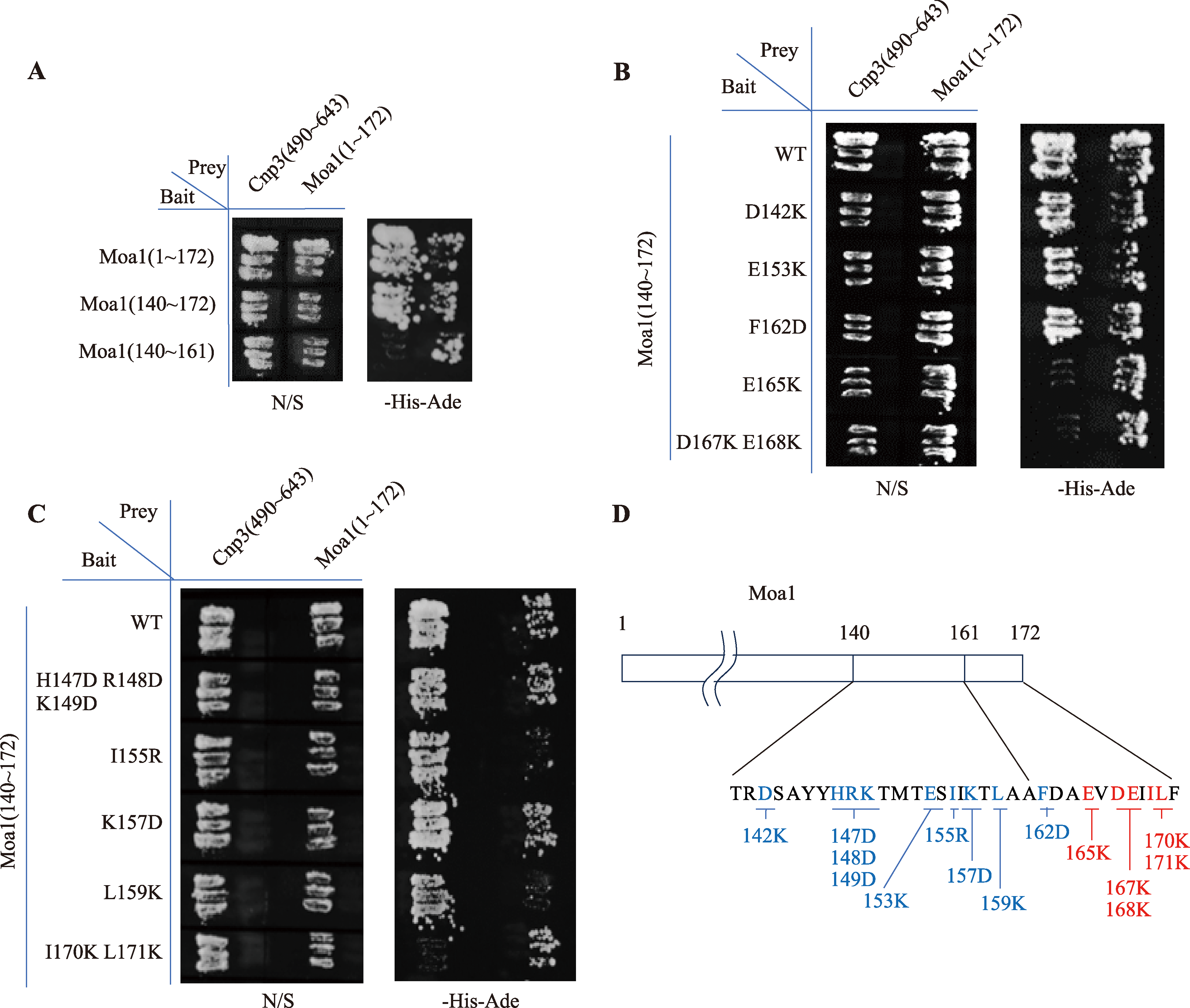
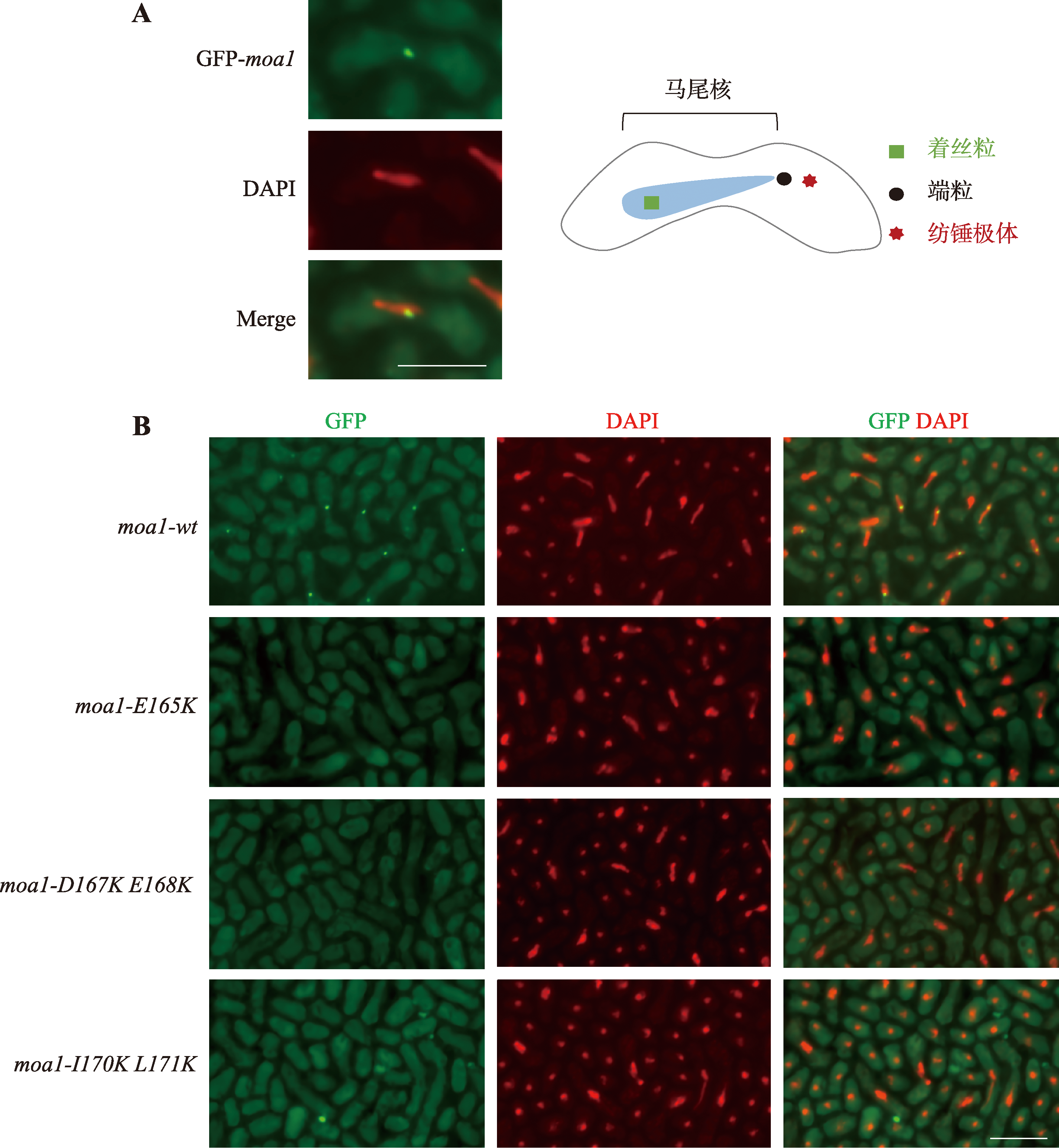
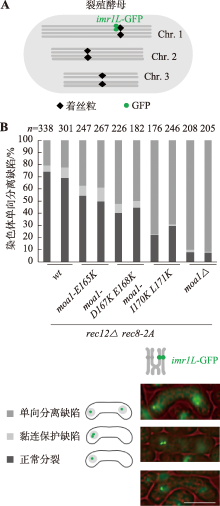
Hereditas(Beijing) ›› 2024, Vol. 46 ›› Issue (8): 649-660.doi: 10.16288/j.yczz.24-035
• Research Article • Previous Articles Next Articles
Functional roles of the interaction of Moa1 with CENP-C and Rec8 in meiosis of Schizosaccharomyces pombe
Yu Min1,2( ), Zihan Ni1,2, Lingling Ma1,2, Yoshinori Watanabe1,2(
), Zihan Ni1,2, Lingling Ma1,2, Yoshinori Watanabe1,2( )
)
- 1. Science Center for Future Foods, Jiangnan University, Wuxi 214122, China
2. School of Biotechnology, Jiangnan University, Wuxi 214122, China
-
Received:2024-03-08Revised:2024-05-07Online:2024-08-20Published:2024-05-14 -
Contact:Yoshinori Watanabe E-mail:1401577396@qq.com;ywatanabe@jiangnan.edu.cn
Cite this article
Yu Min, Zihan Ni, Lingling Ma, Yoshinori Watanabe. Functional roles of the interaction of Moa1 with CENP-C and Rec8 in meiosis of Schizosaccharomyces pombe[J]. Hereditas(Beijing), 2024, 46(8): 649-660.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
Table 1
The strains and their genotypes used in this study"
| 菌株名称 | 基因型 |
|---|---|
| PW632 | h+ pat1-114 3pk-moa1 |
| PW1 | h+ pat1-114 natMX6-3pk-moa1 |
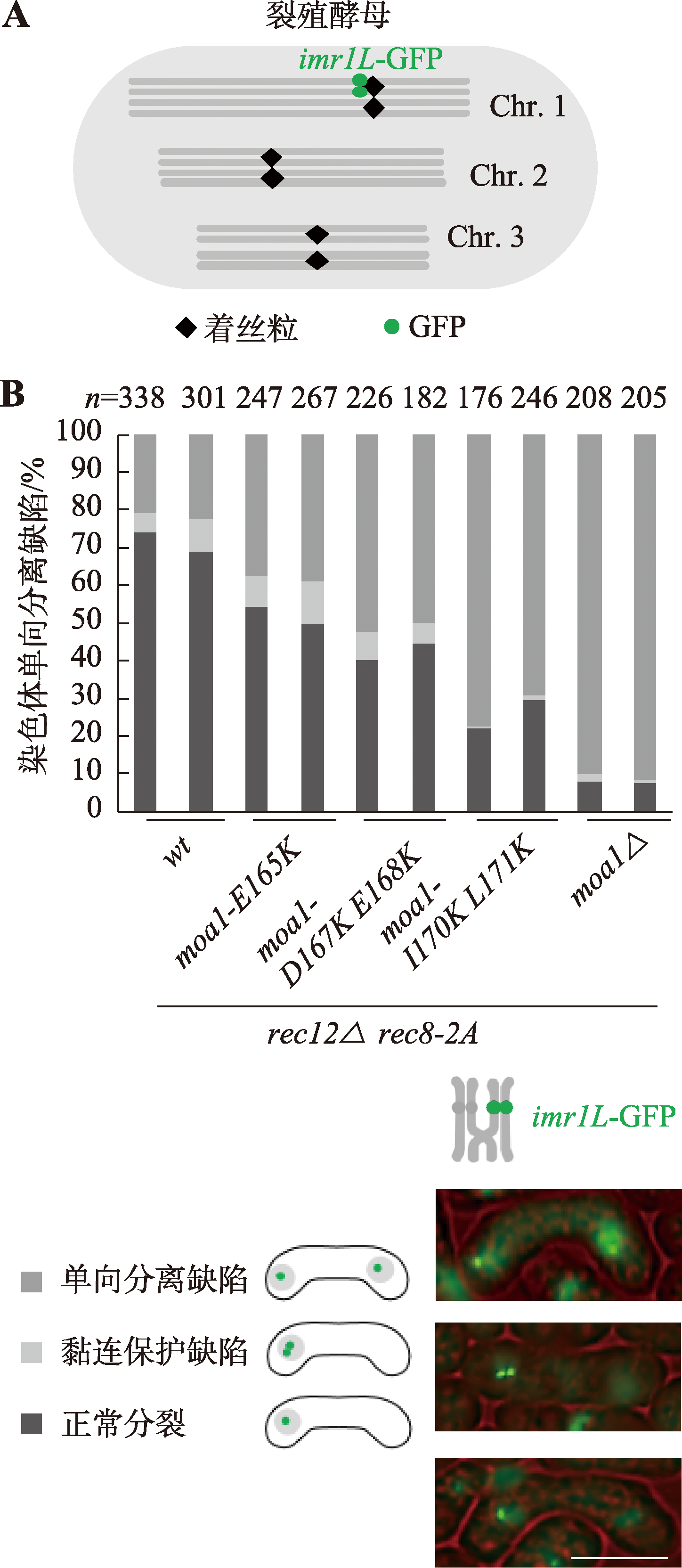
| M1 | h- leu1 imr1L-GFP mes1-B44 rec8-2A<<Kan rec12::hyg |
| M2 | h+ mes1-B44 rec8-2A<<Kan rec12::hyg |
| M3 | h90 mei4::hyg |
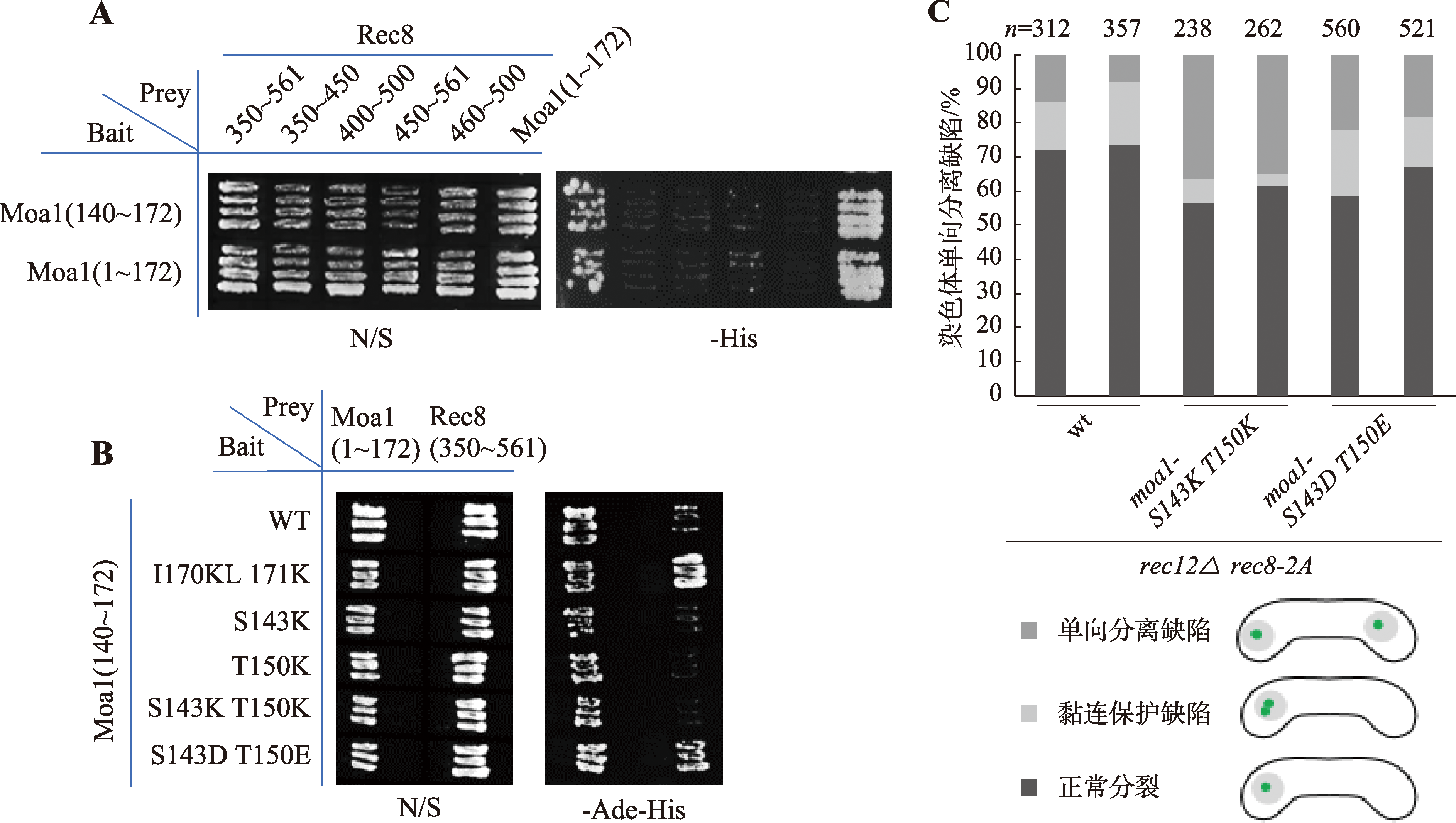
| PM11 | h- leu1 imr1L-GFP mes1-B44 rec8-2A<<Kan rec12::hyg natMX6-3pk-moa1-S143K T150K |
| PM12 | h+ mes1-B44 rec8-2A<<Kan rec12::hyg natMX6-3pk-moa1-S143K T150K |
| PM21 | h- leu1 imr1L-GFP mes1-B44 rec8-2A<<Kan rec12::hyg natMX6-3pk-moa1-S143D T150E |
| PM22 | h+ mes1-B44 rec8-2A<<Kan rec12::hyg natMX6-3pk-moa1-S143D T150E |
| PM31 | h- leu1 imr1L-GFP mes1-B44 rec8-2A<<Kan rec12::hyg natMX6-3pk-moa1-E165K |
| PM32 | h+ mes1-B44 rec8-2A<<Kan rec12::hyg natMX6-3pk-moa1-E165K |
| PM41 | h- leu1 imr1L-GFP mes1-B44 rec8-2A<<Kan rec12::hyg natMX6-3pk-moa1-D167K E168K |
| PM42 | h+ mes1-B44 rec8-2A<<Kan rec12::hyg natMX6-3pk-moa1-D167K E168K |
| PM51 | h- leu1 imr1L-GFP mes1-B44 rec8-2A<<Kan rec12::hyg natMX6-3pk-moa1-I170K L171K |
| PM52 | h+ mes1-B44 rec8-2A<<Kan rec12::hyg natMX6-3pk-moa1-I170K L171K |
| LM1 | h- leu1imr1L-GFP mes1-B44 rec8-2A<<Kan rec12::bsdr moa1::natr |
| LM2 | h+ mes1-B44 rec8-2A<<Kan rec12::bsdr moa1::natr |
| PM-W | h90 mei4::hyg natMX6-GFP-3pk-moa1 |
| PM36 | h90 mei4::hyg natMX6-GFP-3pk-moa1-E165K |
| PM37 | h90 mei4::hyg natMX6-GFP-3pk-moa1-D167K E168K |
| PM38 | h90 mei4::hyg natMX6-GFP-3pk-moa1-I170K L171K |
| [1] | Börner GV, Hochwagen A, Macqueen AJ. Meiosis in budding yeast. Genetics, 2023, 225(2): 1-33. |
| [2] | Hochwagen A. Meiosis. Curr Biol, 2008, 18(15): R641-R645. |
| [3] |
Sakuno T, Watanabe Y. Studies of meiosis disclose distinct roles of cohesion in the core centromere and pericentromeric regions. Chromosome Res, 2009, 17(2): 239-249.
doi: 10.1007/s10577-008-9013-y pmid: 19308704 |
| [4] |
Kagami A, Sakuno T, Yamagishi Y, Ishiguro T, Tsukahara T, Shirahige K, Tanaka K, Watanabe Y. Acetylation regulates monopolar attachment at multiple levels during meiosis I in fission yeast. EMBO Rep, 2011, 12(11): 1189-1195.
doi: 10.1038/embor.2011.188 pmid: 21979813 |
| [5] |
Yokobayashi S, Watanabe Y. The kinetochore protein Moal enables cohesion-mediated monopolar attachment at meiosis I. Cell, 2005, 123(5): 803-817.
pmid: 16325576 |
| [6] |
Lee BH, Kiburz BM, Amon A. Spo13 maintains centromeric cohesion and kinetochore coorientation during meiosis I. Curr Biol, 2004, 14(24): 2168-2182.
pmid: 15620644 |
| [7] | Kim J, Ishiguro K-I, Nambu A, Akiyoshi B, Yokobayashi S, Kagami A, Ishiguro T, Pendas AM, Takeda N, Sakakibara Y, Kitajima TS, Tanno Y, Sakuno T, Watanabe Y. Meikin is a conserved regulator of meiosis-I-specific kinetochore function. Nature, 2014, 517(7535): 466-471. |
| [8] | Hayashi T, Ebe M, Nagao K, Kokubu A, Sajiki K, Yanagida M. Schizosaccharomyces pombe centromere protein Mis19 links Mis16 and Mis18 to recruit CENP-A through interacting with NMD factors and the SWI/SNF complex. Genes Cells, 2014, 19(7): 541-554. |
| [9] |
Earnshaw WC, Rothfield N. Identification of a family of human centromere proteins using autoimmune sera from patients with scleroderma. Chromosoma, 1985, 91(3-4): 313-321.
doi: 10.1007/BF00328227 pmid: 2579778 |
| [10] | She CW, Song YC. Advances in research of the structure and function of plant centromeres. Hereditas(Beijing), 2006, 28(12): 1597-1606. |
| 佘朝文, 宋运淳. 植物着丝粒结构和功能的研究进展. 遗传, 2006, 28(12): 1597-1606. | |
| [11] |
Przewloka MR, Venkei Z, Bolanos-Garcia VM, Debski J, Dadlez M, Glover DM. CENP-C is a structural platform for kinetochore assembly. Curr Biol, 2011, 21(5): 399-405.
doi: 10.1016/j.cub.2011.02.005 pmid: 21353555 |
| [12] | Hara M, Ariyoshi M, Sano T, Nozawa RS, Shinkai S, Onami S, Jansen I, Hirota T, Fukagawa T. Centromere/ kinetochore is assembled through CENP-C oligomerization. Mol Cell, 2023, 83(13): 2188-2205.e13. |
| [13] | Chik JK, Moiseeva V, Goel PK, Meinen BA, Koldewey P, An SJ, Mellone BG, Subramanian L, Cho US. Structures of CENP-C cupin domains at regional centromeres reveal unique patterns of dimerization and recruitment functions for the inner pocket. J Biol Chem, 2019, 294(38): 14119-14134. |
| [14] | Musacchio A, Desai A. A molecular view of kinetochore assembly and function. Biology (Basel), 2017, 6(1): 5. |
| [15] | Kwon M-S, Hori T, Okada M, Fukagawa T. CENP-C is involved in chromosome segregation, mitotic checkpoint function, and kinetochore assembly. Mol Biol Cell, 2007, 18(6): 2155-2168. |
| [16] | Fellmeth JE, Jang JK, Persaud M, Sturm H, Changela N, Parikh A, Mckim KS. A dynamic population of prophase CENP-C is required for meiotic chromosome segregation. PLoS Genet, 2023, 19(11): e1011066. |
| [17] | Heeger S, Leismann O, Schittenhelm R, Schraidt O, Heidmann S, Lehner CF. Genetic interactions of separase regulatory subunits reveal the diverged Drosophila Cenp-C homolog. Genes Dev, 2005, 19(17): 2041-2053. |
| [18] |
Moore LL, Roth MB. Hcp-4, a Cenp-C-like protein in Caenorhabditis elegans, is required for resolution of sister centromeres. J Cell Biol, 2001, 153(6): 1199-1208.
doi: 10.1083/jcb.153.6.1199 pmid: 11402064 |
| [19] |
Fellmeth JE, Mckim KS. Meiotic CENP-C is a shepherd: bridging the space between the centromere and the kinetochore in time and space. Essays Biochem, 2020, 64(2): 251-261.
doi: 10.1042/EBC20190080 pmid: 32794572 |
| [20] |
Hillers KJ, Jantsch V, Martinez-Perez E, Yanowitz JL. Meiosis. WormBook, 2017, 2017: 1-43.
doi: 10.1895/wormbook.1.178.1 pmid: 26694509 |
| [21] | XU XY, Yanagida M. Cohesin organization, dynamics, and subdomain functions revealed by genetic suppressor screening. Proc Jpn Acad Ser B Phys Biol Sci, 2023, 99(3): 61-74. |
| [22] | Zhang N, Zhang J, Lin G. Advances in the study of DNA damage and repair in mammalian oocytes. Hereditas (Beijing), 2023, 45(5): 379-394. |
| 张楠, 张珏, 林戈. 哺乳动物卵母细胞的DNA损伤与修复研究进展. 遗传, 2023, 45(5): 379-394. | |
| [23] | Kuru-Schors M, Haemmerle M, Gutschner T. The cohesin complex and its interplay with non-coding RNAs. Noncoding RNA, 2021, 7(4): 67. |
| [24] | Litwin I, Pilarczyk E, Wysocki R. The emerging role of cohesin in the DNA damage response. Genes (Basel), 2018, 9(12): 581. |
| [25] | Zhang Y, Fang YD. Progresses on the structure and function of cohesin. Hereditas (Beijing), 2020, 42(1): 57-72. |
| 张雨, 方玉达. Cohesin结构及功能研究进展. 遗传, 2020, 42(1): 57-72. | |
| [26] |
Yokobayashi S, Yamamoto M, Watanabe Y. Cohesins determine the attachment manner of kinetochores to spindle microtubules at meiosis I in fission yeast. Mol Cell Biol, 2003, 23(11): 3965-3973.
doi: 10.1128/MCB.23.11.3965-3973.2003 pmid: 12748297 |
| [27] | Rittenhouse NL, Dowen JM. Cohesin regulation and roles in chromosome structure and function. Curr Opin Genet Dev, 2024, 85: 102159. |
| [28] | Lu YJ, Zhou CY, Xiong B. Functional diversity of chromosome cohesion proteins. SCI SIN Vitae, 2022, 52(12): 1844-1857. |
| 卢亚娟, 周长银, 熊波. 染色体黏合蛋白功能的多样性. 中国科学:生命科学, 2022, 52(12): 1844-1857. | |
| [29] | Minamino M, Higashi TL, Bouchoux C, Uhlmann F. Topological in vitro loading of the budding yeast cohesin ring onto DNA. Life Sci Alliance, 2018, 1(5): e201800143. |
| [30] |
Kitajima TS, Miyazaki Y, Yamamoto M, Watanabe Y. Rec8 cleavage by separase is required for meiotic nuclear divisions in fission yeast. EMBO J, 2003, 22(20): 5643-5653.
pmid: 14532136 |
| [31] |
Takahashi TS, Yiu PY, Chou MF, Gygi S, Walter JC. Recruitment of xenopus Scc2 and cohesin to chromatin requires the pre-replication complex. Nat Cell Biol, 2004, 6(10): 991-996.
doi: 10.1038/ncb1177 pmid: 15448702 |
| [32] |
Parisi S, Mckay MJ, Molnar M, Thompson MA, van der Spek PJ, van Drunen-Schoenmaker E, Kanaar R, Lehmann E, Hoeijmakers JH, Kohli J. Rec8p, a meiotic recombination and sister chromatid cohesion phosphoprotein of the Rad21p family conserved from fission yeast to humans. Mol Cell Biol, 1999, 19(5): 3515-3528.
doi: 10.1128/MCB.19.5.3515 pmid: 10207075 |
| [33] | Kitajima TS, Kawashima SA, Watanabe Y. The conserved kinetochore protein shugoshin protects centromeric cohesion during meiosis. Nature, 2004, 427(6974): 510-517. |
| [34] | Watanabe Y, Nurse P. Cohesin Rec8 is required for reductional chromosome segregation at meiosis. Nature, 1999, 400(6743): 461-464. |
| [35] |
Miyazaki S, Kim J, Yamagishi Y, Ishiguro T, Okada Y, Tanno Y, Sakuno T, Watanabe Y. Meikin-associated polo-like kinase specifies Bub1 distribution in meiosis I. Genes Cells, 2017, 22(6): 552-567.
doi: 10.1111/gtc.12496 pmid: 28497540 |
| [36] |
Tanaka K, Li Chang H, Kagami A, Watanabe Y. CENP-C functions as a scaffold for effectors with essential kinetochore functions in mitosis and meiosis. Dev Cell, 2009, 17(3): 334-343.
doi: 10.1016/j.devcel.2009.08.004 pmid: 19758558 |
| [37] | Ishiguro KI, Shimada R. MEIOSIN directs initiation of meiosis and subsequent meiotic prophase program during spermatogenesis. Genes Genet Syst, 2022, 97(1): 27-39. |
| [38] | Nimmo ER, Pidoux AL, Perry PE, Allshire RC. Defective meiosis in telomere-silencing mutants of Schizosaccharomyces pombe. Nature, 1998, 392(6678): 825-828. |
| [39] | Ding DQ, Haraguchi T, Hiraoka Y. Chromosomally- retained RNA mediates homologous pairing. Nucleus, 2012, 3(6): 516-519. |
| [40] |
Menees TM, Ross-Macdonald PB, Roeder GS. mei4, a meiosis-specific yeast gene required for chromosome synapsis. Mol Cell Biol, 1992, 12(3): 1340-1351.
doi: 10.1128/mcb.12.3.1340-1351.1992 pmid: 1545815 |
| [41] | Kumar R, Bourbon HM, De Massy B. Functional conservation of Mei4 for meiotic DNA double-strand break formation from yeasts to mice. Genes Dev, 2010, 24(12): 1266-1280. |
| [42] |
Zhou Z, Sang Q, Wang L. Physiological and pathological mechanisms of oocyte meiosis. Hereditas(Beijing), 2023, 45(12): 1087-1099.
doi: 10.16288/j.yczz.23-170 pmid: 38764273 |
| 周舟, 桑庆, 王磊. 人类卵母细胞减数分裂的生理和病理机制. 遗传, 2023, 45(12): 1087-1099. | |
| [43] |
Takahashi K, Murakami S, Chikashige Y, Funabiki H, Niwa O, Yanagida M. A low copy number central sequence with strict symmetry and unusual chromatin structure in fission yeast centromere. Mol Biol Cell, 1992, 3(7): 819-835.
pmid: 1515677 |
| [44] |
Kimata Y, Kitamura K, Fenner N, Yamano H. Mes1 controls the meiosis I to meiosis II transition by distinctly regulating the anaphase-promoting complex/cyclosome coactivators Fzr1/Mfr1 and Slp1 in fission yeast. Mol Biol Cell, 2011, 22(9): 1486-1494.
doi: 10.1091/mbc.E10-09-0774 pmid: 21389117 |
| [45] | Izawa D, Goto M, Yamashita A, Yamano H, Yamamoto M. Fission yeast Mes1p ensures the onset of meiosis II by blocking degradation of cyclin Cdc13p. Nature, 2005, 434(7032): 529-533. |
| [46] |
Ogushi S, Rattani A, Godwin J, Metson J, Schermelleh L, Nasmyth K. Loss of sister kinetochore co-orientation and peri-centromeric cohesin protection after meiosis I depends on cleavage of centromeric REC8. Dev Cell, 2021, 56(22): 3100-3114.e4.
doi: 10.1016/j.devcel.2021.10.017 pmid: 34758289 |
| [47] | Sakuno T, Hiraoka Y. Rec8 cohesin: a structural platform for shaping the meiotic chromosomes. Genes (Basel), 2022, 13(2). 200. |
| [48] | Ma W, Zhou JW, Chen J, Carr AM, Watanabe Y. Meikin synergizes with shugoshin to protect cohesin Rec8 during meiosis I. Genes Dev, 2021, 35(9-10): 692-697. |
| [49] | Mehta G, Anbalagan GK, Bharati AP, Gadre P, Ghosh SK. An interplay between Shugoshin and Spo13 for centromeric cohesin protection and sister kinetochore mono-orientation during meiosis I in Saccharomyces cerevisiae. Curr Genet, 2018, 64(5): 1141-1152. |
| [1] | Zihan Ni, Yu Min, Lingling Ma, Yoshinori Watanabe. The effect of centromere protein Fta2 phosphorylation during meiosis [J]. Hereditas(Beijing), 2024, 46(7): 552-559. |
| [2] | Yan Jingliang, Ma Lingling, Watanabe Yoshinori. Ssu72 phosphatase deficiency leads to spindle crossing during the second meiotic division process [J]. Hereditas(Beijing), 2024, 46(6): 502-508. |
| [3] | Xiangjiang Lv, Jing Guo, Ge Lin. Novel mutations in TRIP13 lead to female infertility with oocyte maturation arrest [J]. Hereditas(Beijing), 2023, 45(6): 514-525. |
| [4] | Zhou Zhou, Qing Sang, Lei Wang. Physiological and pathological mechanisms of oocyte meiosis [J]. Hereditas(Beijing), 2023, 45(12): 1087-1099. |
| [5] | Yuxuan Guo, Shunping Yan, Yingxiang Wang. Recent advances in functional conservation and divergence of recombinase RAD51 and DMC1 [J]. Hereditas(Beijing), 2022, 44(5): 398-413. |
| [6] | Yuanyuan Li, Lei Guo, Zhiming Han. Roles of NEK family in cell cycle regulation [J]. Hereditas(Beijing), 2021, 43(7): 642-653. |
| [7] | Hui Nie, Yiwen Zhang, Jianing Li, Nannan Wang, Lan Xu. Progress on the correlation between the abnormal synaptonemal complex and infertility [J]. Hereditas(Beijing), 2021, 43(12): 1142-1148. |
| [8] | Na Wang, Zhilian Jia, Qiang Wu. RFX5 regulates gene expression of the Pcdhα cluster [J]. Hereditas(Beijing), 2020, 42(8): 760-774. |
| [9] | Yu Zhang, Yuda Fang. Progresses on the structure and function of cohesin [J]. Hereditas(Beijing), 2020, 42(1): 57-72. |
| [10] | Fan Li, Rongpei Yu, Dan Sun, Jihua Wang, Shenchong Li, Jiwei Ruan, Qinli Shan, Pingli Lu, Guoxian Wang. Molecular mechanisms of meiotic recombination suppression in plants [J]. Hereditas(Beijing), 2019, 41(1): 52-65. |
| [11] | Yaping Liao,Chunjing Wang,Meng Liang,Xiaomei Hu,Qi Wu. Analysis of genetic characteristics and reproductive risks of balanced complex chromosome rearrangement carriers in China [J]. Hereditas(Beijing), 2017, 39(5): 396-412. |
| [12] | Yanan Zhai, Quan Xu, Ya Guo, Qiang Wu. Characterization of a cluster of CTCF-binding sites in a protocadherin regulatory region [J]. HEREDITAS(Beijing), 2016, 38(4): 323-336. |
| [13] | Shanshan Yue,Laixin Xia. Identification of C(2)M interacting proteins by yeast two-hybrid screening [J]. HEREDITAS(Beijing), 2015, 37(11): 1160-1166. |
| [14] | ZHANG Bao-Le GAO Dian-Shuai XU Yin-Xue. G protein-coupled receptor 3: a key factor in the regulation of the nervous system and follicle development [J]. HEREDITAS, 2013, 35(5): 578-586. |
| [15] | XIE Wen-Jun, SHI Dian-Yi, CAI Ze-Xi, CHEN Xiao-Yang, JIN Wei-Wei. Organization, function and genetic controlling of synaptonemal complex [J]. HEREDITAS, 2012, 34(2): 167-176. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||