达[J]. 遗传, 2020, 42(1): 100-111.
Xinyuan Wang, Yu Zhang, Nan Yang, He Cheng, Yujie Sun. DNMT3a mediates paclitaxel-induced abnormal expression of LINE-1 by increasing the intragenic methylation[J]. Hereditas(Beijing), 2020, 42(1): 100-111.
表2
甲基化特异性PCR所用引物"
| 引物名称 | 引物序列(5′→3′) |
|---|---|
| LINE1- Promoter-MSP | F: GTCGAATAGGAATAGTTTCGG |
| R: ACTCCCTAACCCCTTACGCT | |
| LINE1-GB1- MSP | F: GTTTAGATTTAGGAAATATAGAGAAC |
| R: CTAACTTATAAAATTTCTACCGAA | |
| LINE1-GB2- MSP | F: GTTGGATGGAGAATGATTTTGAC |
| R: TTAATCACATCGACTCCTAAAAC | |
| Actin-MSP | F: TGGTGATGGAGGAGGTTTAGTAAG |
| R: AACCAATAAAACCTACTCCTCCCTTAA |
表3
染色质免疫共沉淀所用引物"
| 引物名称 | 引物序列(5′→3′) |
|---|---|
| LINE-1-SP | F: TCACTAGGGAGTGCCAGACAG |
| R: ATTTTCCAGGTGCGACCGTCA | |
| LINE-1-SGB1 | F: GTAGATAAAACCACAAAGATG |
| R: TTGACGAGCTGAGAGAAGAAG | |
| LINE-1-SGB2 | F: GGAACGCAGTTCCTCACCAGC |
| R: ATGTATAACTAGAATAACCAA | |
| LINE-1-SGB3 | F: GGCAAAGAAGTTGAAAACTTTG |
| R: TCAGCTCCATCAGCTCCTTTA | |
| LINE-1-SGB4 | F: AAGGAGCTGATGGAGCTGAAA |
| R: CTAAACTTCCCTTCTCGCTTCA | |
| LINE-1-SGB5 | F: CCGATGCGATCAACTGGAAGA |
| R: TAAACTTCCCTTCTCGCTTCA | |
| LINE-1-SGB6 | F: CCAAGAAATATGGGACTATGT |
| R: TAGATTGGGGAAGTTCTCCTG | |
| LINE-1-SGB7 | F: CAGGAGAACTTCCCCAATCTA |
| R: CTGGCTGCCCTTAACATTT | |
| LINE-1-SGB8 | F: CAGATTCACCAAAGTTGAAATG |
| R: CCACTCTCTTCTGGCTTGTAG |

图1
PTX处理上调乳腺癌细胞中DNMT3a蛋白和LINE-1 mRNA表达并提升LINE-1基因内部而非启动子区的甲基化水平 A:Real-time PCR检测PTX处理后MDA-MB-231乳腺癌细胞中LINE-1的mRNA水平;B:MSP检测PTX处理后MDA-MB-231细胞中LINE-1启动子甲基化水平;C:利用甲基化特异性PCR扩增的LINE-1基因内部相应片段(S1和S2)位置示意图,片段位于LINE-1 Orf-1开放阅读框架中长度约500 bp富含CpG的区域;D:MSP检测PTX处理后MDA-MB-231细胞中LINE-1基因内部甲基化水平;E: Western blot检测PTX处理后MDA-MB-231细胞中DNMT3a蛋白的表达及相应的灰度分析结果。*: P<0.05表示有差异; **: P<0.01表示差异显著。"

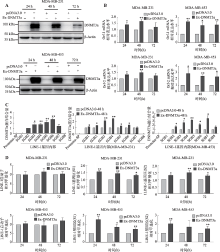
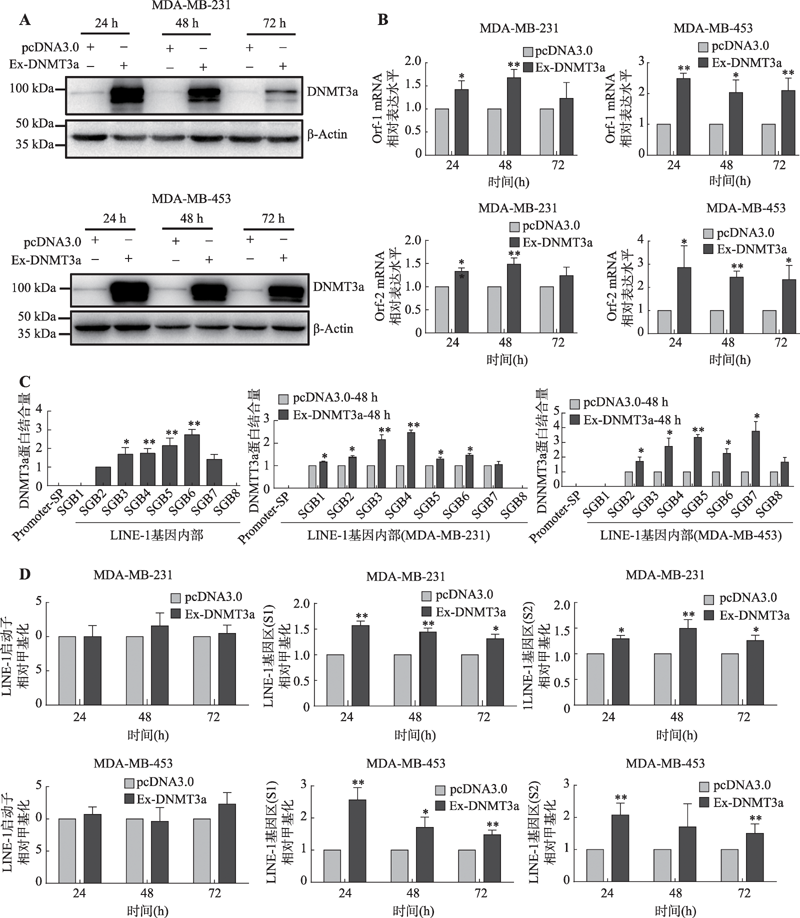
图5
DNMT3a在乳腺癌细胞系中通过提升LINE-1基因内部甲基化水平从而正向调控LINE-1的转录 A:Western blot检测MDA-MB-231和MDA-MB-453乳腺癌细胞中转染DNMT3a过表达质粒后DNMT3a蛋白的表达情况;B:Real-time PCR检测在MDA-MB-231和MDA-MB-453乳腺癌细胞中过表达DNMT3a蛋白后LINE-1 mRNA的表达水平;C:ChIP检测过表达DNMT3a对其与LINE-1基因启动子区和基因内部区域结合情况的影响;D:MSP检测过表达DNMT3a对LINE-1启动子和基因内部甲基化水平的影响。*:P<0.05表示有差异;**:P<0.01表示差异显著。"
| [1] |
Wei R, Lau SSS, Cheung PSY . Breast carcinoma in chinese women: Does age affect treatment choice and outcome? Asian J Surg, 2010,33(2):97-102.
doi: 10.1016/S1015-9584(10)60017-6 |
| [2] |
Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM . Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer, 2010,127(12):2893-2917.
doi: 10.1002/ijc.25516 pmid: 21351269 |
| [3] |
Clarke R, Tyson JJ, Dixon JM. Endocrine resistance in breast cancer--An overview and update. Mol Cell Endocrinol, 2015, 418 Pt 3: 220-234.
doi: 10.1016/j.mce.2015.09.035 pmid: 26455641 |
| [4] |
Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA . Cloning of a novel receptor expressed in rat prostate and ovary. Proc Natl Acad Sci USA, 1996,93(12):5925-5930.
doi: 10.1073/pnas.93.12.5925 pmid: 8650195 |
| [5] |
Rivera E . Management of metastatic breast cancer: monotherapy options for patients resistant to anthracyclines and taxanes. Am J Clin Oncol, 2010,33(2):176-185.
doi: 10.1097/COC.0b013e3181931049 pmid: 19675449 |
| [6] |
Bao WD, Kojima KK, Kohany O . Repbase update, a database of repetitive elements in eukaryotic genomes. Mob DNA, 2015,6:11.
doi: 10.1186/s13100-015-0041-9 pmid: 26045719 |
| [7] |
Belancio VP, Hedges DJ, Deininger P . Mammalian non-LTR retrotransposons: for better or worse, in sickness and in health. Genome Res, 2008,18(3):343-358.
doi: 10.1101/gr.5558208 pmid: 18256243 |
| [8] |
Beck CR, Garcia-Perez JL, Badge RM, Moran JV . LINE-1 elements in structural variation and disease. Annu Rev Genomics Hum Genet, 2011,12:187-215.
doi: 10.1146/annurev-genom-082509-141802 pmid: 21801021 |
| [9] | Liu Q, Wang JH, Li XY, Cen S,. The connection between LINE-1 retrotransposition and human tumorigenesis. Hereditas(Beijing), 2016,38(2):93-102. |
| 刘茜, 王瑾晖, 李晓宇, 岑山 . 逆转录转座子LINE-1与肿瘤的发生和发展. 遗传, 2016,38(2):93-102. | |
| [10] |
Belgnaoui SM, Gosden RG, Semmes OJ, Haoudi A . Human LINE-1 retrotransposon induces DNA damage and apoptosis in cancer cells. Cancer Cell Int, 2006,6:13.
doi: 10.1186/1475-2867-6-13 pmid: 16670018 |
| [11] |
Feng Q, Moran JV, Kazazian HH Jr, Boeke JD . Human L1 retrotransposon encodes a conserved endonuclease required for retrotransposition. Cell, 1996,87(5):905-916.
doi: 10.1016/s0092-8674(00)81997-2 pmid: 8945517 |
| [12] |
Martin SL, Li J, Epperson LE, Lieberman B . Functional reverse transcriptases encoded by A-type mouse LINE-1: defining the minimal domain by deletion analysis. Gene, 1998,215(1):69-75.
doi: 10.1016/s0378-1119(98)00252-2 pmid: 9666081 |
| [13] |
Farkash EA, Kao GD, Horman SR, Prak ET . Gamma radiation increases endonuclease-dependent L1 retrotransposition in a cultured cell assay. Nucleic Acids Res, 2006,34(4):1196-1204.
doi: 10.1093/nar/gkj522 pmid: 16507671 |
| [14] |
Gasior SL, Wakeman TP, Xu B, Deininger PL . The human LINE-1 retrotransposon creates DNA double-strand breaks. . Mol Biol, 2006,357(5):1383-1393.
doi: 10.1016/j.jmb.2006.01.089 pmid: 16490214 |
| [15] |
Garcia-Perez JL, Doucet AJ, Bucheton A, Moran JV, Gilbert N . Distinct mechanisms for trans-mediated mobilization of cellular RNAs by the LINE-1 reverse transcriptase. Genome Res, 2007,17(5):602-611.
doi: 10.1101/gr.5870107 pmid: 17416749 |
| [16] |
Wei W, Gilbert N, Ooi SL, Lawler JF, Ostertag EM, Kazazian HH, Boeke JD, Moran JV . Human L1 retrotransposition: cis preference versus trans complementation. Mol Cell Biol, 2001,21(4):1429-1439.
doi: 10.1128/MCB.21.4.1429-1439.2001 pmid: 11158327 |
| [17] |
Kines KJ, Sokolowski M, deHaro DL, Christian CM, Baddoo M, Smither ME, Belancio VP,. The endonuclease domain of the LINE-1 ORF2 protein can tolerate multiple mutations. Mob DNA, 2016,7:8.
doi: 10.1186/s13100-016-0064-x pmid: 27099633 |
| [18] |
Okano M, Xie S, Li E . Cloning and characterization of a family of novel mammalian DNA (cytosine-5) methyltransferases. Nat Genet, 1998,19(3):219-220.
doi: 10.1038/890 pmid: 9662389 |
| [19] |
Brusa G, Benvenuti M, Mazzacurati L, Mancini M, Pattacini L, Martinelli G, Barbieri E, Greenberger JS, Baccarani M, Santucci MA. p53 loss of function enhances genomic instability and accelerates clonal evolution of murine myeloid progenitors expressing the p(210)BCR-ABL tyrosine kinase. Haematologica, 2003,88(6):622-630.
pmid: 12801837 |
| [20] |
Greaves M, Maley CC . Clonal evolution in cancer. Nature, 2012,481(7381):306-313.
doi: 10.1038/nature10762 |
| [21] |
Ng CK, Pemberton HN, Reis-Filho JS . Breast cancer intratumor genetic heterogeneity: causes and implications. Expert Rev Anticancer Ther, 2012,12(8):1021-1032.
doi: 10.1586/era.12.85 pmid: 23030222 |
| [22] |
Chen L, Dahlstrom JE, Chandra A, Board P, Rangasamy D . Prognostic value of LINE-1 retrotransposon expression and its subcellular localization in breast cancer. Breast Cancer Res Treat, 2012,136(1):129-142.
doi: 10.1007/s10549-012-2246-7 pmid: 23053642 |
| [23] |
Park SY, Seo AN, Jung HY, Gwak JM, Jung N, Cho NY, Kang GH . Alu and LINE-1 hypomethylation is associated with HER2 enriched subtype of breast cancer. PLoS One, 2014,9(6):e100429.
doi: 10.1371/journal.pone.0100429 pmid: 24971511 |
| [24] |
Feng F, Lu YY, Zhang F, Gao XD, Zhang CF, Meredith A, Xu ZX, Yang YT, Chang XJ, Wang H, Qu JH, Zeng Z, Yang JL, Wang CP, Zhu YF, Cui JJ, Yang YP . Long interspersed nuclear element ORF-1 protein promotes proliferation and resistance to chemotherapy in heaptocellular carcinoma. World J Gastroenterol, 2013,19(7):1068-1078.
doi: 10.3748/wjg.v19.i7.1068 pmid: 23466962 |
| [25] |
Chen Y, Zeng Q, Liu X, Fu J, Zeng Z, Zhao Z, Liu Z, Bai W, Dong Z, Liu H, Lu X, Zhu Y, Lu Y . LINE-1 ORF-1p enhances the transcription factor activity of pregnenolone X receptor and promotes sorafenib resistance in hepatocellular carcinoma cells. Cancer Manag Res, 2018,10:4421-4438.
doi: 10.2147/CMAR.S176088 pmid: 30349375 |
| [26] |
Suzuki MM, Bird A . DNA methylation landscapes: provocative insights from epigenomics. Nat Rev Genet, 2008,9(6):465-476.
doi: 10.1038/nrg2341 pmid: 18463664 |
| [27] |
Jin B, Robertson KD . DNA methyltransferases, DNA damage repair, and cancer. Adv Exp Med Biol, 2013,754:3-29.
doi: 10.1007/978-1-4419-9967-2_1 pmid: 22956494 |
| [28] |
Yang L, Rau R, Goodell MA . DNMT3A in haematological malignancies. Nat Rev Cancer, 2015,15(3):152-165.
doi: 10.1038/nrc3895 pmid: 25693834 |
| [29] |
Jeong M, Sun D, Luo M, Huang Y, Challen GA, Rodriguez B, Zhang X, Chavez L, Wang H, Hannah R, Kim SB, Yang L, Ko M, Chen R, Göttgens B, Lee JS, Gunaratne P, Godley LA, Darlington GJ, Rao A, Li W, Goodell MA . Large conserved domains of low DNA methylation maintained by Dnmt3a. Nat Genet, 2014,46(1):17-23.
doi: 10.1038/ng.2836 pmid: 24270360 |
| [30] |
Wu H, Coskun V, Tao J, Xie W, Ge W, Yoshikawa K, Li E, Zhang Y, Sun YE . Dnmt3a-dependent nonpromoter DNA methylation facilitates transcription of neurogenic genes. Science, 2010,329(5990):444-448.
doi: 10.1126/science.1190485 pmid: 20651149 |
| [31] |
Yang X, Han H, De Carvalho DD, Lay FD, Jones PA, Liang G . Gene body methylation can alter gene expression and is a therapeutic target in cancer. Cancer Cell, 2014,26(4):577-590.
doi: 10.1016/j.ccr.2014.07.028 |
| [32] |
Yoder JA, Walsh CP, Bestor TH . Cytosine methylation and the ecology of intragenomic parasites. Trends Genet, 1997,13(8):335-340.
doi: 10.1016/s0168-9525(97)01181-5 pmid: 9260521 |
| [33] |
Ray Chaudhuri A, Callen E, Ding X, Gogola E, Duarte AA, Lee JE, Wong N, Lafarga V, Calvo JA, Panzarino NJ, John S, Day A, Crespo AV, Shen B, Starnes LM, de Ruiter JR, Daniel JA, Konstantinopoulos PA, Cortez D, Cantor SB, Fernandez-Capetillo O, Ge K, Jonkers J, Rottenberg S, Sharan SK, Nussenzweig A, . Replication fork stability confers chemoresistance in BRCA-deficient cells. Nature, 2016,535(7612):382-387.
doi: 10.1038/nature18325 pmid: 27443740 |
| [34] |
Du M, Su XM, Zhang T, Xing YJ . Aberrant promoter DNA methylation inhibits bone morphogenetic protein 2 expression and contributes to drug resistance in breast cancer. Mol Med Rep, 2014,10(2):1051-1055.
doi: 10.3892/mmr.2014.2276 |
| [35] |
Kulis M, Heath S, Bibikova M, Queirós AC, Navarro A, Clot G, Martínez-Trillos A, Castellano G, Brun-Heath I, Pinyol M, Barberán-Soler S, Papasaikas P, Jares P, Beà S, Rico D, Ecker S, Rubio M, Royo R, Ho V, Klotzle B, Hernández L, Conde L, López-Guerra M, Colomer D, Villamor N, Aymerich M, Rozman M, Bayes M, Gut M, Gelpí JL, Orozco M, Fan JB, Quesada V, Puente XS, Pisano DG, Valencia A, López-Guillermo A, Gut I, López-Otín C, Campo E, Martín-Subero JI . Epigenomic analysis detects widespread gene-body DNA hypomethylation in chronic lymphocytic leukemia. Nat Genet, 2012,44(11):1236-1242.
doi: 10.1038/ng.2443 pmid: 23064414 |
| [36] |
Maunakea AK, Nagarajan RP, Bilenky M, Ballinger TJ, D'Souza C, Fouse SD, Johnson BE, Hong C, Nielsen C, Zhao Y, Turecki G, Delaney A, Varhol R, Thiessen N, Shchors K, Heine VM, Rowitch DH, Xing X, Fiore C, Schillebeeckx M, Jones SJ, Haussler D, Marra MA, Hirst M, Wang T, Costello JF,. Conserved role of intragenic DNA methylation in regulating alternative promoters. Nature, 2010,466(7303):253-257.
doi: 10.1038/nature09165 pmid: 20613842 |
| [37] |
Varley KE, Gertz J, Bowling KM, Parker SL, Reddy TE, Pauli-Behn F, Cross MK, Williams BA, Stamatoyannopoulos JA, Crawford GE, Absher DM, Wold BJ, Myers RM . Dynamic DNA methylation across diverse human cell lines and tissues. Genome Res, 2013,23(3):555-567.
doi: 10.1101/gr.147942.112 |
| [38] |
Sridhar VV, Kapoor A, Zhang K, Zhu J, Zhou T, Hasegawa PM, Bressan RA, Zhu JK . Control of DNA methylation and heterochromatic silencing by histone H2B deubiquitination. Nature. 2007,447(7145):735-738.
doi: 10.1038/nature05864 pmid: 17554311 |
| [39] |
Schuettengruber B, Cavalli G . Recruitment of polycomb group complexes and their role in the dynamic regulation of cell fate choice. Development, 2009,136(21):3531-3542.
doi: 10.1242/dev.033902 pmid: 19820181 |
| [40] |
Hawkins RD, Hon GC, Lee LK, Ngo Q, Lister R, Pelizzola M, Edsall LE, Kuan S, Luu Y, Klugman S, Antosiewicz-Bourget J, Ye Z, Espinoza C, Agarwahl S, Shen L, Ruotti V, Wang W, Stewart R, Thomson JA, Ecker JR, Ren B . Distinct epigenomic landscapes of pluripotent and lineage-committed human cells. Cell Stem Cell, 2010,6(5):479-491.
doi: 10.1016/j.stem.2010.03.018 pmid: 20452322 |
| [41] |
Wagner EJ, Carpenter PB . Understanding the language of Lys36 methylation at histone H3. Nat Rev Mol Cell Biol, 2012,13(2):115-126.
doi: 10.1038/nrm3274 pmid: 22266761 |
| [42] |
Venkatesh S, Smolle M, Li H, Gogol MM, Saint M, Kumar S, Natarajan K, Workman JL . Set2 methylation of histone H3 lysine 36 suppresses histone exchange on transcribed genes. Nature, 2012,489(7416):452-455.
doi: 10.1038/nature11326 |
| [43] |
Chen BF, Chan WY . The de novo DNA methyltransferase DNMT3A in development and cancer. Epigenetics, 2014,9(5):669-677.
doi: 10.4161/epi.28324 |
| [44] |
Deng DJ . DNA methylation and demethylation: current status and future per-spective. Hereditas(Beijing), 2014,36(5):403-410.
doi: 10.3724/SP.J.1005.2014.0403 |
|
邓大君 . DNA甲基化和去甲基化的研究现状及思考. 遗传. 2014,36(5):403-410.
doi: 10.3724/SP.J.1005.2014.0403 |
| [1] | 马春辉, 胡海旭, 张丽娟, 刘毅, 刘天懿. 用于循环肿瘤细胞定量分析的CK19数字PCR检测方法的建立及性能验证[J]. 遗传, 2023, 45(3): 250-260. |
| [2] | 常栋, 刘享享, 刘睿, 孙建伟. FSCN1在乳腺癌发生发展中的作用及其调控机制[J]. 遗传, 2023, 45(2): 115-127. |
| [3] | 张强, 顾明亮. 单细胞测序技术及其在乳腺癌研究中的应用[J]. 遗传, 2020, 42(3): 250-268. |
| [4] | 禹奇超,宋彬,邹轩轩,王岭,刘德权,李波,马昆. 乳腺癌癌旁组织特异性表达基因分析[J]. 遗传, 2019, 41(7): 625-633. |
| [5] | 余同露,蔡栋梁,朱根凤,叶晓娟,闵太善,陈红岩,卢大儒,陈浩明. CSN4基因干扰对乳腺癌MDA-MB-231细胞增殖和凋亡的影响[J]. 遗传, 2019, 41(4): 318-326. |
| [6] | 姚传波, 周鑫, 陈策实, 雷群英. Hippo信号通路在乳腺癌中的调控机制及作用[J]. 遗传, 2017, 39(7): 617-629. |
| [7] | 李泰明, 蓝文俊, 黄灿, 张春, 刘晓玫. 近红外荧光蛋白标记乳腺癌细胞外泌体的构建及鉴定[J]. 遗传, 2016, 38(5): 427-435. |
| [8] | 吴新刚,彭姝彬,黄谦. 乳腺癌耐药蛋白基因的转录调控机制[J]. 遗传, 2012, 34(12): 1529-1536. |
| [9] | 程龙,黄翠芬,叶棋浓. 乳腺癌中雌激素受体α表达水平调节的分子机制[J]. 遗传, 2010, 32(3): 191-197. |
| [10] | 王靖,李彦辉,郭政,朱晶,马文财,彭春方,刘庆. 根据蛋白质互作网络预测乳腺癌相关蛋白质的细致功能[J]. 遗传, 2007, 29(9): 1061-1066. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||

www.chinagene.cn
备案号:京ICP备09063187号-4
总访问:,今日访问:,当前在线:
