Hereditas(Beijing) ›› 2023, Vol. 45 ›› Issue (12): 1087-1099.doi: 10.16288/j.yczz.23-170
• Invited Review • Previous Articles Next Articles
Physiological and pathological mechanisms of oocyte meiosis
Zhou Zhou1,2( ), Qing Sang1(
), Qing Sang1( ), Lei Wang1(
), Lei Wang1( )
)
- 1. Institutes of Biomedical Sciences, Fudan University, Shanghai 200032, China
2. Shanghai Institute for Biomedical and Pharmaceutical Technologies, Shanghai 200237, China
-
Received:2023-08-07Revised:2023-10-11Online:2023-12-20Published:2023-10-24 -
Contact:Qing Sang,Lei Wang E-mail:zhouzhoustudy@163.com;sangqing@fudan.edu.cn;wangleiwanglei@fudan.edu.cn -
Supported by:National Natural Science Foundation of China(82288102);National Natural Science Foundation of China(32130029);National Natural Science Foundation of China(81725006);National Natural Science Foundation of China(82171643);National Natural Science Foundation of China(81971450);National Key Research and Development Program of China(2021YFC2700100);China Postdoctoral Science Fund(2022M712147)
Cite this article
Zhou Zhou, Qing Sang, Lei Wang. Physiological and pathological mechanisms of oocyte meiosis[J]. Hereditas(Beijing), 2023, 45(12): 1087-1099.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
Table 1
Pathogenic genes of oocyte meiosis defects"
| 致病基因 | 遗传模式 | 患者表型 | 功能 | 参考文献 |
|---|---|---|---|---|
| TRIP13 | AR | 卵母细胞MI阻滞、合子分裂失败 | 调控同源染色体的分离 | [ |
| REC114 | AR | 受精后多原核、早期胚胎停滞、反复葡萄胎、流产 | 调控同源重组的DSBs | [ |
| MEI1 | AR | 早期胚胎停滞、反复葡萄胎、反复流产 | 调控同源重组的DSBs | [ |
| PATL2 | AR | 卵母细胞GV阻滞、受精失败、早期胚胎停滞 | 介导翻译抑制途径 | [ |
| PABPC1L | AR | 卵母细胞成熟障碍 | 介导翻译激活途径 | [ |
| TBPL2 | AR | 卵母细胞成熟障碍、卵子退化和早期胚胎停滞 | 介导特定基因的转录 | [ |
| ZP1 | AR | 卵子无透明带 | 组装为卵母细胞透明带 | [ |
| ZP3 | AD | 卵子无透明带 | 组装为卵母细胞透明带 | [ |
| ZP2 | AR | 卵子薄透明带、体外受精失败 | 组装为卵母细胞透明带 | [ |
| ASTL | AR | 受精差和早期胚胎停滞 | 阻止多精受精 | [ |
| TUBB8 | AD/AR/新发 | 卵母细胞成熟障碍、受精失败、早期胚胎停滞 | 构成卵母细胞纺锤体 | [ |
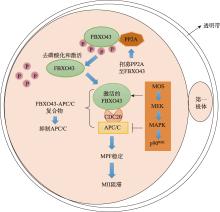
| FBXO43 | AR | 早期胚胎停滞 | 维持卵母细胞MII阻滞 | [ |
| CDC20 | AR | 卵母细胞MI阻滞、受精失败、早期胚胎停滞 | 维持卵母细胞MII阻滞 | [ |
| MOS | AR | 早期胚胎停滞 | 维持卵母细胞MII阻滞 | [ |
| WEE2 | AR | 受精失败 | 抑制MPF的活性 | [ |
| BTG4 | AR | 合子分裂失败 | 介导母源mRNA的降解 | [ |
| ZFP36L2 | AR | 早期胚胎停滞 | 介导母源mRNA的降解 | [ |
| KPNA7 | AR | 早期胚胎停滞 | 介导核转运过程 | [ |
| CHK1 | AD | 合子分裂失败 | 抑制MPF的活性 | [ |
| TLE6 | AR | 受精失败或早期胚胎停滞 | 调控胞质晶格形成 | [ |
| PADI6 | AR | 早期胚胎停滞 | 调控胞质晶格形成 | [ |
| NLRP2 | AR | 早期胚胎停滞 | 调控胞质晶格形成 | [ |
| NLRP5 | AR | 受精失败或早期胚胎停滞 | 调控胞质晶格形成 | [ |
| KHDC3L | AR | 早期胚胎停滞或反复葡萄胎 | 调控胞质晶格形成 | [ |
| [1] |
Sen A, Caiazza F. Oocyte maturation: a story of arrest and release. Front Biosci (Schol Ed), 2013, 5(2): 451-477.
doi: 10.2741/s383 pmid: 23277062 |
| [2] |
He MN, Zhang T, Yang Y, Wang C. Mechanisms of oocyte maturation and related epigenetic regulation. Front Cell Dev Biol, 2021, 9: 654028.
doi: 10.3389/fcell.2021.654028 |
| [3] |
Pei ZL, Deng K, Xu CJ, Zhang S. The molecular regulatory mechanisms of meiotic arrest and resumption in oocyte development and maturation. Reprod Biol Endocrinol, 2023, 21(1): 90.
doi: 10.1186/s12958-023-01143-0 |
| [4] |
Sang Q, Zhou Z, Mu J, Wang L. Genetic factors as potential molecular markers of human oocyte and embryo quality. J Assist Reprod Genet, 2021, 38(5): 993-1002.
doi: 10.1007/s10815-021-02196-z |
| [5] |
Baerwald AR, Adams GP, Pierson RA. Ovarian antral folliculogenesis during the human menstrual cycle: a review. Hum Reprod Update, 2012, 18(1): 73-91.
doi: 10.1093/humupd/dmr039 pmid: 22068695 |
| [6] |
Orisaka M, Miyazaki Y, Shirafuji A, Tamamura C, Tsuyoshi H, Tsang BK, Yoshida Y. The role of pituitary gonadotropins and intraovarian regulators in follicle development: a mini-review. Reprod Med Biol, 2021, 20(2): 169-175.
doi: 10.1002/rmb2.12371 pmid: 33850449 |
| [7] |
Zhao Y, Feng HW, Zhang YH, Zhang JV, Wang XH, Liu DT, Wang TR, Li RHW, Ng EHY, Yeung WSB, Rodriguez-Wallberg KA, Liu K. Current understandings of core pathways for the activation of mammalian primordial follicles. Cells, 2021, 10(6): 1491.
doi: 10.3390/cells10061491 |
| [8] |
Rienzi L, Balaban B, Ebner T, Mandelbaum J. The oocyte. Hum Reprod, 2012, 27(Suppl 1): i2-i21.
doi: 10.1093/humrep/des200 |
| [9] |
Gougeon A. Human ovarian follicular development: from activation of resting follicles to preovulatory maturation. Ann Endocrinol (Paris), 2010, 71(3): 132-143.
doi: 10.1016/j.ando.2010.02.021 pmid: 20362973 |
| [10] |
Suarez SS. Mammalian sperm interactions with the female reproductive tract. Cell Tissue Res, 2016, 363(1): 185-194.
doi: 10.1007/s00441-015-2244-2 pmid: 26183721 |
| [11] |
Sanders JR, Swann K. Molecular triggers of egg activation at fertilization in mammals. Reproduction, 2016, 152(2): R41-R50.
doi: 10.1530/REP-16-0123 |
| [12] | Ahmed TA, Ahmed SM, El-Gammal Z, Shouman S, Ahmed A, Mansour R, El-Badri N. Oocyte aging: the role of cellular and environmental factors and impact on female fertility. Adv Exp Med Biol, 2020, 1247(8): 109-123. |
| [13] | Hunter N. Meiotic recombination: the essence of heredity. Cold Spring Harb Perspect Biol, 2015, 7(12): a016618. |
| [14] |
de Massy B. Initiation of meiotic recombination: how and where? Conservation and specificities among eukaryotes. Annu Rev Genet, 2013, 47: 563-599.
doi: 10.1146/annurev-genet-110711-155423 pmid: 24050176 |
| [15] |
Powers NR, Parvanov ED, Baker CL, Walker M, Petkov PM, Paigen K. The meiotic recombination activator PRDM9 trimethylates both H3K36 and H3K4 at recombination hotspots in vivo. PLoS Genet, 2016, 12(6): e1006146.
doi: 10.1371/journal.pgen.1006146 |
| [16] |
Stanzione M, Baumann M, Papanikos F, Dereli I, Lange J, Ramlal A, Tränkner D, Shibuya H, de Massy B, Watanabe Y, Jasin M, Keeney S, Tóth A. Meiotic DNA break formation requires the unsynapsed chromosome axis-binding protein IHO1 (CCDC36) in mice. Nat Cell Biol, 2016, 18(11): 1208-1220.
doi: 10.1038/ncb3417 pmid: 27723721 |
| [17] |
Moreau S, Ferguson JR, Symington LS. The nuclease activity of Mre11 is required for meiosis but not for mating type switching, end joining, or telomere maintenance. Mol Cell Biol, 1999, 19(1): 556-566.
doi: 10.1128/MCB.19.1.556 pmid: 9858579 |
| [18] |
Cloud V, Chan YL, Grubb J, Budke B, Bishop DK. Rad 51 is an accessory factor for Dmc1-mediated joint molecule formation during meiosis. Science, 2012, 337(6099): 1222-1225.
doi: 10.1126/science.1219379 |
| [19] |
Adhikari D, Liu K. The regulation of maturation promoting factor during prophase I arrest and meiotic entry in mammalian oocytes. Mol Cell Endocrinol, 2014, 382(1): 480-487.
doi: S0303-7207(13)00321-3 pmid: 23916417 |
| [20] |
Conti M, Hsieh M, Zamah AM, Oh JS. Novel signaling mechanisms in the ovary during oocyte maturation and ovulation. Mol Cell Endocrinol, 2012, 356(1-2): 65-73.
doi: 10.1016/j.mce.2011.11.002 pmid: 22101318 |
| [21] |
Su YQ, Wigglesworth K, Pendola FL, O'Brien MJ, Eppig JJ. Mitogen-activated protein kinase activity in cumulus cells is essential for gonadotropin-induced oocyte meiotic resumption and cumulus expansion in the mouse. Endocrinology, 2002, 143(6): 2221-2232.
doi: 10.1210/endo.143.6.8845 |
| [22] |
Sela-Abramovich S, Chorev E, Galiani D, Dekel N. Mitogen-activated protein kinase mediates luteinizing hormone-induced breakdown of communication and oocyte maturation in rat ovarian follicles. Endocrinology, 2005, 146(3): 1236-1244.
pmid: 15576461 |
| [23] | Sha QQ, Dai XX, Dang YJ, Tang FC, Liu JP, Zhang YL, Fan HY. A MAPK cascade couples maternal mRNA translation and degradation to meiotic cell cycle progression in mouse oocytes. Development, 2017, 144(3): 452-463. |
| [24] |
Wu JQ, Kornbluth S. Across the meiotic divide—CSF activity in the post-Emi2/XErp1 era. J Cell Sci, 2008, 121(Pt 21): 3509-3514.
doi: 10.1242/jcs.036855 |
| [25] | Dupré A, Haccard O, Jessus C. Mos in the oocyte: how to use MAPK independently of growth factors and transcription to control meiotic divisions. J Signal Transduct, 2011, 2011: 350412. |
| [26] |
Zhang YL, Liu XM, Ji SY, Sha QQ, Zhang J, Fan HY. ERK1/2 activities are dispensable for oocyte growth but are required for meiotic maturation and pronuclear formation in mouse. J Genet Genomics, 2015, 42(9): 477-485.
doi: 10.1016/j.jgg.2015.07.004 |
| [27] |
Krauchunas AR, Wolfner MF. Molecular changes during egg activation. Curr Top Dev Biol, 2013, 102: 267-292.
doi: 10.1016/B978-0-12-416024-8.00010-6 pmid: 23287037 |
| [28] |
Sang Q, Ray PF, Wang L. Understanding the genetics of human infertility. Science, 2023, 380(6641): 158-163.
doi: 10.1126/science.adf7760 pmid: 37053320 |
| [29] |
Zhang ZH, Li B, Fu J, Li R, Diao FY, Li CH, Chen BB, Du J, Zhou Z, Mu J, Yan Z, Wu L, Liu S, Wang WJ, Zhao L, Dong J, He L, Liang XZ, Kuang YP, Sun XX, Sang Q, Wang L. Bi-allelic missense pathogenic variants in TRIP13 cause female infertility characterized by oocyte maturation arrest. Am J Hum Genet, 2020, 107(1): 15-23.
doi: S0002-9297(20)30148-8 pmid: 32473092 |
| [30] |
Wang WJ, Dong J, Chen BB, Du J, Kuang YP, Sun XX, Fu J, Li B, Mu J, Zhang ZH, Zhou Z, Lin Z, Wu L, Yan Z, Mao XY, Li QL, He L, Wang L, Sang Q. Homozygous mutations in REC114 cause female infertility characterised by multiple pronuclei formation and early embryonic arrest. J Med Genet, 2020, 57(3): 187-194.
doi: 10.1136/jmedgenet-2019-106379 |
| [31] |
Dong J, Zhang H, Mao XY, Zhu JH, Li D, Fu J, Hu JJ, Wu L, Chen BB, Sun YM, Mu J, Zhang ZH, Sun XX, Zhao L, Wang WJ, Wang WJ, Zhou Z, Zeng Y, Du J, Li QL, He L, Jin L, Kuang YP, Wang L, Sang Q. Novel biallelic mutations in MEI1: expanding the phenotypic spectrum to human embryonic arrest and recurrent implantation failure. Hum Reprod, 2021, 36(8): 2371-2381.
doi: 10.1093/humrep/deab118 |
| [32] |
Christou-Kent M, Kherraf ZE, Amiri-Yekta A, Le Blévec E, Karaouzène T, Conne B, Escoffier J, Assou S, Guttin A, Lambert E, Martinez G, Boguenet M, Fourati Ben Mustapha S, Cedrin Durnerin I, Halouani L, Marrakchi O, Makni M, Latrous H, Kharouf M, Coutton C, Thierry- Mieg N, Nef S, Bottari SP, Zouari R, Issartel JP, Ray PF, Arnoult C. PATL2 is a key actor of oocyte maturation whose invalidation causes infertility in women and mice. EMBO Mol Med, 2018, 10(5): e8515.
doi: 10.15252/emmm.201708515 |
| [33] |
Chen BB, Zhang ZH, Sun XX, Kuang YP, Mao XY, Wang XQ, Yan Z, Li B, Xu Y, Yu M, Fu J, Mu J, Zhou Z, Li QL, Jin L, He L, Sang Q, Wang L. Biallelic mutations in PATL2 cause female infertility characterized by oocyte maturation arrest. Am J Hum Genet, 2017, 101(4): 609-615.
doi: S0002-9297(17)30340-3 pmid: 28965849 |
| [34] |
Wu L, Chen H, Li D, Song D, Chen BB, Yan Z, Lyu QF, Wang L, Kuang YP, Li B, Sang Q. Novel mutations in PATL2: expanding the mutational spectrum and corresponding phenotypic variability associated with female infertility. J Hum Genet, 2019, 64(5): 379-385.
doi: 10.1038/s10038-019-0568-6 pmid: 30765866 |
| [35] |
Wang WJ, Guo J, Shi JZ, Li Q, Chen BB, Pan ZQ, Qu RG, Fu J, Shi R, Xue X, Mu J, Zhang ZH, Wu TY, Wang WJ, Zhao L, Li QL, He L, Sun XX, Sang Q, Lin G, Wang L. Bi-allelic pathogenic variants in PABPC1L cause oocyte maturation arrest and female infertility. EMBO Mol Med, 2023, 15(6): e17177.
doi: 10.15252/emmm.202217177 |
| [36] |
Yang P, Chen TL, Wu KL, Hou ZZ, Zou Y, Li M, Zhang XZ, Xu JT, Zhao H. A homozygous variant in TBPL2 was identified in women with oocyte maturation defects and infertility. Hum Reprod, 2021, 36(7): 2011-2019.
doi: 10.1093/humrep/deab094 |
| [37] |
Huang HL, Lv C, Zhao YC, Li W, He XM, Li P, Sha AG, Tian X, Papasian CJ, Deng HW, Lu GX, Xiao HM. Mutant ZP1 in familial infertility. N Engl J Med, 2014, 370(13): 1220-1226.
doi: 10.1056/NEJMoa1308851 |
| [38] |
Chen TL, Bian YH, Liu XM, Zhao SG, Wu KL, Yan L, Li M, Yang ZL, Liu HB, Zhao H, Chen ZJ. A recurrent missense mutation in ZP3 causes empty follicle syndrome and female infertility. Am J Hum Genet, 2017, 101(3): 459-465.
doi: S0002-9297(17)30323-3 pmid: 28886344 |
| [39] |
Dai C, Hu L, Gong F, Tan YQ, Cai SF, Zhang SP, Dai J, Lu CF, Chen J, Chen YZ, Lu GX, Du J, Lin G. ZP2 pathogenic variants cause in vitro fertilization failure and female infertility. Genet Med, 2019, 21(2): 431-440.
doi: 10.1038/s41436-018-0064-y |
| [40] |
Maddirevula S, Coskun S, Al-Qahtani M, Aboyousef O, Alhassan S, Aldeery M, Alkuraya FS. ASTL is mutated in female infertility. Hum Genet, 2022, 141(1): 49-54.
doi: 10.1007/s00439-021-02388-8 |
| [41] |
Chen BB, Li B, Li D, Yan Z, Mao XY, Xu Y, Mu J, Li QL, Jin L, He L, Kuang YP, Sang Q, Wang L. Novel mutations and structural deletions in TUBB8: expanding mutational and phenotypic spectrum of patients with arrest in oocyte maturation, fertilization or early embryonic development. Hum Reprod, 2017, 32(2): 457-464.
doi: 10.1093/humrep/dew322 |
| [42] |
Feng RZ, Sang Q, Kuang YP, Sun XX, Yan Z, Zhang SZ, Shi JZ, Tian GL, Luchniak A, Fukuda Y, Li B, Yu M, Chen JL, Xu Y, Guo L, Qu RG, Wang XQ, Sun ZG, Liu M, Shi HJ, Wang HY, Feng Y, Shao RJ, Chai RJ, Li QL, Xing QH, Zhang R, Nogales E, Jin L, He L, Gupta ML, Cowan NJ, Wang L. Mutations in TUBB8 and human oocyte meiotic arrest. N Engl J Med, 2016, 374(3): 223-232.
doi: 10.1056/NEJMoa1510791 |
| [43] |
Wang WJ, Wang WJ, Xu Y, Shi JZ, Fu J, Chen BB, Mu J, Zhang ZH, Zhao L, Lin J, Du J, Li QL, He L, Jin L, Sun XX, Wang L, Sang Q. FBXO 43 variants in patients with female infertility characterized by early embryonic arrest. Hum Reprod, 2021, 36(8): 2392-2402.
doi: 10.1093/humrep/deab131 |
| [44] |
Zhao L, Xue SG, Yao ZY, Shi JZ, Chen BB, Wu L, Sun LH, Xu Y, Yan Z, Li B, Mao XY, Fu J, Zhang ZH, Mu J, Wang WJ, Du J, Liu S, Dong J, Wang WJ, Li Q, He L, Jin L, Liang XZ, Kuang YP, Sun XX, Wang L, Sang Q. Biallelic mutations in CDC20 cause female infertility characterized by abnormalities in oocyte maturation and early embryonic development. Protein Cell, 2020, 11(12): 921-927.
doi: 10.1007/s13238-020-00756-0 |
| [45] |
Zhang YL, Zheng W, Ren PP, Hu HL, Tong XM, Zhang SP, Li X, Wang HC, Jiang JC, Jin JM, Yang WJ, Cao LR, He YL, Ma YR, Zhang YY, Gu YF, Hu L, Luo KL, Gong F, Lu GX, Lin G, Fan HY, Zhang SY. Biallelic mutations in MOS cause female infertility characterized by human early embryonic arrest and fragmentation. EMBO Mol Med, 2021, 13(12): e14887.
doi: 10.15252/emmm.202114887 |
| [46] |
Zeng Y, Shi JZ, Xu SR, Shi R, Wu TH, Li HY, Xue X, Zhu YC, Chen BB, Sang Q, Wang L. Bi-allelic mutations in MOS cause female infertility characterized by preimplantation embryonic arrest. Hum Reprod, 2022, 37(3): 612-620.
doi: 10.1093/humrep/deab281 |
| [47] |
Zhang YL, Zheng W, Ren PP, Jin JM, Hu ZH, Liu Q, Fan HY, Gong F, Lu GX, Lin G, Zhang SY, Tong XM. Biallelic variants in MOS cause large polar body in oocyte and human female infertility. Hum Reprod, 2022, 37(8): 1932-1944.
doi: 10.1093/humrep/deac120 |
| [48] |
Sang Q, Li B, Kuang YP, Wang XQ, Zhang ZH, Chen BB, Wu L, Lyu QF, Fu YL, Yan Z, Mao XY, Xu Y, Mu J, Li QL, Jin L, He L, Wang L. Homozygous mutations in WEE2 cause fertilization failure and female infertility. Am J Hum Genet, 2018, 102(4): 649-657.
doi: S0002-9297(18)30060-0 pmid: 29606300 |
| [49] |
Zheng W, Zhou Z, Sha QQ, Niu XL, Sun XX, Shi JZ, Zhao L, Zhang SP, Dai J, Cai SF, Meng F, Hu L, Gong F, Li XR, Fu J, Shi R, Lu GX, Chen BB, Fan HY, Wang L, Lin G, Sang Q. Homozygous mutations in BTG4 cause zygotic cleavage failure and female infertility. Am J Hum Genet, 2020, 107(1): 24-33.
doi: S0002-9297(20)30157-9 pmid: 32502391 |
| [50] |
Zheng W, Sha QQ, Hu HL, Meng F, Zhou QW, Chen XQ, Zhang SP, Gu YF, Yan X, Zhao L, Zong YR, Hu L, Gong F, Lu GX, Fan HY, Lin G. Biallelic variants in ZFP36L2 cause female infertility characterised by recurrent preimplantation embryo arrest. J Med Genet, 2022, 59(9): 850-857.
doi: 10.1136/jmedgenet-2021-107933 |
| [51] |
Wang WJ, Miyamoto Y, Chen BB, Shi JZ, Diao FY, Zheng W, Li Q, Yu L, Li L, Xu Y, Wu L, Mao XY, Fu J, Li B, Yan Z, Shi R, Xue X, Mu J, Zhang ZH, Wu TY, Zhao L, Wang WJ, Zhou Z, Dong J, Li QL, Jin L, He L, Sun XX, Lin G, Kuang YP, Wang L, Sang Q. Karyopherin α deficiency contributes to human preimplantation embryo arrest. J Clin Invest, 2023, 133(2): e159951.
doi: 10.1172/JCI159951 |
| [52] |
Zhang HH, Chen TL, Wu KL, Hou ZZ, Zhao SG, Zhang CX, Gao Y, Gao M, Chen ZJ, Zhao H. Dominant mutations in CHK1 cause pronuclear fusion failure and zygote arrest that can be rescued by CHK1 inhibitor. Cell Res, 2021, 31(7): 814-817.
doi: 10.1038/s41422-021-00507-8 pmid: 33953335 |
| [53] |
Alazami AM, Awad SM, Coskun S, Al-Hassan S, Hijazi H, Abdulwahab FM, Poizat C, Alkuraya FS. TLE6 mutation causes the earliest known human embryonic lethality. Genome Biol, 2015, 16: 240.
doi: 10.1186/s13059-015-0792-0 pmid: 26537248 |
| [54] |
Xu Y, Shi YL, Fu J, Yu M, Feng RZ, Sang Q, Liang B, Chen BB, Qu RG, Li B, Yan Z, Mao XY, Kuang YP, Jin L, He L, Sun XX, Wang L. Mutations in PADI6 cause female infertility characterized by early embryonic arrest. Am J Hum Genet, 2016, 99(3): 744-752.
doi: S0002-9297(16)30228-2 pmid: 27545678 |
| [55] |
Mu J, Wang WJ, Chen BB, Wu L, Li B, Mao XY, Zhang ZH, Fu J, Kuang YP, Sun XX, Li QL, Jin L, He L, Sang Q, Wang L. Mutations in NLRP2 and NLRP5 cause female infertility characterised by early embryonic arrest. J Med Genet, 2019, 56(7): 471-480.
doi: 10.1136/jmedgenet-2018-105936 |
| [56] |
Zhang WD, Chen ZL, Zhang DF, Zhao B, Liu L, Xie ZY, Yao YG, Zheng P. KHDC3L mutation causes recurrent pregnancy loss by inducing genomic instability of human early embryonic cells. PLoS Biol, 2019, 17(10): e3000468.
doi: 10.1371/journal.pbio.3000468 |
| [57] |
Jiang ZY, Fan HY. Five questions toward mRNA degradation in oocytes and preimplantation embryos: when, who, to whom, how, and why? Biol Reprod, 2022, 107(1): 62-75.
doi: 10.1093/biolre/ioac014 |
| [58] |
Sha QQ, Zhang J, Fan HY. A story of birth and death: mRNA translation and clearance at the onset of maternal-to-zygotic transition in mammals. Biol Reprod, 2019, 101(3): 579-590.
doi: 10.1093/biolre/ioz012 |
| [59] |
Abou-Haila A, Bendahmane M, Tulsiani DR. Significance of egg's zona pellucida glycoproteins in sperm-egg interaction and fertilization. Minerva Ginecol, 2014, 66(4): 409-419.
pmid: 25020059 |
| [60] | Yu C, Ji SY, Sha QQ, Dang YJ, Zhou JJ, Zhang YL, Liu Y, Wang ZW, Hu BQ, Sun QY, Sun SC, Tang FC, Fan HY. BTG 4 is a meiotic cell cycle-coupled maternal-zygotic- transition licensing factor in oocytes. Nat Struct Mol Biol, 2016, 23(5): 387-394. |
| [61] |
Sha QQ, Yu JL, Guo JX, Dai XX, Jiang JC, Zhang YL, Yu C, Ji SY, Jiang Y, Zhang SY, Shen L, Ou XH, Fan HY. CNOT6L couples the selective degradation of maternal transcripts to meiotic cell cycle progression in mouse oocyte. Embo J, 2018, 37(24): e99333.
doi: 10.15252/embj.201899333 |
| [62] |
Bebbere D, Albertini DF, Coticchio G, Borini A, Ledda S. The subcortical maternal complex: emerging roles and novel perspectives. Mol Hum Reprod, 2021, 27(7): gaab043.
doi: 10.1093/molehr/gaab043 |
| [63] |
da Silveira JC, de Ávila ACFCM, Garrett HL, Bruemmer JE, Winger QA, Bouma GJ. Cell-secreted vesicles containing microRNAs as regulators of gamete maturation. J Endocrinol, 2018, 236(1): R15-R27.
doi: 10.1530/JOE-17-0200 |
| [64] |
Eckersley-Maslin MA, Alda-Catalinas C, Reik W. Dynamics of the epigenetic landscape during the maternal-to-zygotic transition. Nat Rev Mol Cell Biol, 2018, 19(7): 436-450.
doi: 10.1038/s41580-018-0008-z |
| [65] | Xu Y, Su GH, Ma D, Xiao Y, Shao ZM, Jiang YZ. Technological advances in cancer immunity: from immunogenomics to single-cell analysis and artificial intelligence. Signal Transduct Target Ther, 2021, 6(1): 312. |
| [1] | Xiangjiang Lv, Jing Guo, Ge Lin. Novel mutations in TRIP13 lead to female infertility with oocyte maturation arrest [J]. Hereditas(Beijing), 2023, 45(6): 514-525. |
| [2] | Nan Zhang, Jue Zhang, Ge Lin. Advances in the study of DNA damage and repair in mammalian oocytes [J]. Hereditas(Beijing), 2023, 45(5): 379-394. |
| [3] | Wenlong Wang, Chunxia Zhang. Research progress on the study of transcriptome-wide poly(A) tails in mammalian oocytes and early embryos [J]. Hereditas(Beijing), 2023, 45(4): 273-278. |
| [4] | Wenbing Liu, Dan Liu, Jin Yan, Xin Liu, Qianfei Wang. Genetic predisposition in patients with severe COVID-19 [J]. Hereditas(Beijing), 2022, 44(8): 672-681. |
| [5] | Yuxuan Guo, Shunping Yan, Yingxiang Wang. Recent advances in functional conservation and divergence of recombinase RAD51 and DMC1 [J]. Hereditas(Beijing), 2022, 44(5): 398-413. |
| [6] | Yuanyuan Li, Lei Guo, Zhiming Han. Roles of NEK family in cell cycle regulation [J]. Hereditas(Beijing), 2021, 43(7): 642-653. |
| [7] | Yige Li, Dandan Zhang. Progress on functional mechanisms of colorectal cancer causal SNPs in post-GWAS [J]. Hereditas(Beijing), 2021, 43(3): 203-214. |
| [8] | Hui Nie, Yiwen Zhang, Jianing Li, Nannan Wang, Lan Xu. Progress on the correlation between the abnormal synaptonemal complex and infertility [J]. Hereditas(Beijing), 2021, 43(12): 1142-1148. |
| [9] | Guoqing Qian. Advances in genome-wide association study of chronic obstructive pulmonary disease [J]. Hereditas(Beijing), 2020, 42(9): 832-846. |
| [10] | Xinyu Yang,Zhenwei Jia. The role of EGF-like factor signaling pathway in granulosa cells in regulation of oocyte maturation and development [J]. Hereditas(Beijing), 2019, 41(2): 137-145. |
| [11] | Fan Li, Rongpei Yu, Dan Sun, Jihua Wang, Shenchong Li, Jiwei Ruan, Qinli Shan, Pingli Lu, Guoxian Wang. Molecular mechanisms of meiotic recombination suppression in plants [J]. Hereditas(Beijing), 2019, 41(1): 52-65. |
| [12] | Junyu Zhang,Shan Lv,Huimin Niu,Anmin Lei. Research progress on the asymmetric division in mammalian oocytes [J]. Hereditas(Beijing), 2018, 40(4): 279-291. |
| [13] | Xingwei Huang, Xiangrong Cheng, Nan Wang, Yuwei Zhang, Chen Liao, Lianhong Jin, Lei. Histone variant H3.3 and its functions in reprogramming [J]. Hereditas(Beijing), 2018, 40(3): 186-196. |
| [14] | Xinqi Lin,Rong Liang,Junguo Zhang,Lucheng Pi,Sidong Chen,Li Liu,Yanhui Gao. Comparison of common burden tests for genetic association studies of rare variants [J]. Hereditas(Beijing), 2018, 40(2): 162-169. |
| [15] | Ying Huang,Qi Liu,Lianjiang Chi,Chengmin Shi,Zhen Wu,Min Hu,Hong Shi,Hua Chen. Application of BIG-Annotator in the genome sequencing data functional annotation and genetic diagnosis [J]. Hereditas(Beijing), 2018, 40(11): 1015-1023. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||