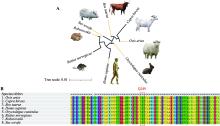
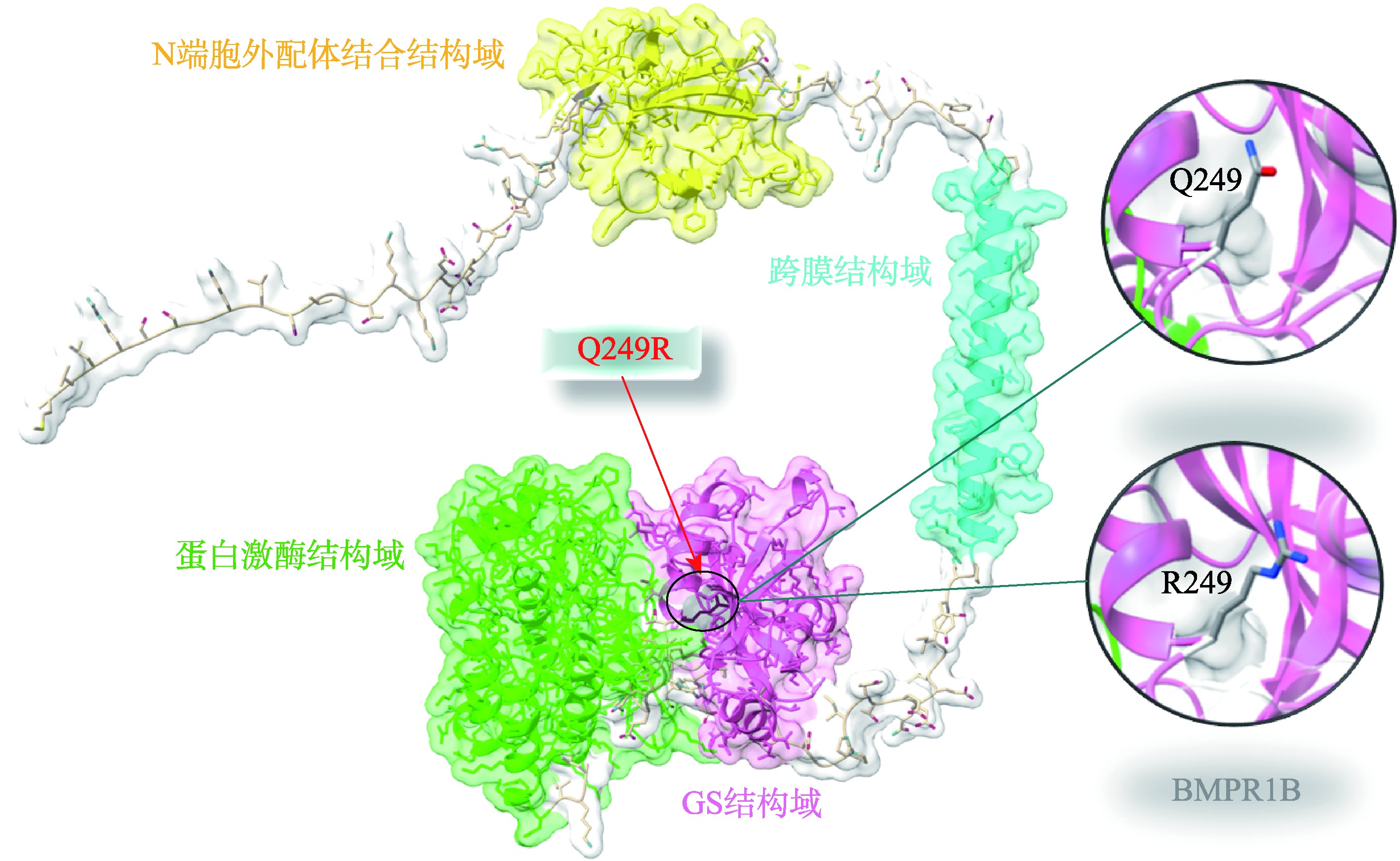
Hereditas(Beijing) ›› 2023, Vol. 45 ›› Issue (4): 295-305.doi: 10.16288/j.yczz.22-366
• Review • Previous Articles Next Articles
Progress on the effect of FecB mutation on BMPR1B activity and BMP/SMAD pathway in sheep
Yiming Gong( ), Xiangyu Wang, Xiaoyun He, Yufang Liu, Ping Yu, Mingxing Chu(
), Xiangyu Wang, Xiaoyun He, Yufang Liu, Ping Yu, Mingxing Chu( ), Ran Di(
), Ran Di( )
)
- Key Laboratory of Animal Genetics, Breeding and Reproduction of Ministry of Agriculture and Rural Affairs, Institute of Animal Science, Chinese Academy of Agricultural Sciences, Beijing 100193, China
-
Received:2022-11-17Revised:2023-02-06Online:2023-04-20Published:2023-03-28 -
Contact:Chu Mingxing,Di Ran E-mail:g18080010835@163.com;dirangirl@163.com;mxchu@263.net -
Supported by:National Natural Science Foundation of China(32272838);National Natural Science Foundation of China(31861143012);Earmarked Fund for China Agriculture Research System of MOF and MARA(CARS-38);Agricultural Science and Technology Innovation Program of China(CAAS-ZDRW202106);Agricultural Science and Technology Innovation Program of China(ASTIP-IAS13)
Cite this article
Yiming Gong, Xiangyu Wang, Xiaoyun He, Yufang Liu, Ping Yu, Mingxing Chu, Ran Di. Progress on the effect of FecB mutation on BMPR1B activity and BMP/SMAD pathway in sheep[J]. Hereditas(Beijing), 2023, 45(4): 295-305.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
| [1] | Piper LR, Bindon BM. The Booroola Merino and the performance of medium non-Peppin crosses at Armidale. Wool Technol Sheep Breed, 1983, 31(1): 14-19, 33. |
| [2] | Davis GH, Kelly RW. Segregation of a major gene influencing ovulation rate in progeny of Booroola sheep in commercial and research flocks. Proc N Z Soc Anim Prod, 1983, 43: 197-200. |
| [3] | Piper LR, Bindon BM, Davis GH. The single gene inheritance of the high litter size of the Booroola Merino. London: Butterworths, 1985, 115-125. |
| [4] | Singh RV, Sivakumar A, Sivashankar S, Das G. Evaluation of the Booroola (FecB) gene in Muzaffarnagari sheep. ACIAR Proc, 2009, 133: 223-224. |
| [5] |
Montgomery GW, Lord EA, Penty JM, Dodds KG, Broad TE, Cambridge L, Sunden SL, Stone RT, Crawford AM.The Booroola fecundity (FecB) gene maps to sheep chromosome 6. Genomics, 1994, 22(1): 148-153.
pmid: 7959761 |
| [6] |
Souza CJ, MacDougall C, MacDougall C, Campbell BK, McNeilly AS, Baird DT. The Booroola (FecB) phenotype is associated with a mutation in the bone morphogenetic receptor type 1 B (BMPR1B) gene. J Endocrinol, 2001, 169(2): R1-R6.
doi: 10.1677/joe.0.169r001 pmid: 11312159 |
| [7] |
Wilson T, Wu XY, Juengel JL, Ross IK, Lumsden JM, Lord EA, Dodds KG, Walling GA, McEwan JC, O'Connell AR, McNatty KP, Montgomery GW. Highly prolific Booroola sheep have a mutation in the intracellular kinase domain of bone morphogenetic protein IB receptor (ALK-6) that is expressed in both oocytes and granulosa cells. Biol Reprod, 2001, 64(4): 1225-1235.
doi: 10.1095/biolreprod64.4.1225 pmid: 11259271 |
| [8] |
Mulsant P, Lecerf F, Fabre S, Schibler L, Monget P, Lanneluc I, Pisselet C, Riquet J, Monniaux D, Callebaut I, Cribiu E, Thimonier J, Teyssier J, Bodin L, Cognié Y, Chitour N, Elsen JM. Mutation in bone morphogenetic protein receptor-IB is associated with increased ovulation rate in Booroola Mérino ewes. Proc Natl Acad Sci USA, 2001, 98(9): 5104-5109.
doi: 10.1073/pnas.091577598 pmid: 11320249 |
| [9] | Davis GH. The Booroola gene: origin, distribution, use and management of the FecB mutation. ACIAR Proc, 2009, 133: 22-31. |
| [10] | Walkden-Brown SW, Werf JHJ van der, Nimbkar C, Gupta VS.Proceedings of the Helen Newton Turner Memorial International Workshop, Pune, Maharashtra, India, 10-12 November, 2008. ACIAR Proc, 2009, 133: 238. |
| [11] |
Chong YQ, Jiang XP, Liu GQ. An ancient positively selected BMPRIB missense variant increases litter size of Mongolian sheep populations following latitudinal gradient. Mol Genet Genomics, 2022, 297(1): 155-167.
doi: 10.1007/s00438-021-01828-4 pmid: 35013854 |
| [12] |
Persani L, Rossetti R, Di Pasquale E, Cacciatore C, Fabre S. The fundamental role of bone morphogenetic protein 15 in ovarian function and its involvement in female fertility disorders. Hum Reprod Update, 2014, 20(6): 869-883.
doi: 10.1093/humupd/dmu036 pmid: 24980253 |
| [13] |
Chang HM, Qiao J, Leung PCK. Oocyte-somatic cell interactions in the human ovary-novel role of bone morphogenetic proteins and growth differentiation factors. Hum Reprod Update, 2016, 23(1): 1-18.
doi: 10.1093/humupd/dmw039 |
| [14] |
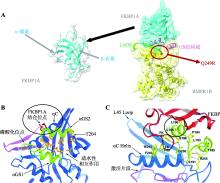
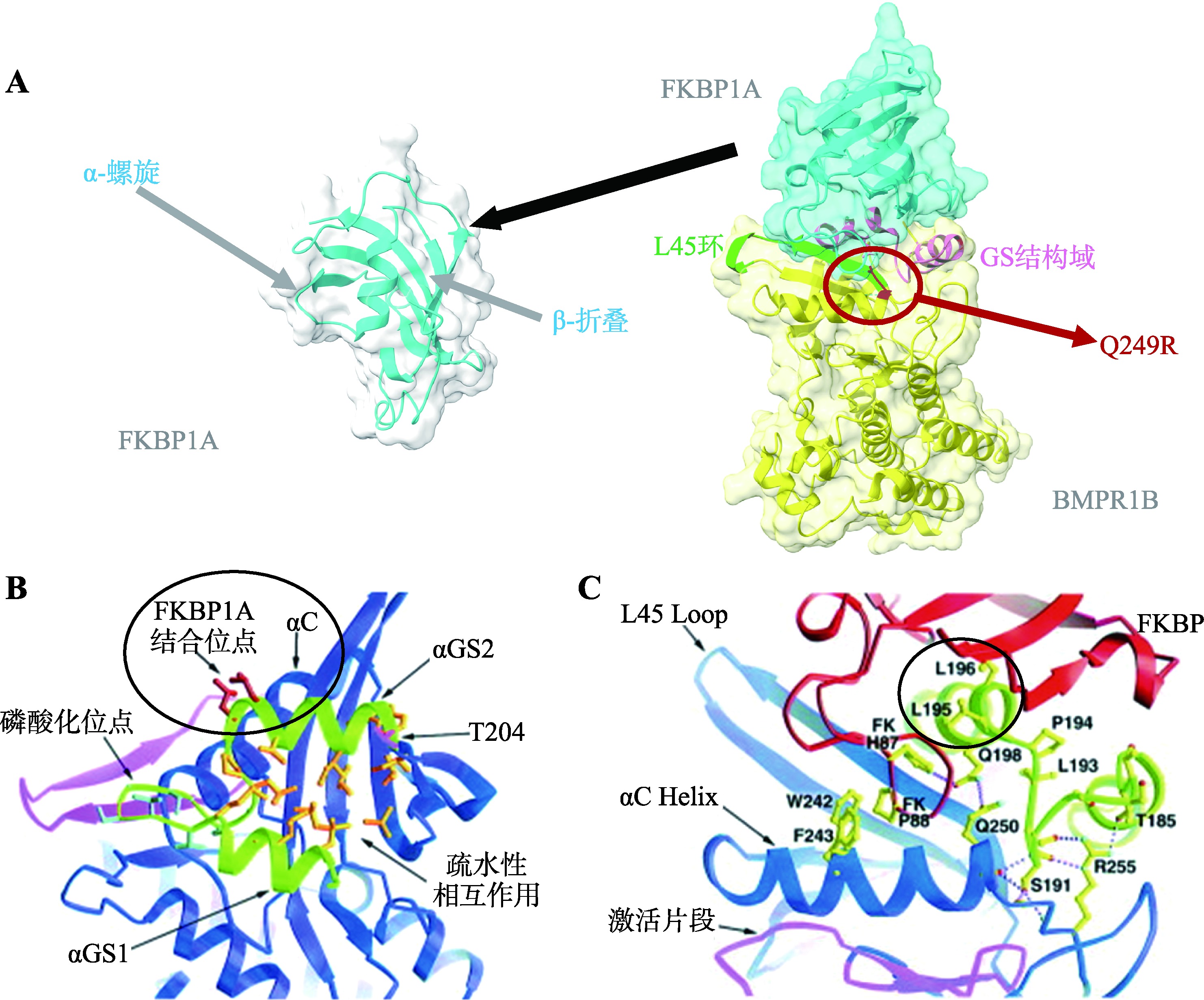
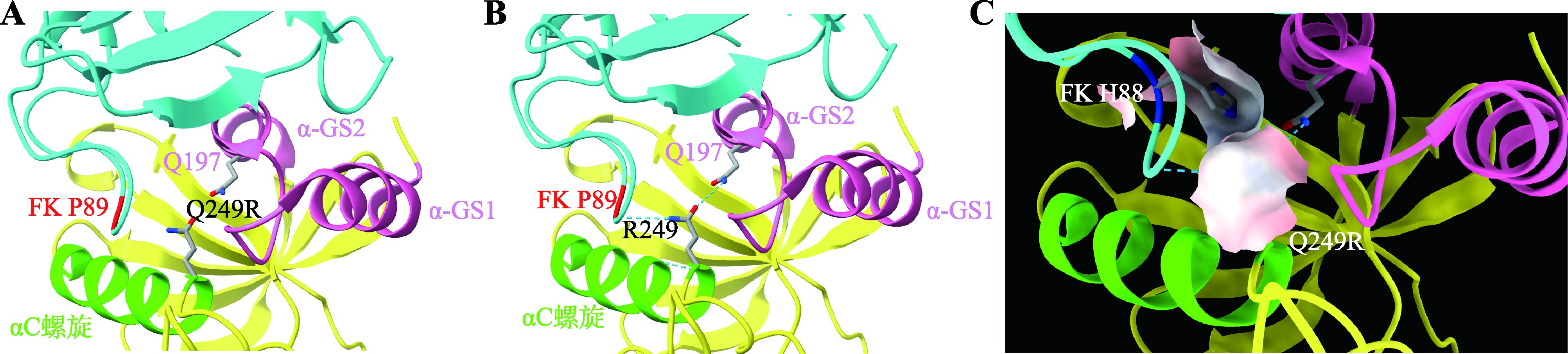
Huse M, Chen YG, Massagué J, Kuriyan J.Crystal structure of the cytoplasmic domain of the type I TGF beta receptor in complex with FKBP12. Cell, 1999, 96(3): 425-436.
doi: 10.1016/s0092-8674(00)80555-3 pmid: 10025408 |
| [15] |
Akhatayeva Z, Bi Y, He YW, Khan R, Li J, Li HX, Pan CY, Lan XY. Survey of the relationship between polymerphisms within the BMPR1B gene and sheep reproductive traits. Anim Biotechnol, 2021, 29: 1-10.
doi: 10.1080/10495398.2016.1276926 |
| [16] |
Luong HTT, Chaplin J, McRae AF, Medland SE, Willemsen G, Nyholt DR, Henders AK, Hoekstra C, Duffy DL, Martin NG, Boomsma DI, Montgomery GW, Painter JN. Variation in BMPR1B, TGFRB1 and BMPR2 and control of dizygotic twinning. Twin Res Hum Genet, 2011, 14(5): 408-416.
doi: 10.1375/twin.14.5.408 pmid: 21962132 |
| [17] | Renault L, Patiño LC, Magnin F, Delemer B, Young J, Laissue P, Binart N, Beau I. BMPR1A and BMPR1B missense mutations cause primary ovarian insufficiency. J Clin Endocrinol Metab, 2020, 105(4): dgz226. |
| [18] |
Sun XJ, Mei SQ, Tao H, Wang GD, Su LN, Jiang SW, Deng CY, Xiong YZ, Li FG. Microarray profiling for differential gene expression in PMSG-hCG stimulated preovulatory ovarian follicles of Chinese Taihu and Large White sows. BMC Genomics, 2011, 12: 111.
doi: 10.1186/1471-2164-12-111 pmid: 21324170 |
| [19] |
Haas CS, Oliveira FC, Rovani MT, Ferst JG, Vargas SF, Vieira AD, Mondadori RG, Pegoraro LMC, Gonçalves PBD, Bordignon V, Ferreira R, Gasperin BG. Bone morphogenetic protein 15 intrafollicular injection inhibits ovulation in cattle. Theriogenology, 2022, 182: 148-154.
doi: 10.1016/j.theriogenology.2022.02.010 pmid: 35176680 |
| [20] |
Yang CX, Zi XD, Wang Y, Yang DQ, Ma L, Lu JY, Niu HR, Xiao X. Cloning and mRNA expression levels of GDF9, BMP15, and BMPR1B genes in prolific and non-prolific goat breeds. Mol Reprod Dev, 2012, 79(1): 2.
doi: 10.1002/mrd.21386 |
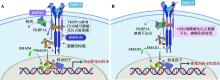
| [21] |
Kumar S, Stecher G, Li M, Knyaz C, Tamura K. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol, 2018, 35(6): 1547-1549.
doi: 10.1093/molbev/msy096 pmid: 29722887 |
| [22] |
Wen YL, Guo XF, Ma L, Zhang XS, Zhang JL, Zhao SG, Chu MX. The expression and mutation of BMPR1B and its association with litter size in small-tail Han sheep (Ovis aries). Arch Anim Breed, 2021, 64(1): 211-221.
doi: 10.5194/aab-64-211-2021 |
| [23] |
Zhang XY, Zhang LP, Sun WB, Lang X, Wu JP, Zhu CY, Jia JL, Jin JP, La Y, Casper DP. Study on the correlation between BMPR1B protein in sheep blood and reproductive performance. J Anim Sci, 2020, 98(5): skaa100.
doi: 10.1093/jas/skaa100 |
| [24] |
Jiang Y, Xie M, Chen WB, Talbot R, Maddox JF, Faraut T, Wu CH, Muzny DM, Li YX, Zhang WG, Stanton JA, Brauning R, Barris WC, Hourlier T, Aken BL, Searle SMJ, Adelson DL, Bian C, Cam GR, Chen YL, Cheng SF, DeSilva U, Dixen K, Dong Y, Fan GY, Franklin IR, Fu SY, Guan R, Highland MA, Holder ME, Huang GD, Ingham AB, Jhangiani SN, Kalra D, Kovar CL, Lee SL, Liu WQ, Liu X, Lu CX, Lv T, Mathew T, McWilliam S, Menzies M, Pan SK, Robelin D, Servin B, Townley D, Wang WL, Wei B, White SN, Yang XH, Ye C, Yue YJ, Zeng P, Zhou Q, Hansen JB, Kristensen K, Gibbs RA, Flicek P, Warkup CC, Jones HE, Oddy VH, Nicholas FW, McEwan JC, Kijas J, Wang J, Worley KC, Archibald AL, Cockett N, Xu X, Wang W, Dalrymple BP. The sheep genome illuminates biology of the rumen and lipid metabolism. Science, 2014, 344(6188): 1168-1173.
doi: 10.1126/science.1252806 pmid: 24904168 |
| [25] |
Sanfins A, Rodrigues P, Albertini DF. GDF-9 and BMP-15 direct the follicle symphony. J Assist Reprod Genet, 2018, 35(10): 1741-1750.
doi: 10.1007/s10815-018-1268-4 |
| [26] |
Paradis F, Novak S, Murdoch GK, Dyck MK, Dixon WT, Foxcroft GR. Temporal regulation of BMP2, BMP6, BMP15, GDF9, BMPR1A, BMPR1B, BMPR2 and TGFBR1 mRNA expression in the oocyte, granulosa and theca cells of developing preovulatory follicles in the pig. Reproduction, 2009, 138(1): 115-129.
doi: 10.1530/REP-08-0538 pmid: 19359354 |
| [27] | Pan ZY, Di R, Liu QY, Hu WP, Wang XY, Guo XF, Chu MX. Bioinformatics analysis on BMPR1B gene of sheep. China Herbivore Sci, 2015, 35(6): 1-5. |
| 潘章源, 狄冉, 刘秋月, 胡文萍, 王翔宇, 郭晓飞, 储明星. 绵羊BMPR1B基因生物信息学分析. 中国草食动物科学, 2015, 35(6): 1-5. | |
| [28] |
Deeds EJ, Shakhnovich EI. A structure-centric view of protein evolution, design, and adaptation. Adv Enzymol Relat Areas Mol Biol, 2007, 75: 133-191, xi-xii.
pmid: 17124867 |
| [29] |
Hess GP, Rupley JA. Structure and function of proteins. Annu Rev Biochem, 1971, 40: 1013-1044.
pmid: 4333476 |
| [30] |
Abdoli R, Zamani P, Deljou A, Rezvan H. Association of BMPR-1B and GDF9 genes polymorphisms and secondary protein structure changes with reproduction traits in Mehraban ewes. Gene, 2013, 524(2): 296-303.
doi: 10.1016/j.gene.2013.03.133 pmid: 23583795 |
| [31] |
Liu JY, Du X, Zhou JL, Pan ZX, Liu HL, Li QF.MicroRNA-26b functions as a proapoptotic factor in porcine follicular Granulosa cells by targeting Sma-and Mad-related protein 4. Biol Reprod, 2014, 91(6): 146.
doi: 10.1095/biolreprod.114.122788 pmid: 25395673 |
| [32] |
Wrana JL, Attisano L, Wieser R, Ventura F, Massagué J. Mechanism of activation of the TGF-beta receptor. Nature, 1994, 370(6488): 341-347.
doi: 10.1038/370341a0 |
| [33] |
Attisano L, Wrana JL. Signal transduction by members of the transforming growth factor-beta superfamily. Cytokine Growth Factor Rev, 1996, 7(4): 327-339.
doi: 10.1016/S1359-6101(96)00042-1 |
| [34] |
Massagué J, Blain SW, Lo RS. TGFbeta signaling in growth control, cancer, and heritable disorders. Cell, 2000, 103(2): 295-309.
doi: 10.1016/s0092-8674(00)00121-5 pmid: 11057902 |
| [35] |
Galat A. Functional diversity and pharmacological profiles of the FKBPs and their complexes with small natural ligands. Cell Mol Life Sci, 2013, 70(18): 3243-3275.
doi: 10.1007/s00018-012-1206-z pmid: 23224428 |
| [36] |
Standaert RF, Galat A, Verdine GL, Schreiber SL. Molecular cloning and overexpression of the human FK506-binding protein FKBP. Nature, 1990, 346(6285): 671-674.
doi: 10.1038/346671a0 |
| [37] |
Xing MY, Wang J, Yang Q, Wang Y, Li JS, Xiong J, Zhou S. FKBP 12 is a predictive biomarker for efficacy of anthracycline-based chemotherapy in breast cancer. Cancer Chemother Pharmacol, 2019, 84(4): 861-872.
doi: 10.1007/s00280-019-03923-1 |
| [38] |
Michnick SW, Rosen MK, Wandless TJ, Karplus M, Schreiber SL. Solution structure of FKBP, a rotamase enzyme and receptor for FK506 and rapamycin. Science, 1991, 252(5007): 836-839.
pmid: 1709301 |
| [39] |
Moore JM, Peattie DA, Fitzgibbon MJ, Thomson JA.Solution structure of the major binding protein for the immunosuppressant FK506. Nature, 1991, 351(6323): 248-250.
doi: 10.1038/351248a0 |
| [40] |
Van Duyne GD, Standaert RF, Karplus PA, Schreiber SL, Clardy J. Atomic structure of FKBP-FK506, an immunophilin-immunosuppressant complex. Science, 1991, 252(5007): 839-842.
pmid: 1709302 |
| [41] |
Van Duyne GD, Standaert RF, Karplus PA, Schreiber SL, Clardy J. Atomic structures of the human immunophilin FKBP-12 complexes with FK506 and rapamycin. J Mol Biol, 1993, 229(1): 105-124.
doi: 10.1006/jmbi.1993.1012 pmid: 7678431 |
| [42] |
Chaikuad A, Bullock AN. Structural basis of intracellular TGF-β signaling: receptors and Smads. Cold Spring Harb Perspect Biol, 2016, 8(11): a022111.
doi: 10.1101/cshperspect.a022111 |
| [43] |
Aghdasi B, Ye K, Resnick A, Huang A, Ha HC, Guo X, Dawson TM, Dawson VL, Snyder SH. FKBP12, the 12-kDa FK506-binding protein, is a physiologic regulator of the cell cycle. Proc Natl Acad Sci USA, 2001, 98(5): 2425-2430.
pmid: 11226255 |
| [44] |
Van Acker K, Bultynck G, Rossi D, Sorrentino V, Boens N, Missiaen L, De Smedt H, Parys JB, Callewaert G. The 12 kDa FK506-binding protein, FKBP12, modulates the Ca(2+)-flux properties of the type-3 ryanodine receptor. J Cell Sci, 2004, 117(Pt 7): 1129-1137.
pmid: 14970260 |
| [45] |
Yamaguchi T, Kurisaki A, Yamakawa N, Minakuchi K, Sugino H. FKBP12 functions as an adaptor of the Smad7-Smurf1 complex on activin type I receptor. J Mol Endocrinol, 2006, 36(3): 569-579.
pmid: 16720724 |
| [46] |
Miyakawa AA, Girão-Silva T, Krieger JE, Edelman ER. Rapamycin activates TGF receptor independently of its ligand: implications for endothelial dysfunction. Clin Sci (Lond), 2018, 132(4): 437-447.
doi: 10.1042/CS20171457 pmid: 29343616 |
| [47] |
Wojciech S, Ahmad R, Belaid-Choucair Z, Journé AS, Gallet S, Dam J, Daulat A, Ndiaye-Lobry D, Lahuna O, Karamitri A, Guillaume JL, Do Cruzeiro M, Guillonneau F, Saade A, Clément N, Courivaud T, Kaabi N, Tadagaki K, Delagrange P, Prévot V, Hermine O, Prunier C, Jockers R. The orphan GPR50 receptor promotes constitutive TGFβ receptor signaling and protects against cancer development. Nat Commun, 2018, 9(1): 1216.
doi: 10.1038/s41467-018-03609-x pmid: 29572483 |
| [48] |
Wang TW, Donahoe PK. The immunophilin FKBP12: a molecular guardian of the TGF-beta family type I receptors. Front Biosci, 2004, 9: 619-631.
doi: 10.2741/1095 |
| [49] |
Otsuka F, Moore RK, Iemura S, Ueno N, Shimasaki S.Follistatin inhibits the function of the oocyte-derived factor BMP-15. Biochem Biophys Res Commun, 2001, 289(5): 961-966.
doi: 10.1006/bbrc.2001.6103 |
| [50] |
Erickson GF, Shimasaki S. The spatiotemporal expression pattern of the bone morphogenetic protein family in rat ovary cell types during the estrous cycle. Reprod Biol Endocrinol, 2003, 1: 9.
doi: 10.1186/1477-7827-1-9 |
| [51] |
Lee WS, Otsuka F, Moore RK, Shimasaki S. Effect of bone morphogenetic protein-7 on folliculogenesis and ovulation in the rat. Biol Reprod, 2001, 65(4): 994-999.
doi: 10.1095/biolreprod65.4.994 pmid: 11566718 |
| [52] |
Otsuka F, Moore RK, Shimasaki S. Biological function and cellular mechanism of bone morphogenetic protein-6 in the ovary. J Biol Chem, 2001, 276(35): 32889-32895.
doi: 10.1074/jbc.M103212200 pmid: 11447221 |
| [53] |
Kaivo-oja N, Jeffery LA, Ritvos O, Mottershead DG. Smad signalling in the ovary. Reprod Biol Endocrinol, 2006, 4: 21.
doi: 10.1186/1477-7827-4-21 |
| [54] |
Weiss A, Attisano L. The TGFbeta superfamily signaling pathway. Wiley Interdiscip Rev Dev Biol, 2013, 2(1): 47-63.
doi: 10.1002/wdev.86 |
| [55] |
Saneyasu T, Honda K, Kamisoyama H. Myostatin increases Smad2 phosphorylation and atrogin-1 expression in chick embryonic myotubes. J Poult Sci, 2019, 56(3): 224-230.
doi: 10.2141/jpsa.0180092 pmid: 32055218 |
| [56] |
Li QL. Inhibitory SMADs: potential regulators of ovarian function. Biol Reprod, 2015, 92(2): 50.
doi: 10.1095/biolreprod.114.125203 pmid: 25550343 |
| [57] |
Yao YL, Reheman A, Xu YF, Li QF. miR-125b contributes to ovarian granulosa cell apoptosis through targeting BMPR1B, a major gene for sheep prolificacy. Reprod Sci, 2019, 26(2): 295-305.
doi: 10.1177/1933719118770544 pmid: 29661099 |
| [58] |
Shimizu T, Kayamori T, Murayama C, Miyamoto A. Bone morphogenetic protein (BMP)-4 and BMP-7 suppress granulosa cell apoptosis via different pathways: BMP-4 via PI3K/PDK-1/Akt and BMP-7 via PI3K/PDK-1/PKC. Biochem Biophys Res Commun, 2012, 417(2): 869-873.
doi: 10.1016/j.bbrc.2011.12.064 |
| [59] |
Wijayanti D, Zhang SH, Yang YT, Bai YY, Akhatayeva Z, Pan CY, Zhu HJ, Qu L, Lan XY. Goat SMAD family member 1 (SMAD1): mRNA expression, genetic variants, and their associations with litter size. Theriogenology, 2022, 193: 11-19.
doi: 10.1016/j.theriogenology.2022.09.001 pmid: 36116245 |
| [60] |
Xu SS, Gao L, Xie XL, Ren YL, Shen ZQ, Wang F, Shen M, Eyϸórsdóttir E, Hallsson JH, Kiseleva T, Kantanen J, Li MH. Genome-wide association analyses highlight the potential for different genetic mechanisms for litter size among sheep breeds. Front Genet, 2018, 9: 118.
doi: 10.3389/fgene.2018.00118 |
| [61] |
Wu J, Chen X, Sehgal P, Zhang TW, Jackson-Weaver O, Gou YC, Bautch V, Frenkel B, Sun HC, Xu J. Arginine methylation of R81 in Smad6 confines BMP-induced Smad1 signaling. J Biol Chem, 2021, 296: 100496.
doi: 10.1016/j.jbc.2021.100496 |
| [62] |
Abdurahman A, Du X, Yao YL, Sulaiman Y, Aniwashi J, Li QF. Smad4 feedback enhances BMPR1B transcription in ovine granulosa cells. Int J Mol Sci, 2019, 20(11): 2732.
doi: 10.3390/ijms20112732 |
| [63] |
Miyazawa K, Miyazono K. Regulation of TGF-β family signaling by inhibitory Smads. Cold Spring Harb Perspect Biol, 2017, 9(3): a022095.
doi: 10.1101/cshperspect.a022095 |
| [64] |
Wang GL, Cai J, Zhang JS, Li CY. Mechanism of triptolide in treating ankylosing spondylitis through the anti‑ossification effect of the BMP/Smad signaling pathway. Mol Med Rep, 2018, 17(2): 2731-2737.
doi: 10.3892/mmr.2017.8117 pmid: 29207198 |
| [65] |
Goto K, Kamiya Y, Imamura T, Miyazono K, Miyazawa K. Selective inhibitory effects of Smad6 on bone morphogenetic protein type I receptors. J Biol Chem, 2007, 282(28): 20603-20611.
doi: 10.1074/jbc.M702100200 pmid: 17493940 |
| [66] |
Xu J, Wang AH, Oses-Prieto J, Makhijani K, Katsuno Y, Pei M, Yan LL, Zheng YG, Burlingame A, Brückner K, Derynck R. Arginine methylation initiates BMP-induced Smad signaling. Mol Cell, 2013, 51(1): 5-19.
doi: 10.1016/j.molcel.2013.05.004 pmid: 23747011 |
| [67] |
Hata A, Lagna G, Massagué J, Hemmati-Brivanlou A. Smad 6 inhibits BMP/Smad1 signaling by specifically competing with the Smad4 tumor suppressor. Genes Dev, 1998, 12(2): 186-197.
doi: 10.1101/gad.12.2.186 |
| [68] |
Bai S, Shi X, Yang X, Cao X. Smad 6 as a transcriptional corepressor. J Biol Chem, 2000, 275(12): 8267-8270.
doi: 10.1074/jbc.275.12.8267 pmid: 10722652 |
| [69] |
Lin X, Liang YY, Sun BH, Liang M, Shi YJ, Brunicardi FC, Shi Y, Feng XH. Smad 6 recruits transcription corepressor CtBP to repress bone morphogenetic protein- induced transcription. Mol Cell Biol, 2003, 23(24): 9081-9093.
doi: 10.1128/MCB.23.24.9081-9093.2003 |
| [70] |
Bahire SV, Rajput PK, Kumar V, Kumar D, Kataria M, Kumar S. Quantitative expression of mRNA encoding BMP/SMAD signalling genes in the ovaries of Booroola carrier and non-carrier GMM sheep. Reprod Domest Anim, 2019, 54(10): 1375-1383.
doi: 10.1111/rda.13535 pmid: 31356698 |
| [71] |
Abdurahman A, Aierken W, Zhang F, Obulkasim R, Aniwashi J, Sulayman A. miR-1306 induces cell apoptosis by targeting BMPR1B gene in the ovine granulosa cells. Front Genet, 2022, 13: 989912.
doi: 10.3389/fgene.2022.989912 |
| [72] |
Feary ES, Juengel JL, Smith P, French MC, O'Connell AR, Lawrence SB, Galloway SM, Davis GH, McNatty KP. Patterns of expression of messenger RNAs encoding GDF9, BMP15, TGFBR1, BMPR1B, and BMPR2 during follicular development and characterization of ovarian follicular populations in ewes carrying the Woodlands FecX2W mutation. Biol Reprod, 2007, 77(6): 990-998.
doi: 10.1095/biolreprod.107.062752 pmid: 17715428 |
| [73] |
Broege A, Pham L, Jensen ED, Emery A, Huang TH, Stemig M, Beppu H, Petryk A, O'Connor M, Mansky K, Gopalakrishnan R. Bone morphogenetic proteins signal via SMAD and mitogen-activated protein (MAP) kinase pathways at distinct times during osteoclastogenesis. J Biol Chem, 2013, 288(52): 37230-37240.
doi: 10.1074/jbc.M113.496950 pmid: 24235143 |
| [74] |
Zanotti S, Smerdel-Ramoya A, Stadmeyer L, Canalis E.Activation of the ERK pathway in osteoblastic cells, role of gremlin and BMP-2. J Cell Biochem, 2008, 104(4): 1421-1426.
doi: 10.1002/jcb.21715 pmid: 18286547 |
| [75] |
Chan MC, Nguyen PH, Davis BN, Ohoka N, Hayashi H, Du KY, Lagna G, Hata A. A novel regulatory mechanism of the bone morphogenetic protein (BMP) signaling pathway involving the carboxyl-terminal tail domain of BMP type II receptor. Mol Cell Biol, 2007, 27(16): 5776-5789.
doi: 10.1128/MCB.00218-07 pmid: 17576816 |
| [76] |
Gomez-Puerto MC, Iyengar PV, de Vinuesa AG, Ten Dijke P, Sanchez-Duffhues G. Bone morphogenetic protein receptor signal transduction in human disease. J Pathol, 2019, 247(1): 9-20.
doi: 10.1002/path.5170 |
| [77] |
Derynck R, Zhang YE. Smad-dependent and Smad- independent pathways in TGF-beta family signalling. Nature, 2003, 425(6958): 577-584.
doi: 10.1038/nature02006 |
| [1] | Haitao Wang, Tingting Li, Xun Huang, Runlin Ma, Qiuyue Liu. Application of genetic modification technologies in molecular design breeding of sheep [J]. Hereditas(Beijing), 2021, 43(6): 580-600. |
| [2] | Xiaohong He, Lin Jiang, Yabin Pu, Qianjun Zhao, Yuehui Ma. Progress on genetic mapping and genetic mechanism of cattle and sheep horns [J]. Hereditas(Beijing), 2021, 43(1): 40-51. |
| [3] | Bingyuan Wang, Yulian Mu, Kui Li, Zhiguo Liu. Research progress of stem cells in agricultural animals [J]. Hereditas(Beijing), 2020, 42(11): 1073-1080. |
| [4] | Zhida Zhao,Li Zhang. Applications of genome selection in sheep breeding [J]. Hereditas(Beijing), 2019, 41(4): 293-303. |
| [5] | Qing Xia, Qiuyue Liu, Xiangyu Wang, Wenping Hu, Chunyan Li, Xiaoyun He, Mingxing Chu, Ran Di. The molecular mechanism of sheep seasonal breeding and artificial regulatory techniques for estrus and mating in anestrus [J]. Hereditas(Beijing), 2018, 40(5): 369-377. |
| [6] | Tongyu Zhang,Caiye Zhu,Lixin Du,Fuping Zhao. Advances in genome-wide association studies for important traits in sheep and goats [J]. Hereditas(Beijing), 2017, 39(6): 491-500. |
| [7] | Zhao Yongxin, Li Menghua. Research advances on the origin, evolution and genetic diversity of Chinese native sheep breeds [J]. Hereditas(Beijing), 2017, 39(11): 958-973. |
| [8] | Wei Wang, Yushuang Wang, Lanlan Huang, Zijian Jian, Xinhua Wang, Shouren Liu, Wenhui Pi. Increasing the efficiency of homologous recombination vector-mediated end joining repair by inhibition of Lig4 gene using siRNA in sheep embryo fibroblasts [J]. Hereditas(Beijing), 2016, 38(9): 831-839. |
| [9] | Tianzhi Chen, Bingling Zhao, Yu Liu, Yuanyuan Zhao, Haidong Wang, Ruiwen Fan, Pengchao Wang, Changsheng Dong. Expression and localization of GPR143 in sheep skin [J]. HEREDITAS(Beijing), 2016, 38(7): 658-665. |
| [10] | Lei Gao,Min Shen,Shangquan Gan,Jingquan Yang,Yiyuan Zhang. Molecular cloning and tissue expression of the CCNG1 gene in sheep [J]. HEREDITAS(Beijing), 2015, 37(4): 374-381. |
| [11] | Ruixia Xu,Lei Gao,Weili Zhao,Wei Zhang,Guangchao Song,Shangquan Gan,Guoqing Shi. Analysis of FABP4 expression pattern in rump fat deposition and metabolism of Altay sheep [J]. HEREDITAS(Beijing), 2015, 37(2): 174-182. |
| [12] | WANG Chun-Ling MENG Chen-Ling CAO Shao-Xian ZHANG Jun MENG Chun-Hua WANG Hui-Li FANG Yong-Fei ZHU Dong-Dong MAO Da-Gan. Single nuclear polymorphisms in exon 3 of POMC gene and the asso-ciation with growth traits in Hu sheep and East Friesian × Hu crossbred sheep [J]. HEREDITAS, 2013, 35(9): 1095-1100. |
| [13] | ZHANG Wei, SHEN Min, LI Huan, GAO Lei, LIANG Yao-Wei, YANG Jing-Quan, LIU Shou-Ren, WANG Xin-Hua, GAN Shang-Quan. Detection and analysis of polymorphisms of 59571364 and 59912586 loci on X chromosome in fat-tail and thin-tail sheep flocks [J]. HEREDITAS, 2013, 35(12): 1384-1390. |
| [14] | GAN Shang-Quan ZHANG Wei SHEN Min LI Huan YANG Jing-Quan LIANG Yao-Wei GAO Lei LIU Shou-Ren WANG Xin-Hua. Correlation analysis between polymorphism of the 59383635th locus on X chromosome and fat-tail trait in sheep [J]. HEREDITAS, 2013, 35(10): 1209-1216. |
| [15] | GAO Lei GAN Shang-Quan YANG Jin-Quan YANG Jian-Bo LIANG Yao-Wei Abdulla Aini·Nula Hong SHEN Min. Relative quantification of mRNA transcription of Cry1 in dif-ferent tissues of sheep in oestrous cycle by real-time quantitative PCR [J]. HEREDITAS, 2013, 35(1): 85-92. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||