Hereditas(Beijing) ›› 2020, Vol. 42 ›› Issue (4): 374-379.doi: 10.16288/j.yczz.19-271
• Research Article • Previous Articles Next Articles
Study on hereditary susceptibility genetic markers to anti-tuberculosis drug induced liver injury in Chinese population
Chenxi Zhou, Mo Li, Cong Huai, Lin He, Shengying Qin( )
)
- Bio-X Institute, Shanghai Jiaotong University, Shanghai 200030, China
-
Received:2019-11-24Revised:2019-12-26Online:2020-04-20Published:2020-02-26 -
Contact:Qin Shengying E-mail:chinsir@sjtu.edu.cn -
Supported by:Supported by the National Natural Science Foundation of China Nos(81773818);Supported by the National Natural Science Foundation of China Nos(81273596);Supported by the National Natural Science Foundation of China Nos(30900799);Supported by the National Natural Science Foundation of China Nos(81671326);Shanghai Pujiang Program No(17PJD020)
Cite this article
Chenxi Zhou, Mo Li, Cong Huai, Lin He, Shengying Qin. Study on hereditary susceptibility genetic markers to anti-tuberculosis drug induced liver injury in Chinese population[J]. Hereditas(Beijing), 2020, 42(4): 374-379.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
Table 1
109 candidate genes of anti-turberculosis drug induced liver injury"
| 基因类型 | 基因家族 | 目标捕获基因 |
|---|---|---|
| 一相药物 代谢基因 | CYP家族基因 | CYP1A1、CYP1A2、CYP1B1、CYP2A13、CYP2A6、CYP2A7、CYP2B6、CYP2C18、CYP2C19 CYP2C8、CYP2C9、CYP2D6、CYP2E1、CYP2F1、CYP2J2、CYP2R1、CYP2S1、CYP2U1、 CYP2W1、CYP3A4、CYP3A43、CYP3A5、CYP3A、CYP4V2、CYP4X1和CYP4Z1 |
| 超氧化物歧化酶 | SOD1、SOD2和SOD3 | |
| 含黄素单加氧化酶 | FMO1、FMO2、FMO3、FMO4和FMO5 | |
| 其他 | TPMT | |
| 二相药物 代谢基因 | UGT家族 | UGT1A1、UGT1A3、UGT1A4、UGT1A6、UGT1A9、UGT2B15和UGT2B7 |
| GST家族 | GSTM1和GSTT1 | |
| NAT家族 | NAT1和NAT2 | |
| 三相药物 转运蛋白 | ATP结合盒式 转运蛋白 | ABCB1、ABCB11、ABCB4、ABCC1、ABCC10、ABCC1、ABCC12、ABCC2、ABCC3、ABCC4、ABCC5和ABCC6 |
| 其他转运蛋白 | SLCO1B1、POU2F1和POU5F1 | |
| 调控受体 | 调控受体 | AHR、ARNT、ESR1、ESR2、FOXA1、FOXA2、FOXA3、HNF1A、HNF1B、HNF4A、HNF4G、NR1H2、NR1H3、NR1H4、NR1I、NR1I3、PPARA、PPARD、PPARG、RARA、RARB、RARG和VDR |
| 固有免疫 | 白细胞介素 | IL10、IL12A、IL12B、IL13、IL18、IL1A、IL1B、IL2、IL4、IL5、IL6、IL7和IL9 |
| 其他细胞激素 | CCL2、IFNG和TNF | |
| 适应性免疫 | 人类白细胞抗原 | HLA-A、HLA-B、HLA-C、HLA-DPA1、HLA-DPB1、HLA-DQA1、HLA-DQB1、HLA-DRA和HLA-DRB1 |
Table 2
Significant SNP/SNVs associated with anti-tuberculosis drug induced liver injury"
| 基因 | 编号 | 频数 REF/ALT | 比值比(OR) | 95%置信区间(95%CI) | P值 | |
|---|---|---|---|---|---|---|
| 结核病人 | 健康人 | |||||
| UGT1A4 | rs2011404 | 6/74 | 23/55 | 0.1939 | 0.0740~0.5083 | 0.0004 |
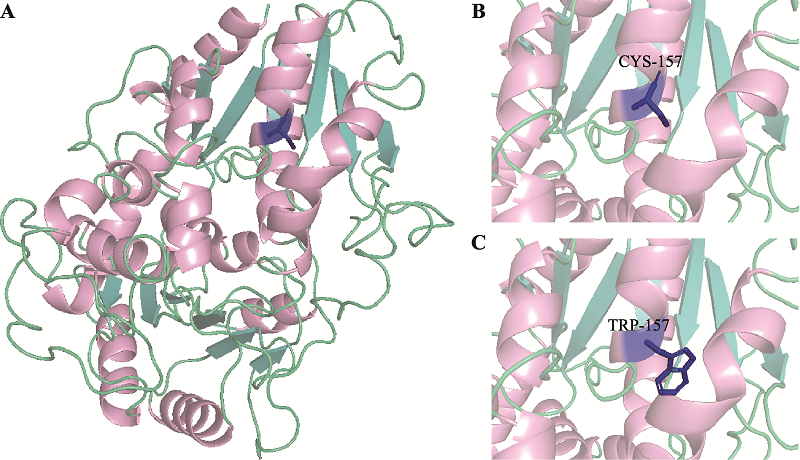
| CYP2D6 | rs16947 | 64/12 | 70/2 | 6.5620 | 1.4140~30.4500 | 0.0069 |
| CYP2S1 | rs338599 | 71/9 | 56/18 | 0.3944 | 0.1646~0.9446 | 0.0330 |
Table 3
Validation of significant SNP/SNVs associated with anti-tuberculosis drug induced liver injury"
| 基因 | 编号 | 等位基因 REF/ALT | 等位基因频率 | 卡方(χ2) | P值 | |
|---|---|---|---|---|---|---|
| 结核病人 | 东亚健康人群数据库 | |||||
| UGT1A4 | rs2011404 | T/G | 0.0750/0.9250 | 0.0258/0.9742 | 4.6809 | 0.0305 |
| CYP2D6 | rs16947 | G/A | 0.8421/0.1579 | 0.8601/0.1399 | 0.0698 | 0.7917 |
| CYP2S1 | rs338599 | C/G | 0.8875/0.1125 | 0.7897/0.2103 | 3.7978 | 0.0513 |
| [1] | WHO. Global tuberculosis report 2018. 2018. |
| [2] | Zheng W, Ji LD, Xing WH, Tu WW, Xu J . Advances in genome-wide association study of tuberculosis. Hereditas (Beijing), 2013,35(7):823-829. |
| 郑伟, 季林丹, 邢文华, 涂巍巍, 徐进 , 肺结核全基因组关联研究进展. 遗传, 2013,35(7):823-829. | |
| [3] | 肺结核诊断和治疗指南(2001年订). 内科急危重症杂志, 2002,8(4):225-229. |
| [4] | Xia YY, Zhan SY . Systematic review of anti-tuberculosis drug induced adverse reactions in China. Chin J Tubercul Respir Dis, 2007,30(6):419-423. |
| 夏愔愔, 詹思延 , 国内抗结核药物不良反应发生率的综合分析. 中华结核和呼吸杂志, 2007,30(6):419-423. | |
| [5] | Shang PH, Xia YY, Liu FY, Wang XM, Yuan YL, Hu DY, Tu DH, Chen YX, Deng PY, Cheng SM, Zhou L, Ma Y, Zhu LZ, Gao WW, Wang HY, Chen DF, Yang L, He PP, Wu SS, Tang SW, Lv XZ, Shu Z, Zhang Y, Yang ZR, Chen Y, Li N, Sun F, Li XT, He YJ, Garner P, Zhan SY . Incidence, clinical features and impact on anti-tuberculosis treatment of anti-tuberculosis drug induced liver injury (atli) in china. PLoS One, 2011,6(7):e21836. |
| [6] | Chen R, Wang J, Zhang Y, Tang SW, Zhan SY . Key factors of susceptibility to anti-tuberculosis drug-induced hepatotoxicity. Arch Toxicol, 2015,89(6):883-897. |
| [7] | Russmann S, Kullak-Ublick GA, Grattagliano I . Current concepts of mechanisms in drug-induced hepatotoxicity. Curr Med Chem, 2009,16(23):3041-3053. |
| [8] | Metushi I, Uetrecht J, Phillips E . Mechanism of isoniazid- induced hepatotoxicity: Then and now. Br J Clin Pharmacol, 2016,81(6):1030-1036. |
| [9] | Benichou C, Danan G, Flahault A . Causality assessment of adverse reactions to drugs-ii. An original model for validation of drug causality assessment methods: case reports with positive rechallenge. J Clin Epidemiol, 1993,46(11):1331-1336. |
| [10] | Hoofnagle JH, Björnsson ES . Drug-induced liver injury - types and phenotypes. N Engl J Med, 2019,381(3):264-273. |
| [11] | Yu LC, Mao YM, Chen CW . Guidelines for the management of drug-induced liver injury. J Clin Hepatol, 2015,31(11):1752-1769. |
| 于乐成, 茅益民, 陈成伟 . 药物性肝损伤诊治指南. 临床肝胆病杂志, 2015,31(11):1752-1769. | |
| [12] | Wen ZD, Hu GX, Yang J, Chen ZM . Retrospective analysis of 7018 cases of drug-induced liver injury literature. Chin Foreign Med Res, 2016,14(12):64-65. |
| 温祝杜, 胡国信, 杨剑, 陈智明 . 7018例药物性肝损伤文献回顾性分析. 中外医学研究, 2016,14(12):64-65. | |
| [13] | Huang YS, Chern HD, Su WJ, Wu JC, Chang SC, Chiang CH, Chang FY, Lee SD . Cytochrome p450 2e1 genotype and the susceptibility to antituberculosis drug-induced hepatitis. Hepatology, 2003,37(4):924-930. |
| [14] | Huang YS, Chern HD, Su WJ, Wu JC, Lai SL, Yang SY, Chang FY, Lee SD . Polymorphism of the n-acetyltransferase 2 gene as a susceptibility risk factor for antituberculosis drug-induced hepatitis. Hepatology, 2002,35(4):883-889. |
| [15] | Roy B, Chowdhury A, Kundu S, Santra A, Dey B, Chakraborty M, Majumder PP . Increased risk of antituberculosis drug-induced hepatotoxicity in individuals with glutathione s-transferase m1 ‘null’ mutation. J Gastroenterol Hepatol, 2001,16(9):1033-1037. |
| [16] | Yimer G, Ueda N, Habtewold A, Amogne W, Suda A, Riedel KD, Burhenne J, Aderaye G, Lindquist L, Makonnen E, Aklillu E . Pharmacogenetic & pharmacokinetic biomarker for efavirenz based arv and rifampicin based anti-tb drug induced liver injury in tb-hiv infected patients. PLoS One, 2011,6(12):e27810. |
| [17] | Li LM, Chen L, Deng GH, Tan WT, Dan YJ, Wang RQ, Chen WS . SLCO1B1*15 haplotype is associated with rifampin-induced liver injury. Mol Med Rep, 2012,6(1):75-82. |
| [18] | Sharma SK, Balamurugan A, Saha PK, Pandey RM, Mehra NK . Evaluation of clinical and immunogenetic risk factors for the development of hepatotoxicity during antituberculosis treatment. Am J Respir Crit Care Med, 2002,166(7):916-919. |
| [19] | Benoit-Biancamano MO, Adam JP, Bernard O, Court MH, Leblanc MH, Caron P, Guillemette C . A pharmacogenetics study of the human glucuronosyltransferase UGT1A4. Pharmacogenet Genomics, 2009,19(12):945-954. |
| [20] | Chang JC, Liu EH, Lee CN, Lin YC, Yu MC, Bai KJ, Chen HY . UGT1A1 polymorphisms associated with risk of induced liver disorders by anti-tuberculosis medications. Int J Tuberc Lung Dis, 2012,16(3):376-378. |
| [21] | Gufford BT, Robarge JD, Eadon MT, Gao H, Lin H, Liu Y, Desta Z, Skaar TC . Rifampin modulation of xeno- and endobiotic conjugating enzyme mrna expression and associated micrornas in human hepatocytes. Pharmacol Res Perspect, 2018,6(2):e00386. |
| [22] | Lee SY, Lee JY, Kim YM, Kim SK, Oh SJ . Expression of hepatic cytochrome P450s and UDP-glucuronosyltransferases in PXR and CAR double humanized mice treated with rifampicin. Toxicol Lett, 2015,235(2):107-115. |
| [23] | Cao L, Greenblatt DJ, Kwara A . Inhibitory effects of selected antituberculosis drugs on common human hepatic cytochrome P450 and UDP-glucuronosyltransferase enzymes. Drug Metab Dispos, 2017,45(9):1035-1043. |
| [1] | Yidan Sun, Zizhao Tian, Wei Zhou, Mo Li, Cong Huai, Lin He, Shengying Qin. Genome-wide association study on liver function tests in Chinese [J]. Hereditas(Beijing), 2021, 43(3): 249-260. |
| [2] | Tingyu Shan, Wen Shi, Yiting Wang, Ziyi Cao, Baohua Wang, Hui Fang. Genome-wide association study and candidate gene prediction of salt tolerance related traits in maize [J]. Hereditas(Beijing), 2021, 43(12): 1159-1169. |
| [3] | Guoqing Qian. Advances in genome-wide association study of chronic obstructive pulmonary disease [J]. Hereditas(Beijing), 2020, 42(9): 832-846. |
| [4] | Chaoliang Wen, Congjiao Sun, Ning Yang. The concepts and research progress: from heritability to microbiability [J]. Hereditas(Beijing), 2019, 41(11): 1023-1040. |
| [5] | Chao Yang, Ruifu Yang, Yujun Cui. Bacterial genome-wide association study: methodologies and applications [J]. Hereditas(Beijing), 2018, 40(1): 57-65. |
| [6] | Yuyan Wang,Zixing Wang,Yaoda Hu,Lei Wang,Ning Li,Biao Zhang,Wei Han,Jingmei Jiang. Current status of pathway analysis in genome-wide association study [J]. Hereditas(Beijing), 2017, 39(8): 707-716. |
| [7] | Pengfei Hu, Jiaping Xu, Cheng Ai, Xiujuan Shao, Hongliang Wang, Yimeng Dong, Xuezhe Cui, Fuhe Yang, Xiumei Xing. Screening weight related genes of velvet antlers by whole genome re-sequencing [J]. Hereditas(Beijing), 2017, 39(11): 1090-1101. |
| [8] | Kaixu Chen, Weilan Wang, Fuchun Zhang, Xiufen Zheng. Progress in genetic research of human height [J]. HEREDITAS(Beijing), 2015, 37(8): 741-755. |
| [9] | Qiwen Wang,Cuifang Chang,Ningning Gu,Cuiyun Pan,Cunshuan Xu. Effect of autophagy on liver regeneration [J]. HEREDITAS(Beijing), 2015, 37(11): 1116-1124. |
| [10] | Li Wang, Yanmei Xu, Zhujun Cheng, Zhaoping Xiong, Libin Deng. The progress of genetics of cholesterol metabolism disorder [J]. HEREDITAS(Beijing), 2014, 36(9): 857-863. |
| [11] | Jiapeng Zhou, Zhiyong Pei, Yubao Chen, Runsheng Chen. Strategies of genome-wide association study based on high-throughput sequencing [J]. HEREDITAS(Beijing), 2014, 36(11): 1099-1111. |
| [12] | Qingfeng Song, Hongxing Zhang, Yilong Ma, Gangqiao Zhou. Fine mapping of complex disease susceptibility loci [J]. HEREDITAS, 2014, 36(1): 2-10. |
| [13] | LUO Xu-Hong LIU Zhi-Fang DONG Chang-Zheng. Advances on gene-based association analysis [J]. HEREDITAS, 2013, 35(9): 1065-1071. |
| [14] | ZHENG Wei JI Lin-Dan XING Wen-Hua TU Wei-Wei XU Jin. Advances in genome-wide association study of tuberculosis [J]. HEREDITAS, 2013, 35(7): 823-829. |
| [15] | XU Rui-Wei, YAN Wei-Li. Advances in genome-wide association studies on essential hypertension [J]. HEREDITAS, 2012, 34(7): 793-809. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||