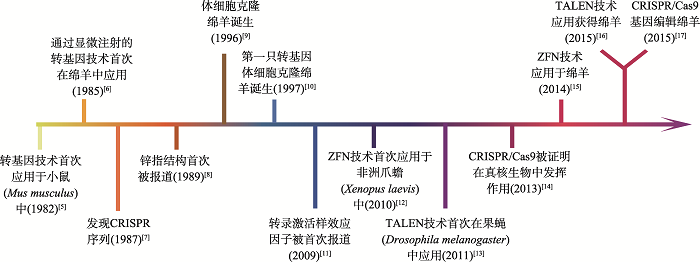
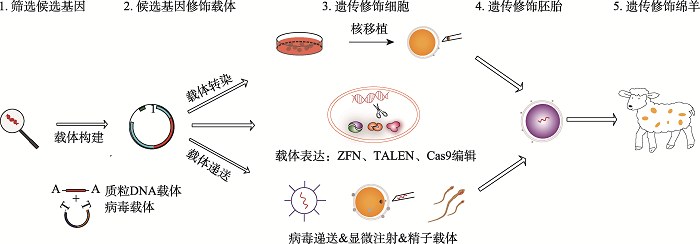
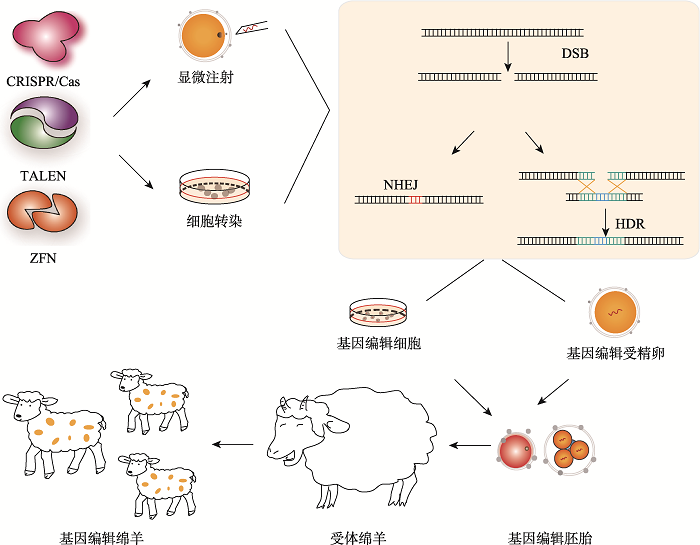
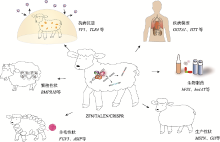
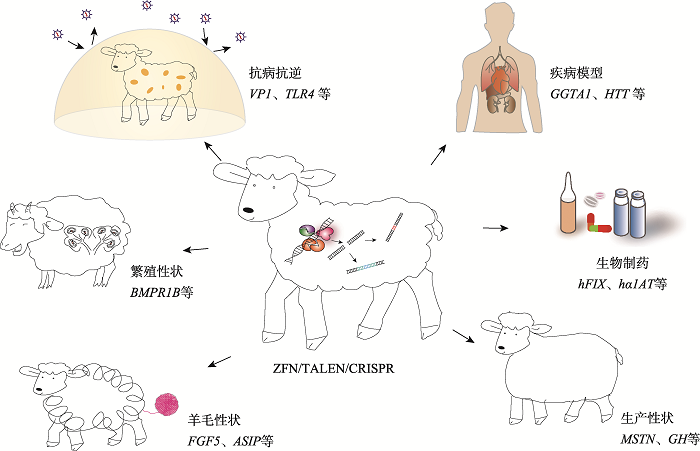
Hereditas(Beijing) ›› 2021, Vol. 43 ›› Issue (6): 580-600.doi: 10.16288/j.yczz.21-087
• Review • Previous Articles Next Articles
Application of genetic modification technologies in molecular design breeding of sheep
Haitao Wang1,2( ), Tingting Li3, Xun Huang1,2, Runlin Ma1,2,4(
), Tingting Li3, Xun Huang1,2, Runlin Ma1,2,4( ), Qiuyue Liu1(
), Qiuyue Liu1( )
)
- 1. Innovation Academy for Seed Design, Chinese Academy of Sciences, Beijing 100101, China
2. State Key Laboratory of Molecular Developmental Biology, Institute of Genetics and Developmental Biology, Chinese Academy of Sciences, Beijing 100101, China
3. Zhejiang A&F University, Hangzhou 311300, China;
4. University of Chinese Academy of Sciences, Beijing 100049, China
-
Received:2021-03-08Revised:2021-04-14Online:2021-06-20Published:2021-04-30 -
Contact:Ma Runlin,Liu Qiuyue E-mail:wanght@genetics.ac.cn;rlma@ucas.ac.cn;qyliu@genetics.ac.cn -
Supported by:Supported by the Strategic Priority Research Program of Chinese Academy of Sciences No(XDA24030205)
Cite this article
Haitao Wang, Tingting Li, Xun Huang, Runlin Ma, Qiuyue Liu. Application of genetic modification technologies in molecular design breeding of sheep[J]. Hereditas(Beijing), 2021, 43(6): 580-600.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
Table 1
Genetic modified sheep generated by microinjection and somatic cell nuclear transfer"
| 制备方法 | 基因名称 | 序列 | 编辑结果 | 表达情况 | 参考文献 |
|---|---|---|---|---|---|
| 显微注射 | hGH | GH cDNA | 随机整合 | 无 | [ |
| oGH (ovine growth hormone) | GH cDNA | 随机整合 | 表达 | [ | |
| vLRT/vvENV (virus long terminal repeat/visna virus envelope) | 病毒LTR区 | 随机整合 | 表达 | [ | |
| hGFR (human growth hormone-releasing factor)/ bGH (bovine growth hormone) | GH cDNA | 随机整合 | 无 | [ | |
| hGFR/bGH | minigene | 随机整合 | 表达 | [ | |
| hα1AT (human α1-antitrypsin) | minigene | 随机整合 | 表达 | [ | |
| hAF IX (human antihemophilic factor IX) | cDNA | 随机整合 | 表达 | [ | |
| hBCF VIII (human blood clotting factor VIII) | cDNA | 随机整合 | 表达 | [ | |
| CAT (chloramphenicol acetyl transferase) | 编码序列 | 随机整合 | 表达 | [ | |
| HTT (Huntington) | 人HD cDNA | 随机整合 | 表达 | [ | |
| IGF1 (insulin-like growth factors) | cDNA | 随机整合 | 表达 | [ | |
| oTLR4 (ovine Toll-like receptor 4) | cDNA | 随机整合 | 表达 | [ | |
| 体细胞 核移植 | hF IX (human factor IX) | 完整编码区 | 随机整合 | 无 | [ |
| oPRP和GGTA1 | cDNA | 定点敲除 | GGTA1和PRP敲除 | [ | |
| COL1A1位点插入AAT基因 | cDNA | 定点插入 | AAT表达 | [ | |
| oTLR4 | cDNA | 随机整合 | 表达 | [ | |
| Caenorhabditis elegans fat-1 | cDNA | 随机整合 | 被沉默 | [ |
Table 2
Studies on genetic modifications by using viral, sperm or transposon vectors in sheep"
| 修饰方法 | 基因名称 | 辅助方法 | 编辑结果 | 参考文献 |
|---|---|---|---|---|
| 精子载体 | SLC7A11 | 睾丸内注射 | 基因表达 | [ |
| PPARγ | 睾丸内注射 | 基因表达 | [ | |
| LPL | 睾丸内注射 | 基因表达 | [ | |
| eGFP | 慢病毒介导 | 基因表达 | [ | |
| 病毒载体 | eGFP | 显微注射 | 基因表达 | [ |
| FGF5 | 显微注射 | 异位表达 | [ | |
| GFP | 显微注射 | 基因表达 | [ | |
| tdTomato、zYellow和acGFP | 显微注射 | 基因表达 | [ | |
| 转座子载体 | Tn5和SB转座子 | 显微注射 | GFP表达 | [ |
| SB转座子 | RNAi | MSTN敲低 | [ |
Table 3
Application of gene editing mediated by nuclease techniques in sheep genetic modification"
| 人工核酸酶 | 基因名称 | 递送方法 | 编辑结果 | 参考文献 |
|---|---|---|---|---|
| ZFN | MSTN | 脂质体转染 | 基因敲除 | [ |
| TALEN | MSTN | 显微注射 | 基因敲除 | [ |
| MSTN | 核移植 | 基因敲除 | [ | |
| CRISPR/Cas9 | MSTN | 显微注射 | 基因敲除 | [ |
| MSTN、ASIP (agouti gene)和BCO2 (beta-carotene oxygenase 2β) | 显微注射 | 基因敲除 | [ | |
| TNSALP (tissue-nonspecific alkaline phosphatase) | 显微注射 | 基因敲除 | [ | |
| PDX1 (pancreatic and duodenal homeobox protein 1) | 显微注射 | 基因敲除 | [ | |
| GFP | 显微注射 | 定点敲入 | [ | |
| CFTR (cystic fibrosis transmembrane conductance regulator) | 核移植 | 基因敲除 | [ | |
| MSTN | 核移植 | 基因敲除 | [ | |
| SOCS2 (suppressor of cytokine signaling 2) | 显微注射 | 碱基突变 | [ | |
| ASIP | 显微注射 | 基因敲除 | [ | |
| BMPR1B (bone morphogenetic protein receptor type 1B) | 显微注射 | 基因敲除 | [ | |
| otoferlin | 显微注射 | 重组敲入 | [ | |
| FGF5 | 显微注射 | 基因敲除 | [ | |
| ASMT (acetylserotonin methyltransferase) | 显微注射 | 随机整合 | [ |
Table 4
Studies on crucial genes in genetically modified sheep"
| 性状类型 | 基因名称 | 基因类型 | 基因功能 | 位点变化 | 基因修饰绵羊(预期)表型 | 参考文献 |
|---|---|---|---|---|---|---|
| 生长性状 | GH | 外源基因 | 促进生长 | cDNA随机整合 | 生长加快 | [ |
| MSTN | 内源基因 | 抑制肌肉生长 | RNAi敲低或敲除 | 敲除后肌肉量增加 | [ | |
| SOCS2 | 内源基因 | 促进骨发育 | 单碱基突变 | 点突变体型增大,体重增加 | [ | |
| 羊毛纤维 | CAT | 外源基因 | 氯霉素抗性 | cDNA随机整合 | 羊毛产量 | [ |
| SLC7A11 | 内源基因 | 谷氨酸转移 | cDNA随机整合 | 毛色变化 | [ | |
| FGF5 | 内源基因 | 调节毛发生长 | 定点敲除(移码突变) | 敲除后毛产量增加 | [ | |
| ASIP | 内源基因 | 黑皮质素拮抗剂 | 定点敲除(移码突变) | 改变毛色 | [ | |
| IGF1 | 外源基因 | 生长调节 | cDNA随机整合 | 外源基因表达后毛产量增加 | [ | |
| 抗病抗逆 | vLRT/vvENV | 外源基因 | ENV蛋白 | 随机整合 | 产生抗体 | [ |
| TLR4 | 外源基因 | 炎症反应 | cDNA随机整合 | 增强抗病能力 | [ | |
| VP1 shRNA | 外源基因 | 口蹄疫病毒蛋白 | 随机整合 | 干扰口蹄疫病毒基因组 | [ | |
| 繁殖性状 | hα1AT | 外源基因 | 蛋白酶抑制剂 | Minigene随机整合 | 外源基因表达 | [ |
| hFIX | 外源基因 | 凝血 | cDNA随机整合 | 外源基因表达 | [ | |
| HTT | 外源基因 | 影响神经系统 | cDNA随机整合 | 亨廷顿舞蹈症 | [ | |
| PPARγ | 内源基因 | 脂肪沉积 | cDNA随机整合 | 增加肌间脂肪 | [ | |
| LPL | 内源基因 | 脂肪沉积 | cDNA随机整合 | 增加肌间脂肪 | [ | |
| TNSALP | 内源基因 | 降低碱性磷酸酶活性 | 单碱基突变 | 人HPP综合征模型 | [ | |
| PDX1 | 内源基因 | 胰腺发育 | 定点敲除(移码突变) | 敲除后胰腺缺失 | [ | |
| CFTR | 外源基因 | 膜信号转导调节 | 定点敲除(移码突变) | 囊性纤维化模型 | [ | |
| BMPR1B | 内源基因 | 卵泡发育 | 定点重组插入 | 增加产羔数 | [ | |
| otoferlin | 外源基因 | 听力形成 | 定点重组插入 | 耳聋模型 | [ | |
| ASMT | 外源基因 | 褪黑激素合成 | cDNA随机整合 | 过表达增加褪黑激素表达量 | [ | |
| 基因表达 | Rosa-26 | 友好位点 | 无 | 定点插入 | 外源基因插入后稳定表达 | [ |
Table 6
Rates of offspring generated by microinjection or SCNT in sheep, goats and pigs"
| 物种 | 方法 | 基因 | 遗传修饰类型 | 移植胚胎数/ 受体数/怀孕 | 基因编辑后代/ 所有后代 | 获得后代 比率(%) | 参考文献 |
|---|---|---|---|---|---|---|---|
| 绵羊 | 原核注射 | hGH | 随机整合 | 1032/192/- | 1/73 | 7.1 | [ |
| hFⅨ | 随机整合 | 67/24/11 | 6/7 | 10.4 | [ | ||
| oGH | 随机整合 | 408/109/39 | 4/44 | 10.7 | [ | ||
| bGH; hGHRF | 随机整合 | 842/98/33; 435/120/51 | 2/47; 9/63 | 5.6; 14.5 | [ | ||
| bGH; hGRF | 随机整合 | 247/-/42 | 11/42 | 17.0; 9.4 | [ | ||
| hα1AT | 随机整合 | 549/152/73 | 5/113 | 20.6 | [ | ||
| hBCFVIII | 随机整合 | 1145/454/276 | 13/434 | 37.9 | [ | ||
| CAT | 随机整合 | 371/76/- | 4/31 | 8.4 | [ | ||
| HTT | 随机整合 | 413/138/- | 6/150 | 36.3 | [ | ||
| MSTN | Cas9敲除 | 213/55/31 | 2/35 | 16.4 | [ | ||
| MSTN、ASIP和BCO2 | Cas9敲除 | 578/82/77 | 35/36 | 7.3 | [ | ||
| SOCS2 | BE3点突变 | 53/8/3 | 3/4 | 7.5 | [ | ||
| ASIP | Cas9敲除 | 92/60/6 | 5/6 | 6.5 | [ | ||
| BMPR1B | Cas9点突变 | 279/39/16 | 7/21 | 7.5 | [ | ||
| FGF5 | 基因敲除 | 100/53/14 | 3/18 | 18 | [ | ||
| FGF5 | 基因敲除 | 63/43/18 | 20/23 | 36.5 | [ | ||
| 徒手克隆 | fat-1 | 随机整合 | 53/29/4 | 3/3 | 5.7 | [ | |
| 体细胞 核移植 | AAT | 定点插入 | 80/42/20 | 13/14 | 17.5 | [ | |
| oTLR4 | 随机整合 | 859/113/12; 1009/108/7 | 9/9 | 0.7; 0.3 | [ | ||
| fat-1 | 随机整合 | 128/16/3; 147/20/3 | 5/5 | 1.6; 2.0 | [ | ||
| MSTN | TALEN敲除 | 70/5/1 | 1/1 | 1.4 | [ | ||
| MSTN | TALEN敲除 | 282/37/28 | 13/23 | 1.4 | [ | ||
| 山羊 | 原核注射 | MSTN | Cas9敲除 | 416/137/64 | 26/93 | 22.4 | [ |
| MSTN | Cas9敲除 | 18/5/3 | 1/4 | 22.2 | [ | ||
| FGF5 | BE3点突变 | 22/7/3 | 5/5 | 22.7 | [ | ||
| BLG (β-lactoglobulin) | Cas9敲除 | 103/67/18 | 4 | 25.2 | [ | ||
| GDF9 | Cas9敲除 | 56/17/13 | 6/8 | 32.1 | [ | ||
| 山羊 | 原核注射 | MSTN | TALEN敲除 | 403/29/7 | 3/3 | 0.7 | [ |
| MSTN | Cas9敲除 | 269/21/7 | 3/3 | 2.2 | [ | ||
| EDAR (ectodysplasin receptor) | Cas9敲除 | 257/79/5 | 2/6 | 2.3 | [ | ||
| 猪 | 体细胞 核移植 | - | 纯体细胞克隆 | 586/10/2 | 0/7 | 1.2 | [ |
| LDLR (low-density lipoprotein receptor) | 敲除 | 2276/18/9 | 57/57 | 2.5 | [ | ||
| PPARγ | ZFN敲除 | 1134/8/4 | 2/10 | 0.2 | [ | ||
| GGTA1 | TALEN敲除 | 2411/12/9 | 30/30 | 1.2 | [ | ||
| FBXO40 | Cas9敲除 | 5424/16/7 | 17/17 | 0.3 | [ | ||
| CD163 | Cas9敲除 | 2192/6/4 | 26/26 | 1.2 | [ | ||
| 显微注射 | DMD (henne muscle dystrophy) | Cas9敲除 | 98/8/1 | 1/2 | 2.0 | [ | |
| TMPRSS2 (ransmembrane protease, serine S1, member 2) | Cas9敲除 | 135/2/2 | 12/12 | 8.8 | [ | ||
| SRY (sex determining region Y) | Cas9敲除 | 66/4/2 | 12/12 | 18.2 | [ | ||
| NANOS2 | Cas9敲除 | 84/4/3 | 18/18 | 21.4 | [ | ||
| GGTA1 | Cas9敲除 | 94/4/2 | 4/15 | 16.0 | [ |
| [1] | Chessa B, Pereira F, Arnaud F, Amorim A, Goyache F, Mainland I, Kao RR, Pemberton JM, Beraldi D, Stear MJ, Alberti A, Pittau M, Iannuzzi L, Banabazi MH, Kazwala RR, Zhang YP, Arranz JJ, Ali BA, Wang ZL, Uzun M, Dione MM, Olsaker I, Holm LE, Saarma U, Ahmad S, Marzanov N, Eythorsdottir E, Holland MJ, Ajmone-Marsan P, Bruford MW, Kantanen J, Spencer TE, Palmarini M. Revealing the history of sheep domestication using retrovirus integrations. Science, 2009,324(5926):532-536. |
| [2] | Zhao JG, Lai LX, Ji WZ, Zhou Q. Genome editing in large animals: current status and future prospects. Natl Sci Rev, 2019,6(3):402-420. |
| [3] | Iiizumi S, Kurosawa A, So S, Ishii Y, Chikaraishi Y, Ishii A, Koyama H, Adachi N. Impact of non-homologous end-joining deficiency on random and targeted DNA integration: implications for gene targeting. Nucleic Acids Res, 2008,36(19):6333-6342. |
| [4] | Choulika A, Perrin A, Dujon B, Nicolas JF. Induction of homologous recombination in mammalian chromosomes by using the I-SceI system of Saccharomyces cerevisiae. Mol Cell Biol, 1995,15(4):1968-1973. |
| [5] | Gordon JW, Scangos GA, Plotkin DJ, Barbosa JA, Ruddle FH. Genetic transformation of mouse embryos by microinjection of purified DNA. Proc Natl Acad Sci USA, 1980,77(12):7380-7384. |
| [6] | Hammer RE, Pursel VG, Rexroad CE, Wall RJ, Bolt DJ, Ebert KM, Palmiter RD, Brinster RL. Production of transgenic rabbits, sheep and pigs by microinjection. Nature, 1985,315(6021):680-683. |
| [7] | Ishino Y, Shinagawa H, Makino K, Amemura M, Nakata A. Nucleotide sequence of the iap gene, responsible for alkaline phosphatase isozyme conversion in Escherichia coli, and identification of the gene product. J Bacteriol, 1987,169(12):5429-5433. |
| [8] | Lee MS, Gippert GP, Soman KV, Case DA, Wright PE. Three-dimensional solution structure of a single zinc finger DNA-binding domain. Science, 1989,245(4918):635-637. |
| [9] | Campbell KH, McWhir J, Ritchie WA, Wilmut I. Sheep cloned by nuclear transfer from a cultured cell line. Nature, 1996,380(6569):64-66. |
| [10] | Schnieke AE, Kind AJ, Ritchie WA, Mycock K, Scott AR, Ritchie M, Wilmut I, Colman A, Campbell KH. Human factor IX transgenic sheep produced by transfer of nuclei from transfected fetal fibroblasts. Science, 1997,278(5346):2130-2133. |
| [11] | Moscou MJ, Bogdanove AJ. A simple cipher governs DNA recognition by TAL effectors. Science, 2009,326(5959):1501. |
| [12] | Rémy S, Tesson L, Ménoret S, Usal C, Scharenberg AM, Anegon I. Zinc-finger nucleases: a powerful tool for genetic engineering of animals. Transgenic Res, 2010,19(3):363-371. |
| [13] | Sander JD, Cade L, Khayter C, Reyon D, Peterson RT, Joung JK, Yeh JRJ. Targeted gene disruption in somatic zebrafish cells using engineered TALENs. Nat Biotechnol, 2011,29(8):697-698. |
| [14] | Cong L, Ran FA, Cox D, Lin SL, Barretto R, Habib N, Hsu PD, Wu XB, Jiang WY, Marraffini LA, Zhang F. Multiplex genome engineering using CRISPR/Cas systems. Science, 2013,339(6121):819-823. |
| [15] | Salabi F, Nazari M, Chen Q, Nimal J, Tong JM, Cao WG. Myostatin knockout using zinc-finger nucleases promotes proliferation of ovine primary satellite cells in vitro. J Biotechnol 2014, 192 Pt A: 268-280. |
| [16] | Proudfoot C, Carlson DF, Huddart R, Long CR, Pryor JH, King TJ, Lillico SG, Mileham AJ, McLaren DG, Whitelaw CBA, Fahrenkrug SC. Genome edited sheep and cattle. Transgenic Res, 2015,24(1):147-153. |
| [17] | Crispo M, Mulet AP, Tesson L, Barrera N, Cuadro F, dos Santos-Neto PC, Nguyen TH, Crénéguy A, Brusselle L, Anegón I, Menchaca A. Efficient generation of myostatin knock-out sheep using CRISPR/Cas9 technology and microinjection into zygotes. PLoS One, 2015,10(8):e0136690. |
| [18] | Murray JD, Nancarrow CD, Marshall JT, Hazelton IG, Ward KA. Production of transgenic merino sheep by microinjection of ovine metallothionein-ovine growth hormone fusion genes. Reprod Fertil Dev, 1989,1(2):147-155. |
| [19] | Adams NR, Briegel JR, Ward KA. The impact of a transgene for ovine growth hormone on the performance of two breeds of sheep. J Anim Sci, 2002,80(9):2325-2333. |
| [20] | Clements JE, Wall RJ, Narayan O, Hauer D, Schoborg R, Sheffer D, Powell A, Carruth LM, Zink MC, Rexroad CE. Development of transgenic sheep that express the visna virus envelope gene. Virology, 1994,200(2):370-380. |
| [21] | Rexroad CE, Hammer RE, Bolt DJ, Mayo KE, Frohman LA, Palmiter RD, Brinster RL. Production of transgenic sheep with growth-regulating genes. Mol Reprod Dev, 1989,1(3):164-169. |
| [22] | Rexroad CE, Mayo K, Bolt DJ, Elsasser TH, Miller KF, Behringer RR, Palmiter RD, Brinster RL. Transferrin- and albumin-directed expression of growth-related peptides in transgenic sheep. J Anim Sci, 1991,69(7):2995-3004. |
| [23] | Wright G, Carver A, Cottom D, Reeves D, Scott A, Simons P, Wilmut I, Garner I, Colman A. High level expression of active human alpha-1-antitrypsin in the milk of transgenic sheep. Biotechnology (NY), 1991,9(9):830-834. |
| [24] | Clark AJ, Bessos H, Bishop JO, Brown P, Harris S, Lathe R, McClenaghan M, Prowse C, Simons JP, Whitelaw CBA, Wilmut I. Expression of human anti-hemophilic factor IX in the milk of transgenic sheep. Bio Tech, 1989,7:487-492. |
| [25] | Niemann H, Halter R, Carnwath JW, Herrmann D, Lemme E, Paul D. Expression of human blood clotting factor VIII in the mammary gland of transgenic sheep. Transgenic Res, 1999,8(3):237-247. |
| [26] | Damak S, Jay NP, Barrell GK, Bullock DW. Targeting gene expression to the wool follicle in transgenic sheep. Biotechnology (NY), 1996,14(2):181-184. |
| [27] | Jacobsen JC, Bawden CS, Rudiger SR, McLaughlan CJ, Reid SJ, Waldvogel HJ, MacDonald ME, Gusella JF, Walker SK, Kelly JM, Webb GC, Faull RLM, Rees MI, Snell RG. An ovine transgenic Huntington's disease model. Hum Mol Genet, 2010,19(10):1873-1882. |
| [28] | Bawden CS, Powell BC, Walker SK, Rogers GE. Expression of a wool intermediate filament keratin transgene in sheep fibre alters structure. Transgenic Res, 1998,7(4):273-287. |
| [29] | Deng SL, Yu K, Wu Q, Li Y, Zhang XS, Zhang BL, Liu GS, Liu YX, Lian ZX. Toll-like receptor 4 reduces oxidative injury via glutathione activity in sheep. Oxid Med Cell Longev, 2016,2016:9151290. |
| [30] | Denning C, Burl S, Ainslie A, Bracken J, Dinnyes A, Fletcher J, King T, Ritchie M, Ritchie WA, Rollo M, de Sousa P, Travers A, Wilmut I, Clark AJ. Deletion of the alpha(1,3)galactosyl transferase (GGTA1) gene and the prion protein (PrP) gene in sheep. Nat Biotechnol, 2001,19(6):559-562. |
| [31] | McCreath KJ, Howcroft J, Campbell KH, Colman A, Schnieke AE, Kind AJ. Production of gene-targeted sheep by nuclear transfer from cultured somatic cells. Nature, 2000,405(6790):1066-1069. |
| [32] | Deng SL, Li GG, Zhang JL, Zhang XS, Cui MS, Guo Y, Liu GS, Li GP, Feng JZ, Lian ZX. Transgenic cloned sheep overexpressing ovine toll-like receptor 4. Theriogenology, 2013,80(1):50-57. |
| [33] | Duan B, Cheng L, Gao Y, Yin FX, Su GH, Shen QY, Liu K, Hu X, Liu X, Li GP. Silencing of fat-1 transgene expression in sheep may result from hypermethylation of its driven cytomegalovirus (CMV) promoter. Theriogenology, 2012,78(4):793-802. |
| [34] | He X, Li HT, Zhou ZY, Zhao ZS, Li W. Production of brown/yellow patches in the SLC7A11 transgenic sheep via testicular injection of transgene. J Genet Genomics, 2012,39(6):281-285. |
| [35] | Qin YR, Chen H, Zhang YN, Zhu CY, Gao B, Yin YH, Li W, Shi QQ, Zheng MM, Xu Q, Song JZ, Li BC. Cloning of the Xuhuai goat PPARγ gene and the preparation of transgenic sheep. Biochem Genet, 2013,51(7-8):543-553. |
| [36] | Qin YR, Zhang YN, Yin YH, Xu F, Gao B, Shi QQ, Zhu CY, Li W, Li BC. Cloning of Xuhuai goat lipoprotein lipase gene and the preparation of transgenic sheep. Mol Biol Rep, 2012,39(8):8439-8446. |
| [37] | Ritchie WA, King T, Neil C, Carlisle AJ, Lillico S, McLachlan G, Whitelaw CBA. Transgenic sheep designed for transplantation studies. Mol Reprod Dev, 2009,76(1):61-64. |
| [38] | Crispo M, Vilariño M, dos Santos-Neto PC, Núñez- Olivera R, Cuadro F, Barrera N, Mulet AP, Nguyen TH, Anegón I, Menchaca A. Embryo development, fetal growth and postnatal phenotype of eGFP lambs generated by lentiviral transgenesis. Transgenic Res, 2015,24(1):31-41. |
| [39] | Liu CX, Wang LQ, Li WR, Zhang XM, Tian YZ, Zhang N, He SG, Chen T, Huang JC, Liu MJ. Highly efficient generation of transgenic sheep by lentivirus accompanying the alteration of methylation status. PLoS One, 2013,8(1):e54614. |
| [40] | Li WR, He SG, Liu CX, Zhang XM, Wang LQ, Lin JP, Chen L, Han B, Huang JC, Liu MJ. Ectopic expression of FGF5s induces wool growth in Chinese merino sheep. Gene, 2017,627:477-483. |
| [41] | Ritchie WA, King T, Neil C, Carlisle AJ, Lillico S, McLachlan G, Whitelaw CBA. Transgenic sheep designed for transplantation studies. Mol Reprod Dev, 2009,76(1):61-64. |
| [42] | Tian YZ, Li WR, Wang LQ, Liu CX, Lin JP, Zhang XM, Zhang N, He SG, Huang JC, Jia B, Liu MJ. Expression of 2A peptide mediated tri-fluorescent protein genes were regulated by epigenetics in transgenic sheep. Biochem Biophys Res Commun, 2013,434(3):681-687. |
| [43] | Platt RJ, Chen SD, Zhou Y, Yim MJ, Swiech L, Kempton HR, Dahlman JE, Parnas O, Eisenhaure TM, Jovanovic M, Graham DB, Jhunjhunwala S, Heidenreich M, Xavier RJ, Langer R, Anderson DG, Hacohen N, Regev A, Feng GP, Sharp PA, Zhang F. CRISPR-Cas9 knockin mice for genome editing and cancer modeling. Cell, 2014,159(2):440-455. |
| [44] | Bevacqua RJ, Fernandez-Martin R, Canel NG, Gibbons A, Texeira D, Lange F, Vans Landschoot G, Savy V, Briski O, Hiriart MI, Grueso E, Ivics Z, Taboga O, Kues WA, Ferraris S, Salamone DF. Assessing Tn5 and Sleeping Beauty for transpositional transgenesis by cytoplasmic injection into bovine and ovine zygotes. PLoS One, 2017,12(3):e0174025. |
| [45] | Hu SW, Ni W, Sai WJF, Zhang H, Cao XD, Qiao J, Sheng JL, Guo F, Chen CF. Sleeping Beauty-mediated knockdown of sheep myostatin by RNA interference. Biotechnol Lett, 2011,33(10):1949-1953. |
| [46] | Rodriguez-Sosa JR, Silvertown JD, Foster RA, Medin JA, Hahnel A. Transduction and transplantation of spermatogonia into the testis of ram lambs through the extra-testicular rete. Reprod Domest Anim, 2009,44(4):612-620. |
| [47] | Zhang XM, Wang LQ, Wu YS, Li WR, An J, Zhang FC, Liu MJ. Knockout of myostatin by zinc-finger nuclease in sheep fibroblasts and embryos. Asian-Australas J Anim Sci, 2016,29(10):1500-1507. |
| [48] | Zhao XX, Ni W, Chen CF, Sai WJF, Qiao J, Sheng JL, Zhang H, Li GZ, Wang DW, Hu SW. Targeted editing of myostatin gene in sheep by transcription activator-like effector nucleases. Asian-Australas J Anim Sci, 2016,29(3):413-418. |
| [49] | Li HH, Wang G, Hao ZQ, Zhang GZ, Qing YB, Liu SH, Qing LL, Pan WR, Chen L, Liu GC, Zhao RP, Jia BY, Zeng LY, Guo JX, Zhao LX, Zhao H, Lv CX, Xu KX, Cheng WM, Li HS, Zhao HY, Wang W, Wei HJ. Generation of biallelic knock-out sheep via gene-editing and somatic cell nuclear transfer. Sci Rep, 2016,6:33675. |
| [50] | Han HB, Ma YH, Wang T, Lian T, Tian XZ, Hu R, Deng SL, Li KP, Wang F, Li N, Liu GS, Zhao YF, Lian ZX. One-step generation of myostatin gene knockout sheep via the CRISPR/Cas9 system. Front Agr Sci & Eng, 2014,1(1):2-5. |
| [51] | Wang XL, Niu YY, Zhou JK, Yu HH, Kou QF, Lei AM, Zhao XE, Yan HL, Cai B, Shen QY, Zhou SW, Zhu HJ, Zhou GX, Niu WZ, Hua JL, Jiang Y, Huang XX, Ma BH, Chen YL. Multiplex gene editing via CRISPR/Cas9 exhibits desirable muscle hypertrophy without detectable off-target effects in sheep. Sci Rep, 2016,6:32271. |
| [52] | Williams DK, Pinzón C, Huggins S, Pryor JH, Falck A, Herman F, Oldeschulte J, Chavez MB, Foster BL, White SH, Westhusin ME, Suva LJ, Long CR, Gaddy D. Genetic engineering a large animal model of human hypophosphatasia in sheep. Sci Rep, 2018,8(1):16945. |
| [53] | Vilarino M, Rashid ST, Suchy FP, McNabb BR, van der Meulen T, Fine EJ, Ahsan SD, Mursaliyev N, Sebastiano V, Diab SS, Huising MO, Nakauchi H, Ross PJ. CRISPR/Cas9 microinjection in oocytes disables pancreas development in sheep. Sci Rep, 2017,7(1):17472. |
| [54] | Wu MM, Wei CH, Lian ZX, Liu RZ, Zhu CY, Wang HH, Cao JX, Shen YL, Zhao FP, Zhang L, Mu Z, Wang YY, Wang XG, Du LX, Wang CD. Rosa26-targeted sheep gene knock-in via CRISPR-Cas9 system. Sci Rep, 2016,6:24360. |
| [55] | Fan ZQ, Perisse IV, Cotton CU, Regouski M, Meng QG, Domb C, Van Wettere AJ, Wang ZD, Harris A, White KL, Polejaeva IA. A sheep model of cystic fibrosis generated by CRISPR/Cas9 disruption of the CFTR gene. JCI Insight, 2018,3(19):e123529. |
| [56] | Zhang YN, Wang YJ, Bi YL, Tang BB, Wang M, Zhang C, Zhang WH, Jin J, Li TT, Zhao RF, Yu XJ, Zuo QS, Li BC. CRISPR/Cas9-mediated sheep MSTN gene knockout and promote sSMSCs differentiation. J Cell Biochem, 2018. |
| [57] | Zhou SW, Cai B, He C, Wang Y, Ding Q, Liu J, Liu Y, Ding YG, Zhao XE, Li GW, Li C, Yu HH, Kou QF, Niu WZ, Petersen B, Sonstegard T, Ma BH, Chen YL, Wang XL. Programmable base editing of the sheep genome revealed no genome-wide off-target mutations. Front Genet, 2019,10:215. |
| [58] | Zhang XM, Li WR, Liu CX, Peng XR, Lin JP, He SG, Li XJ, Han B, Zhang N, Wu YS, Chen L, Wang LQ, Ma YL, Huang JC, Liu MJ. Alteration of sheep coat color pattern by disruption of ASIP gene via CRISPR Cas9. Sci Rep, 2017,7(1):8149. |
| [59] | Zhou SW, Yu HH, Zhao XE, Cai B, Ding Q, Huang Y, Li YX, Li Y, Niu YY, Lei AM, Kou QF, Huang XX, Petersen B, Ma BH, Chen YL, Wang XL. Generation of gene-edited sheep with a defined Booroola fecundity gene (FecBB) mutation in bone morphogenetic protein receptor type 1B (BMPR1B) via clustered regularly interspaced short palindromic repeat (CRISPR)/CRISPR- associated (Cas) 9. Reprod Fertil Dev, 2018,30(12):1616-1621. |
| [60] | Zhang XM, Li WR, Wu YS, Peng XR, Lou B, Wang LQ, Liu MJ. Disruption of the sheep BMPR-IB gene by CRISPR/Cas9 in in vitro-produced embryos. Theriogenology, 2017, 91: 163-172. e2. |
| [61] | Menchaca A, Dos Santos-Neto PC, Souza-Neves M, Cuadro F, Mulet AP, Tesson L, Chenouard V, Guiffès A, Heslan JM, Gantier M, Anegón I, Crispo M. Otoferlin gene editing in sheep via CRISPR-assisted ssODN- mediated homology directed repair. Sci Rep, 2020,10(1):5995. |
| [62] | Hu R, Fan ZY, Wang BY, Deng SL, Zhang XS, Zhang JL, Han HB, Lian ZX. Rapid communication: generation of FGF5 knockout sheep via the CRISPR/Cas9 system. J Anim Sci, 2017,95(5):2019-2024. |
| [63] | Li WR, He SG, Liu CX, Zhang XM, Wang LQ, Lin JP, Chen L, Han B, Huang JC, Liu MJ. Ectopic expression of FGF5s induces wool growth in Chinese merino sheep. Gene, 2017,627:477-483. |
| [64] | Ma T, Tao JL, Yang MH, He CJ, Tian XZ, Zhang XS, Zhang JL, Deng SL, Feng JZ, Zhang ZZ, Wang J, Ji PY, Song YK, He PL, Han HB, Fu JC, Lian ZX, Liu GS. An AANAT/ASMT transgenic animal model constructed with CRISPR/Cas9 system serving as the mammary gland bioreactor to produce melatonin-enriched milk in sheep. J Pineal Res, 2017,63(1). |
| [65] | Kim YG, Cha J, Chandrasegaran S. Hybrid restriction enzymes: zinc finger fusions to Fok I cleavage domain. Proc Natl Acad Sci USA, 1996,93(3):1156-1160. |
| [66] | Wolfe SA, Nekludova L, Pabo CO. DNA recognition by Cys2His2 zinc finger proteins. Annu Rev Biophys Biomol Struct, 2000,29:183-212. |
| [67] | Smith J, Berg JM, Chandrasegaran S. A detailed study of the substrate specificity of a chimeric restriction enzyme. Nucleic Acids Res, 1999,27(2):674-681. |
| [68] | Valerie K, Povirk LF. Regulation and mechanisms of mammalian double-strand break repair. Oncogene, 2003,22(37):5792-5812. |
| [69] | Bibikova M, Golic M, Golic KG, Carroll D. Targeted chromosomal cleavage and mutagenesis in Drosophila using zinc-finger nucleases. Genetics, 2002,161(3):1169-1175. |
| [70] | Miller J, McLachlan AD, Klug A. Repetitive zinc- binding domains in the protein transcription factor IIIA from Xenopus oocytes. EMBO J, 1985,4(6):1609-1614. |
| [71] | Hwang WY, Fu YF, Reyon D, Maeder ML, Tsai SQ, Sander JD, Peterson RT, Yeh JRJ, Joung JK. Efficient genome editing in zebrafish using a CRISPR-Cas system. Nat Biotechnol, 2013,31(3):227-229. |
| [72] | Komor AC, Kim YB, Packer MS, Zuris JA, Liu DR. Programmable editing of a target base in genomic DNA without double-stranded DNA cleavage. Nature, 2016,533(7603):420-424. |
| [73] | Rees HA, Liu DR. Base editing: precision chemistry on the genome and transcriptome of living cells. Nat Rev Genet, 2018,19(12):770-788. |
| [74] | Jin S, Zong Y, Gao Q, Zhu ZX, Wang YP, Qin P, Liang CZ, Wang DW, Qiu JL, Zhang F, Gao CX. Cytosine, but not adenine, base editors induce genome-wide off-target mutations in rice. Science, 2019,364(6437):292-295. |
| [75] | Zuo EW, Sun YD, Wei W, Yuan TL, Ying WQ, Sun H, Yuan LY, Steinmetz LM, Li YX, Yang H. Cytosine base editor generates substantial off-target single-nucleotide variants in mouse embryos. Science, 2019,364(6437):289-292. |
| [76] | Batool A, Malik F, Andrabi KI. Expansion of the CRISPR/Cas genome-sculpting toolbox: innovations, applications and challenges. Mol Diagn Ther, 2021,25(1):41-57. |
| [77] | Liu JJ, Orlova N, Oakes BL, Ma EB, Spinner HB, Baney KLM, Chuck J, Tan D, Knott GJ, Harrington LB, Al-Shayeb B, Wagner A, Brötzmann J, Staahl BT, Taylor KL, Desmarais J, Nogales E, Doudna JA. CasX enzymes comprise a distinct family of RNA-guided genome editors. Nature, 2019,566(7743):218-223. |
| [78] | Su HY, Jay NP, Gourley TS, Kay GW, Damak S. Wool production in transgenic sheep: results from first- generation adults and second-generation lambs. Anim Biotechnol, 1998,9(2):135-147. |
| [79] | Zhang P, Liu P, Dou HW, Chen L, Chen LX, Lin L, Tan PP, Vajta G, Gao JF, Du YT, Ma RZ. Handmade cloned transgenic sheep rich in omega-3 Fatty acids. PLoS One, 2013,8(2):e55941. |
| [80] | Deng SL, Li GD, Yu K, Tian XZ, Wang F, Li WT, Jiang WQ, Ji PY, Han HB, Fu JC, Zhang XS, Zhang JL, Liu YX, Lian ZX, Liu GS. RNAi combining Sleeping Beauty transposon system inhibits ex vivo expression of foot-and-mouth disease virus VP1 in transgenic sheep cells. Sci Rep, 2017,7(1):10065. |
| [81] | Hanrahan JP, Gregan SM, Mulsant P, Mullen M, Davis GH, Powell R, Galloway SM. Mutations in the genes for oocyte-derived growth factors GDF9 and BMP15 are associated with both increased ovulation rate and sterility in Cambridge and Belclare sheep (Ovis aries). Biol Reprod, 2004,70(4):900-909. |
| [82] | Zhang ZF, Sun YW, Du W, He SG, Liu MJ, Tian CY. Effects of vertebral number variations on carcass traits and genotyping of Vertnin candidate gene in Kazakh sheep. Asian-Australas J Anim Sci, 2017,30(9):1234-1238. |
| [83] | Valette M, Poitou C, Le Beyec J, Bouillot JL, Clement K, Czernichow S. Melanocortin-4 receptor mutations and polymorphisms do not affect weight loss after bariatric surgery. PLoS One, 2012,7(11):e48221. |
| [84] | Chmurzyńska A. The multigene family of fatty acid-binding proteins (FABPs): function, structure and polymorphism. J Appl Genet, 2006,47(1):39-48. |
| [85] | Wang X, Niu Y, Zhou J, Zhu H, Ma B, Yu H, Yan H, Hua J, Huang X, Qu L, Chen Y. CRISPR/Cas9-mediated MSTN disruption and heritable mutagenesis in goats causes increased body mass. Anim Genet, 2018,49(1):43-51. |
| [86] | Guo RH, Wan YJ, Xu D, Cui LB, Deng MT, Zhang GM, Jia RX, Zhou WJ, Wang Z, Deng KP, Huang MR, Wang F, Zhang YL. Generation and evaluation of Myostatin knock-out rabbits and goats using CRISPR/Cas9 system. Sci Rep, 2016,6:29855. |
| [87] | Li GW, Zhou SW, Li C, Cai B, Yu HH, Ma BH, Huang Y, Ding YG, Liu Y, Ding Q, He C, Zhou JK, Wang Y, Zhou GX, Li Y, Yan Y, Hua JL, Petersen B, Jiang Y, Sonstegard T, Huang XX, Chen YL, Wang XL. Base pair editing in goat: nonsense codon introgression into FGF5 results in longer hair. FEBS J, 2019,286(23):4675-4692. |
| [88] | Zhou WJ, Wan YJ, Guo RH, Deng MT, Deng KP, Wang Z, Zhang YL, Wang F. Generation of beta-lactoglobulin knock-out goats using CRISPR/Cas9. PLoS One, 2017,12(10):e0186056. |
| [89] | Niu YY, Zhao XE, Zhou JK, Li Y, Huang Y, Cai B, Liu YT, Ding Q, Zhou SW, Zhao J, Zhou GX, Ma BH, Huang XX, Wang XL, Chen YL. Efficient generation of goats with defined point mutation (I397V) in GDF9 through CRISPR/Cas9. Reprod Fertil Dev, 2018,30(2):307-312. |
| [90] | Yu BL, Lu R, Yuan YG, Zhang T, Song SZ, Qi ZQ, Shao B, Zhu MM, Mi F, Cheng Y. Efficient TALEN-mediated myostatin gene editing in goats. BMC Dev Biol, 2016,16(1):26. |
| [91] | Ni W, Qiao J, Hu SW, Zhao XX, Regouski M, Yang M, Polejaeva IA, Chen CF. Efficient gene knockout in goats using CRISPR/Cas9 system. PLoS One, 2014,9(9):e106718. |
| [92] | Hao F, Yan W, Li XC, Wang H, Wang YM, Hu X, Liu X, Liang H, Liu DJ. Generation of cashmere goats carrying an EDAR gene mutant using CRISPR-Cas9-mediated genome editing. Int J Biol Sci, 2018,14(4):427-436. |
| [93] | Polejaeva IA, Chen SH, Vaught TD, Page RL, Mullins J, Ball S, Dai Y, Boone J, Walker S, Ayares DL, Colman A, Campbell KH. Cloned pigs produced by nuclear transfer from adult somatic cells. Nature, 2000,407(6800):86-90. |
| [94] | Davis BT, Wang XJ, Rohret JA, Struzynski JT, Merricks EP, Bellinger DA, Rohret FA, Nichols TC, Rogers CS. Targeted disruption of LDLR causes hypercholesterolemia and atherosclerosis in Yucatan miniature pigs. PLoS One, 2014,9(4):e93457. |
| [95] | Yang DS, Yang HQ, Li W, Zhao BT, Ouyang Z, Liu ZM, Zhao Y, Fan NN, Song J, Tian JT, Li F, Zhang JF, Chang L, Pei DQ, Chen YE, Lai LX. Generation of PPARγ mono-allelic knockout pigs via zinc-finger nucleases and nuclear transfer cloning. Cell Res, 2011,21(6):979-982. |
| [96] | Feng C, Li XR, Cui HT, Long C, Liu X, Tian XH, Pan DK, Luo YZ. Highly efficient generation of GGTA1 knockout pigs using a combination of TALEN mRNA and magnetic beads with somatic cell nuclear transfer. J Integr Agr, 2016,15(7):1540-1549. |
| [97] | Zou YL, Li ZY, Zou YJ, Hao HY, Li N, Li QY. An FBXO40 knockout generated by CRISPR/Cas9 causes muscle hypertrophy in pigs without detectable pathological effects. Biochem Biophys Res Commun, 2018,498(4):940-945. |
| [98] | Wang HT, Shen LC, Chen JY, Liu XJ, Tan T, Hu YQ, Bai XF, Li YX, Tian KG, Li N, Hu XX. Deletion of CD163 exon 7 confers resistance to highly pathogenic porcine reproductive and respiratory viruses on pigs. Int J Biol Sci, 2019,15(9):1993-2005. |
| [99] | Yu HH, Zhao H, Qing YB, Pan WR, Jia BY, Zhao HY, Huang XX, Wei HJ. Porcine zygote injection with Cas9/sgRNA results in DMD-modified pig with muscle dystrophy. Int J Mol Sci, 2016,17(10):1668. |
| [100] | Whitworth KM, Benne JA, Spate LD, Murphy SL, Samuel MS, Murphy CN, Richt JA, Walters E, Prather RS, Wells KD. Zygote injection of CRISPR/Cas9 RNA successfully modifies the target gene without delaying blastocyst development or altering the sex ratio in pigs. Transgenic Res, 2017,26(1):97-107. |
| [101] | Zhou XY, Wang LL, Du YN, Xie F, Li L, Liu Y, Liu CH, Wang SQ, Zhang SB, Huang XX, Wang Y, Wei H. Efficient generation of gene-modified pigs harboring precise orthologous human mutation via CRISPR/Cas9- induced homology-directed repair in zygotes. Hum Mutat, 2016,37(1):110-118. |
| [102] | Park KE, Kaucher AV, Powell A, Waqas MS, Sandmaier SES, Oatley MJ, Park CH, Tibary A, Donovan DM, Blomberg LA, Lillico SG, Whitelaw CBA, Mileham A, Telugu BP, Oatley JM. Generation of germline ablated male pigs by CRISPR/Cas9 editing of the NANOS2 gene. Sci Rep, 2017,7:40176. |
| [103] | Appel MJ, van Veen HA, Vietsch H, Salaheddine M, Nuijens JH, Ziere B, de Loos F. Sub-chronic (13-week) oral toxicity study in rats with recombinant human lactoferrin produced in the milk of transgenic cows. Food Chem Toxicol, 2006,44(7):964-973. |
| [104] | Yu DW, Wang J, Zou HY, Feng T, Chen L, Li J, Qi XL, Li ZF, Duan XY, Xu CL, Zhang L, Long X, Lan J, Chen C, Wang C, Xu XY, Ren JL, Zhao YQ, Hu XX, Lian ZX, Men HS, Pan DD, Li N, Capecchi MR, Du XG, Zhao YF, Wu S. Silencing of retrotransposon-derived imprinted gene RTL1 is the main cause for postimplantational failures in mammalian cloning. Proc Natl Acad Sci USA, 2018,115(47):E11071-11080. |
| [105] | Liu Z, Cai YJ, Wang Y, Nie YH, Zhang CC, Xu YT, Zhang XT, Lu Y, Wang ZY, Poo MM, Sun Q. Cloning of macaque monkeys by somatic cell nuclear transfer. Cell, 2018, 172(4): 881-887.e7. |
| [106] | Liang S, Jin YX, Yuan B, Zhang JB, Kim NH. Melatonin enhances the developmental competence of porcine somatic cell nuclear transfer embryos by preventing DNA damage induced by oxidative stress. Sci Rep, 2017,7(1):11114. |
| [107] | Hashimoto M, Takemoto T. Electroporation enables the efficient mRNA delivery into the mouse zygotes and facilitates CRISPR/Cas9-based genome editing. Sci Rep, 2015,5:11315. |
| [108] | Zhang YM, Wang QQ, Liu KL, Gao EE, Guan H, Hou J. Treatment of donor cells with recombinant KDM4D protein improves preimplantation development of cloned ovine embryos. Cytotechnology, 2018,70(5):1469-1477. |
| [109] | Appel MJ, van Veen HA, Vietsch H, Salaheddine M, Nuijens JH, Ziere B, de Loos F. Sub-chronic (13-week) oral toxicity study in rats with recombinant human lactoferrin produced in the milk of transgenic cows. Food Chem Toxicol, 2006,44(7):964-973. |
| [1] | Xiaoping Lian, Guangfu Huang, Yujiao Zhang, Jing Zhang, Fengyi Hu, Shilai Zhang. The discovery and utilization of favorable genes in Oryza longistaminata [J]. Hereditas(Beijing), 2023, 45(9): 765-780. |
| [2] | Yiming Gong, Xiangyu Wang, Xiaoyun He, Yufang Liu, Ping Yu, Mingxing Chu, Ran Di. Progress on the effect of FecB mutation on BMPR1B activity and BMP/SMAD pathway in sheep [J]. Hereditas(Beijing), 2023, 45(4): 295-305. |
| [3] | Zhongsheng Wu, Yu Gao, Yongtao Du, Song Dang, Kangmin He. The protocol of tagging endogenous proteins with fluorescent tags using CRISPR-Cas9 genome editing [J]. Hereditas(Beijing), 2023, 45(2): 165-175. |
| [4] | Meizhen Liu, Liren Wang, Yongmei Li, Xueyun Ma, Honghui Han, Dali Li. Generation of genetically modified rat models via the CRISPR/Cas9 technology [J]. Hereditas(Beijing), 2023, 45(1): 78-87. |
| [5] | Hui Qu, Yi Liu, Yawen Chen, Hui Wang. Alteration of imprinted genes and offspring organ development caused by environmental factors [J]. Hereditas(Beijing), 2022, 44(2): 107-116. |
| [6] | Yangjinghui Zhang, Peiyao Chang, Zishu Yang, Yuhang Xue, Xueqi Li, Yang Zhang. Advances in epigenetic modification affecting anthocyanin synthesis [J]. Hereditas(Beijing), 2022, 44(12): 1117-1127. |
| [7] | Qingwen Zhao, Dongning Pan. Progress on the epigenetic regulation of adipose tissue thermogenesis [J]. Hereditas(Beijing), 2022, 44(10): 867-880. |
| [8] | Wang Ya'nan, Tao Xu, Wanpeng Wang, Qingzhu Zhang, Xie Li'nan. Role of epigenetic modifications in the development of crops essential traits [J]. Hereditas(Beijing), 2021, 43(9): 858-879. |
| [9] | Xiaohong He, Lin Jiang, Yabin Pu, Qianjun Zhao, Yuehui Ma. Progress on genetic mapping and genetic mechanism of cattle and sheep horns [J]. Hereditas(Beijing), 2021, 43(1): 40-51. |
| [10] | Xia Li, Wan Shi, Lizhao Geng, Jianping Xu. Genome editing in plants directed by CRISPR/Cas ribonucleoprotein complexes [J]. Hereditas(Beijing), 2020, 42(6): 556-564. |
| [11] | Ruiying Qin, Pengcheng Wei. Prime editing creates a novel dimension of plant precise genome editing [J]. Hereditas(Beijing), 2020, 42(6): 519-523. |
| [12] | Jie Wu, Jianping Quan, Yong Ye, ZhenFang Wu, Jie Yang, Ming Yang, Enqin Zheng. Advances in assay for transposase-accessible chromatin with high-throughput sequencing [J]. Hereditas(Beijing), 2020, 42(4): 333-346. |
| [13] | Bingyuan Wang, Yulian Mu, Kui Li, Zhiguo Liu. Research progress of stem cells in agricultural animals [J]. Hereditas(Beijing), 2020, 42(11): 1073-1080. |
| [14] | Xinping Yang,Yuan Yu,Cao Xu. De novo domestication to create new crops [J]. Hereditas(Beijing), 2019, 41(9): 827-835. |
| [15] | Jue Wang, Juan Huang, Rui Xu. Seamless genome editing in Drosophila by combining CRISPR/Cas9 and piggyBac technologies [J]. Hereditas(Beijing), 2019, 41(5): 422-429. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||