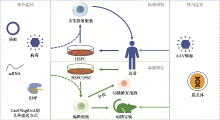
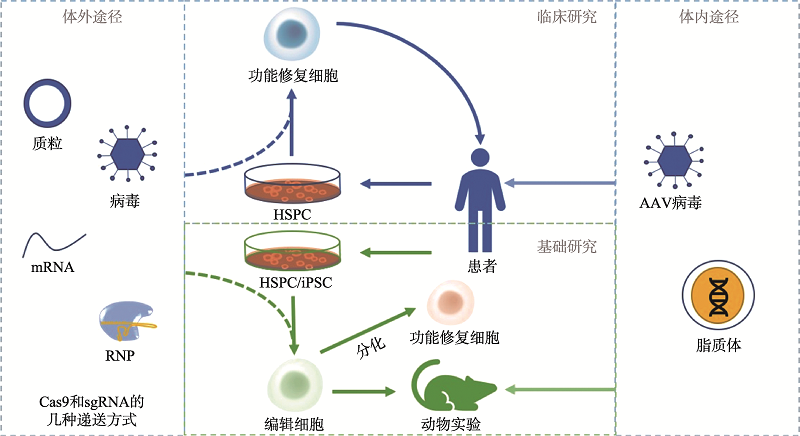
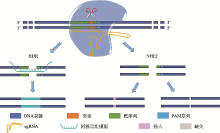
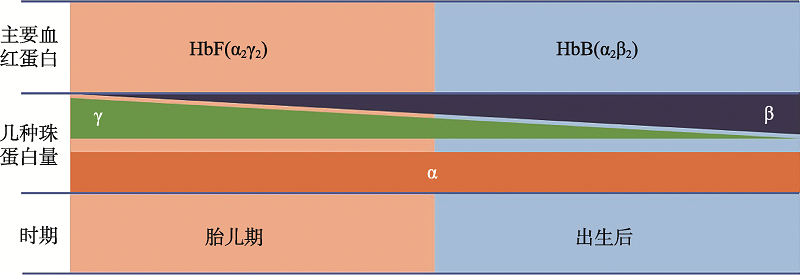
Hereditas(Beijing) ›› 2020, Vol. 42 ›› Issue (10): 949-964.doi: 10.16288/j.yczz.20-110
• Review • Previous Articles Next Articles
Advances in gene therapy for β-thalassemia and hemophilia based on the CRISPR/Cas9 technology
Liwen Bao1( ), Yiye Zhou2, Fanyi Zeng1,2(
), Yiye Zhou2, Fanyi Zeng1,2( )
)
- 1. Shanghai Key Laboratory of Embryo and Reproduction Engineering, Key Laboratory of Embryo Molecular Biology of National Health Commission, Shanghai Institute of Medical Genetics, Shanghai Children’s Hospital, Shanghai Jiao Tong University, Shanghai 200040, China;
2. Department of Histoembryology, Genetics & Development, Shanghai Jiao Tong University College of Basic Medical Sciences, Shanghai 200025, China;
-
Received:2020-04-21Revised:2020-08-25Online:2020-10-20Published:2020-09-15 -
Contact:Zeng Fanyi E-mail:blwbaoliwen@163.com;fzeng@vip.163.com -
Supported by:Supported by the National Key Research and Development Program of China No(2016YFC1000503);Shanghai Key Disciplines Program No(2017ZZ02019);Key Clinical Specialty Projects in Shanghai No(shslczdzk05705)
Cite this article
Liwen Bao, Yiye Zhou, Fanyi Zeng. Advances in gene therapy for β-thalassemia and hemophilia based on the CRISPR/Cas9 technology[J]. Hereditas(Beijing), 2020, 42(10): 949-964.
share this article
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
Table 3
In Situ repair of mutant genes using CRISPR/Cas9"
| 突变 类型 | 主要疾病 | 策略 | 年份 | 编辑 细胞 | 编辑基因 | 修复/编辑效率 | 脱靶率 | 编辑后体外细胞表型 | 参考文献 |
|---|---|---|---|---|---|---|---|---|---|
| 外显子点突变和移码突变 | β-地贫 镰状细胞贫血 血友病B | HDRa | 2014 | β-地贫iPSC | HBB | -28A/G: 7.8% CD41/42 -TCTT: 9.8% 双位点修复效率: 0 | 未检测到脱靶 | HBB mRNA量升高约16倍 | [15] |
| 2015 | β-地贫iPSC | HBB | 16.67% | 未检测到脱靶 | HBB蛋白量由0上升至正常水平的~60% | [29] | |||
| 2016 | β-地贫iPSC | HBB | 0.045% | 未检测到脱靶 | HBB蛋白量接近正常 | [28] | |||
| 2016 | β-地贫iPSC | HBB | na ??ve iPSC组: 57% primed iPSC组: 32% | na ??ve iPSC: 0 primed iPSC: 5% | 未说明 | [74] | |||
| 2017 | β-地贫iPSC | HBB | 双等位基因修复: 54% 单等位基因修复: 25.5% | 未检测到脱靶 | 有HBB mRNA表达 | [30] | |||
| 2018 | β-地贫iPSC | HBB | 2.9% | 3个克隆中2个检测到HBD点突变 | HBB mRNA和HBB蛋白无明显上升 | [32] | |||
| 2019 | 镰状细胞贫血HSPC | HBB | 24.5±7.6% | Cas9: 0.1%~36.7% HiFi Cas9d: ≤0.23% | HbA水平由5.3±1%提升至25.3±13.9% | [31] | |||
| 内含子点突变 | β-地贫 | HDR | 2018 | β-地贫HSC | HBB | 8% | 未检测到脱靶 | 未说明 | [33] |
| NHEJb | 2019 | β-地贫HSPC | HBB | indel产生率: 93% | 未说明 | HbA分数从36.4%上升至75.6% | [35] | ||
| 内含子倒位 | 血友病A | NAHRc | 2015 | 血友病A iPSC | FVIII | 3.7% | 未检测到脱靶 | F8 mRNA量由0上升至正常水平的50%~120% | [36] |
Table 4
Gene expression regulation: basic study on reactivation of HBG by CRISPR/Cas9 in vitro"
| 年份 | 编辑细胞 | 编辑方式 | 编辑效率 | 脱靶率 | 编辑后体外细胞表型 | 参考文献 |
|---|---|---|---|---|---|---|
| 2015 | 正常人HSPC | 破坏BCL11A红系特异增强子 | N/A | N/A | HBG表达水平上升近4倍,分化后HbF+细胞占比升高近2倍 | [49] |
| 2018 | HUDEP-2a | 破坏HBG近端启动子上BCL11A和ZBTB7A结合位点 | N/A | N/A | mRNA水平: γ/(γ+β)%比值由不到5%上升至~70% 蛋白水平: HbF水平由0上升至58.7±9.6% | [47] |
| 2018 | SCD HSPCb | 敲除13.6kb长的可能的γ-珠蛋白基因抑制因子区域 | 缺失: 34.1% 倒位: 28.1% | 0~4.6% | γ链/类β链的比值较对照上升近2.5倍 0%氧气条件下,镰状细胞数由~65%下降至~30% | [48] |
| 2018 | SCD CD34+ 细胞c | 敲除HRId | N/A | N/A | mRNA水平: HBG/(HBG+HBB)%比值由3.2%上升至24% 蛋白水平: HbF上升近4倍 细胞水平: HbF+细胞占比升高近2倍 | [53] |
| 2019 | β-地贫HSPC | 破坏BCL11A红系特异增强子 | 平均84.4% | 未检测 到脱靶 | HbF/(HbF+HbA+HbA2)%比值由~30%上升至~70% | [45] |
| 2019 | HUDEP-2 | 破坏HBG近端启动子上BCL11A结合位点 | N/Ae | N/A | mRNA水平: γ/(γ+β)%比值由~10%上升至~60% 蛋白水平: HbF/(HbF+HbA)%比值由零上升至~40% | [46] |
| 2020 | β-地贫HSPC | 破坏HBG近端启动子上BCL11A结合位点 | 平均85% | 未检测 到脱靶 | γ-珠蛋白的mRNA水平可达α-珠蛋白的126% | [52] |
Table 5
Clinical trials of gene therapy based on CRISPR/Cas9 technology"
| NCT编号t | 国家 | 治疗疾病 | 治疗途径 | 编辑细胞 | 编辑方式 | 试验阶段 | 状态 |
|---|---|---|---|---|---|---|---|
| NCT03745287 | 美国、比利时、加拿大、德国和意大利 | 镰状细胞贫血 | 体外 | 自体CD34+ HSPCa | 破坏BCL11Ab的红系特异增强子 | 临床I/II期 | 招募中 |
| NCT03655678 | 美国、加拿大、德国、意大利和英国 | 输血依赖型β-地贫(非β0/β0) | 体外 | 自体CD34+ HSPC | 破坏BCL11A的红系特异增强子 | 临床I/II期 | 招募中 |
| NCT04205435 | 中国 | 重型β地贫 | 体外 | 自体HSC | 修复HBB IVS2-654(C>T)突变 | 临床I/II期 | 未开始招募 |
| NCT03728322 | N/As | 地贫 | 体外 | 自体HSC | HBB基因突变的原位修复 | 早期I期临床 | 未开始招募 |
| NCT04037566 | 中国 | CD19+白血病/淋巴瘤 | 体外 | 自体T细胞 | 敲除靶向CD19c的CAR-Td细胞内源性HPK1e | 临床I期 | 招募中 |
| NCT04035434 | 美国、澳大利亚和德国 | CD19+ B细胞恶性肿瘤 | 体外 | 异体T细胞 | 敲入靶向CD19的CAR并敲除TCRf和MHC Ig | 临床I/II期 | 招募中 |
| NCT03166878 | 中国 | CD19+ B细胞白血病/淋巴瘤 | 体外 | 异体T细胞 | 敲除靶向CD19的CAR-T细胞内源性TCR和B2Mh | 临床I/II期 | 招募中 |
| NCT03398967 | 中国 | 复发难治性B细胞恶性肿瘤 | 体外 | 异体T细胞 | N/A | 临床I/II期 | 招募中 |
| NCT03690011 | 美国 | T细胞白血病或淋巴瘤 | 体外 | 自体T细胞 | 敲除靶向CD7i和CD28j的CAR-T细胞内源性CD7 | 临床I期 | 未开始招募 |
| NCT04244656 | 美国、澳大利亚和西班牙 | 多发性骨髓瘤 | 体外 | 异体T细胞 | 敲入靶向BCMAk的CAR并敲除TCR和MHC I | 临床I期 | 招募中 |
| NCT03399448 | 美国 | 多发性骨髓瘤 黑色素瘤 滑膜肉瘤 黏液样/圆形细胞脂肪肉瘤 | 体外 | 自体T细胞 | 敲除靶向 NY-ESO-1l阳性癌细胞的T细胞内源性TCR和 PDCD1m | 临床I期 | 终止 |
| NCT03044743 | 中国 | EBVn阳性IV期胃癌 EBV阳性鼻咽癌 EBV阳性淋巴瘤 | 体外 | 自体T细胞 | 敲除T细胞内源性PDCD1 | 临床I/II期 | 招募中 |
| NCT03747965 | 中国 | 间皮素阳性实体瘤 | 体外 | 靶向间皮素的CAR-T细胞 | 敲除靶向间皮素的CAR-T细胞内源性 PDCD1 | 临床I期 | 招募中 |
| NCT03545815 | 中国 | 间皮素阳性实体瘤 | 体外 | 靶向间皮素的CAR-T细胞 | 敲除靶向间皮素的CAR-T细胞内源性 PDCD1和TCR | 临床I期 | 招募中 |
| NCT04426669 | 美国 | 转移性胃肠上皮癌 | 体外 | 自体肿瘤浸润性淋巴细胞 | 破坏肿瘤浸润性淋巴细胞CISHo基因 | 临床I/II期 | 招募中 |
| NCT03081715 | 中国 | 食管癌 | 体外 | 自体T细胞 | 敲除T细胞内源性PDCD1 | N/A | 完成 |
| NCT02863913 | 中国 | 浸润性膀胱癌IV期 | 体外 | 自体T细胞 | 敲除T细胞内源性PDCD1 | 临床I期 | 撤回 |
| NCT04438083 | 澳大利亚 | 肾细胞癌 | 体外 | 异体T细胞 | 敲入靶向CD70的CAR并敲除TCR和MHC I | 临床I期 | 招募中 |
| NCT02867332 | N/A | 转移性肾细胞癌 | 体外 | 自体T细胞 | 敲除T细胞内源性PDCD1 | 临床I期 | 撤回 |
| NCT编号t | 国家 | 治疗疾病 | 治疗途径 | 编辑细胞 | 编辑方式 | 试验阶段 | 状态 |
| NCT02867345 | 中国 | 激素难治性前列腺癌 | 体外 | 自体T细胞 | 敲除T细胞内源性PDCD1 | N/A | 撤回 |
| NCT02793856 | 中国 | 转移性非小细胞肺癌 | 体外 | 自体T细胞 | 敲除T细胞内源性PDCD1 | 临床I期 | 未开始招募 |
| NCT04417764 | 中国 | 晚期肝癌 | 体外 | 自体T细胞 | 敲除T细胞内源性PDCD1 | 临床I期 | 招募中 |
| NCT03057912 | 中国 | 人乳头瘤病毒相关恶性肿瘤 | 阴道栓剂 | 人乳头瘤病毒 | 破坏人乳头瘤病毒E6/E7基因 | 临床I期 | 未知 |
| NCT03164135 | 中国 | HIV-1感染合并有血液肿瘤 | 体外 | 异体CD34+ HSPC | 敲除CCR5p基因 | N/A | 招募中 |
| NCT03855631 | 法国 | 歌舞伎综合征1 | 细胞水平研究 | 自体成纤维细胞 | 表观修饰提升野生KMT2Dq表达 | N/A | 未开始招募 |
| NCT03872479 | 美国 | Leber先天性黑蒙症10型 | 体内(视网膜下注射) | N/A | 破坏CEP290 IVS26 A>G突变r | 临床I/II期 | 招募中 |
| [1] |
Taher AT, Weatherall DJ, Cappellini MD . Thalassaemia. Lancet, 2018,391(10116):155-167.
doi: 10.1016/S0140-6736(17)31822-6 pmid: 28774421 |
| [2] |
Farashi S, Harteveld CL . Molecular basis of α-thalassemia. Blood Cells Mol Dis, 2018,70:43-53.
doi: 10.1016/j.bcmd.2017.09.004 pmid: 29032940 |
| [3] |
Zeng YT, Huang SZ . Disorders of haemoglobin in China. J Med Genet, 1987,24(10):578-583.
doi: 10.1136/jmg.24.10.578 pmid: 3500312 |
| [4] | Liu JW, Hong T, Qin X, Liang YM, Zhang P . Recent advance on genome editing for therapy of β-hemoglobinopathies. Hereditas(Beijing), 2018,40(2):95-103. |
| 刘佳伟, 洪涛, 秦鑫, 梁英民, 张萍 . β-血红蛋白病基因组编辑治疗的研究进展. 遗传, 2018,40(2):95-103. | |
| [5] |
Santagostino E, Fasulo MR . Hemophilia A and hemophilia B: different types of diseases? Semin Thromb Hemost, 2013,39(7):697-701.
doi: 10.1055/s-0033-1353996 pmid: 24014073 |
| [6] |
Blaese RM, Culver KW, Miller AD, Carter CS, Fleisher T, Clerici M, Shearer G, Chang L, Chiang Y, Tolstoshev P, Greenblatt JJ, Rosenberg SA, Klein H, Berger M, Mullen CA, Ramsey WJ, Muul L, Morgan RA, Anderson WF . T lymphocyte-directed gene therapy for ADA- SCID: initial trial results after 4 years. Science, 1995,270(5235):475-480.
doi: 10.1126/science.270.5235.475 pmid: 7570001 |
| [7] | Niu XR, Yin SM, Chen X, Shao TT, Li DL . Gene editing technology and its recent progress in disease therapy. Hereditas(Beijing), 2019,41(7):582-598. |
| 牛煦然, 尹树明, 陈曦, 邵婷婷, 李大力 . 基因编辑技术及其在疾病治疗中的研究进展. 遗传, 2019,41(7):582-598. | |
| [8] |
Nathwani AC, Davidoff AM, Tuddenham EGD . Advances in gene therapy for hemophilia. Hum Gene Ther, 2017,28(11):1004-1012.
doi: 10.1089/hum.2017.167 pmid: 28835123 |
| [9] |
Peyvandi F, Garagiola I . Clinical advances in gene therapy updates on clinical trials of gene therapy in haemophilia. Haemophilia, 2019,25(5):738-746.
doi: 10.1111/hae.13816 pmid: 31282050 |
| [10] |
Zeng YT, Huang SZ, Ren ZR, Lu ZH, Zeng FY, Schechter AN, Rodgers GP . Hydroxyurea therapy in beta-thalassaemia intermedia: improvement in haematological parameters due to enhanced beta-globin synthesis. Br J Haematol, 1995,90(3):557-563.
pmid: 7646994 |
| [11] |
Xie SY, Li W, Ren ZR, Huang SZ, Zeng FY, Zeng YT . Correction of β654-thalassaemia mice using direct intravenous injection of siRNA and antisense RNA vectors. Int J Hematol, 2011,93(3):301-310.
doi: 10.1007/s12185-010-0727-1 |
| [12] |
Xie SY, Ren ZR, Zhang JZ, Guo XB, Wang QX, Wang S, Lin D, Gong XL, Li W, Huang SZ, Zeng FY, Zeng YT . Restoration of the balanced alpha/beta-globin gene expression in beta654-thalassemia mice using combined RNAi and antisense RNA approach. Hum Mol Genet, 2007,16(21):2616-2625.
doi: 10.1093/hmg/ddm218 pmid: 17716993 |
| [13] |
Davis R, Gurumurthy A, Hossain MA, Gunn EM, Bungert J . Engineering globin gene expression. Mol Ther Methods Clin Dev, 2018,12:102-110.
doi: 10.1016/j.omtm.2018.12.004 pmid: 30603654 |
| [14] |
Elalfy MS, Adly AAM, Ismail EA, Elhenawy YI, Elghamry IR . Therapeutic superiority and safety of combined hydroxyurea with recombinant human erythropoietin over hydroxyurea in young β-thalassemia intermedia patients. Eur J Haematol, 2013,91(6):522-533.
doi: 10.1111/ejh.12182 |
| [15] | Xie F, Ye L, Chang JC, Beyer AI, Wang JM, Muench MO, Kan YW . Seamless gene correction of β-thalassemia mutations in patient-specific iPSCs using CRISPR/Cas9 and piggyBac. Genome Res, 2014,24(9):1526-1533. |
| [16] | Ren YX, Xiao RD, Lou XM, Fang XD . Research advance and application in the gene therapy of gene editing technologies. Hereditas (Beijing), 2019,41(1):18-27. |
| 任云晓, 肖茹丹, 娄晓敏, 方向东 . 基因编辑技术及其在基因治疗中的应用. 遗传, 2019,41(1):18-27. | |
| [17] |
Garneau JE, Dupuis MÈ, Villion M, Romero DA, Barrangou R, Boyaval P, Fremaux C, Horvath P, Magadán AH, Moineau S . The CRISPR/Cas bacterial immune system cleaves bacteriophage and plasmid DNA. Nature, 2010,468(7320):67-71.
doi: 10.1038/nature09523 pmid: 21048762 |
| [18] |
Deltcheva E, Chylinski K, Sharma CM, Gonzales K, Chao YJ, Pirzada ZA, Eckert MR, Vogel J, Charpentier E . CRISPR RNA maturation by trans-encoded small RNA and host factor RNase III. Nature, 2011,471(7340):602-607.
doi: 10.1038/nature09886 pmid: 21455174 |
| [19] |
Jinek M, Chylinski K, Fonfara I, Haue M, Doudna JA, Charpentier E . A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science, 2012,337(6096):816-821.
doi: 10.1126/science.1225829 pmid: 22745249 |
| [20] | Mali P, Yang LH, Esvelt KM, Aach J, Guell M , DiCarlo JE, Norville JE, Church GM. RNA-guided human genome engineering via Cas9. Science, 2013,339(6121):823-826. |
| [21] |
Zhang JH, Adikaram P, Pandey M, Genis A, Simonds WF . Optimization of genome editing through CRISPR-Cas9 engineering. Bioengineered, 2016,7(3):166-174.
doi: 10.1080/21655979.2016.1189039 pmid: 27340770 |
| [22] |
Chang HHY, Pannunzio NR, Adachi N, Lieber MR . Non-homologous DNA end joining and alternative pathways to double-strand break repair. Nat Rev Mol Cell Biol, 2017,18(8):495-506.
pmid: 28512351 |
| [23] | Li GL, Zhong CL, Mo JX, Quan R, Wu ZF, Li ZC, Yang HQ, Zhang XW . Advances in site-specific integration of transgene in animal genome. Hereditas (Beijing), 2017,39(2):98-109. |
| 李国玲, 钟翠丽, 莫健新, 全绒, 吴珍芳, 李紫聪, 杨化强, 张献伟 . 动物基因组定点整合转基因技术研究进展. 遗传, 2017,39(2):98-109. | |
| [24] | Zong Y, Gao CX . Progress on base editing systems. Hereditas (Beijing), 2019,41(9):777-800. |
| 宗媛, 高彩霞 . 碱基编辑系统研究进展. 遗传, 2019,41(9):777-800. | |
| [25] |
Liang PP, Sun HW, Sun Y, Zhang XY, Xie XW, Zhang JR, Zhang Z, Chen YX, Ding CH, Xiong YY, Ma WB, Liu D, Huang JJ, Zhou SY . Effective gene editing by high-fidelity base editor 2 in mouse zygotes. Protein Cell, 2017,8(8):601-611.
doi: 10.1007/s13238-017-0418-2 pmid: 28585179 |
| [26] |
Kim K, Ryu SM, Kim ST, Baek G, Kim D, Lim K, Chung E, Kim S, Kim JS . Highly efficient RNA-guided base editing in mouse embryos. Nat Biotechnol, 2017,35(5):435-437.
doi: 10.1038/nbt.3816 pmid: 28244995 |
| [27] |
Lai KT, Huang GF, Su L, He YY . The prevalence of thalassemia in mainland China: evidence from epidemiological surveys. Sci Rep, 2017,7(1):920.
doi: 10.1038/s41598-017-00967-2 pmid: 28424478 |
| [28] |
Niu XH, He WY, Song B, Ou ZH, Fan D, Chen YC, Fan Y, Sun XF . Combining single strand oligodeoxynucleotides and CRISPR/Cas9 to correct gene mutations in β-thalassemia-induced pluripotent stem cells. J Biol Chem, 2016,291(32):16576-16585.
doi: 10.1074/jbc.M116.719237 |
| [29] |
Song B, Fan Y, He WY, Zhu DT, Niu XH, Wang D, Ou ZH, Luo M, Sun XF . Improved hematopoietic differentiation efficiency of gene-corrected beta-thalassemia induced pluripotent stem cells by CRISPR/Cas9 system. Stem Cells Dev, 2015,24(9):1053-1065.
doi: 10.1089/scd.2014.0347 pmid: 25517294 |
| [30] |
Liu YL, Yang Y, Kang XJ, Lin B, Yu Q, Song B, Gao G, Chen YY, Sun XF, Li XP, Bu L, Fan Y . One-step biallelic and scarless correction of a β-thalassemia mutation in patient-specific iPSCs without drug selection. Mol Ther Nucleic Acids, 2017,6:57-67.
doi: 10.1016/j.omtn.2016.11.010 pmid: 28325300 |
| [31] |
Park SH, Lee CM, Dever DP, Davis TH, Camarena J, Srifa W, Zhang YK, Paikari A, Chang AK, Porteus MH, Sheehan VA, Bao G . Highly efficient editing of the β-globin gene in patient-derived hematopoietic stem and progenitor cells to treat sickle cell disease. Nucleic Acids Res, 2019,47(15):7955-7972.
doi: 10.1093/nar/gkz475 pmid: 31147717 |
| [32] |
Wattanapanitch M, Damkham N, Potirat P, Trakarnsanga K, Janan M, U-pratya Y, Kheolamai P, Klincumhom N, Issaragrisil S,. One-step genetic correction of hemoglobin E/beta-thalassemia patient-derived iPSCs by the CRISPR/Cas9 system. Stem Cell Res Ther, 2018,9(1):46.
doi: 10.1186/s13287-018-0779-3 |
| [33] |
Antony JS, Latifi N, Haque AKMA, Lamsfus-Calle A, Daniel-Moreno A, Graeter S, Baskaran P, Weinmann P, Mezger M, Handgretinger R, Kormann MSD . Gene correction of HBB mutations in CD34 + hematopoietic stem cells using Cas9 mRNA and ssODN donors . Mol Cell Pediatr, 2018,5(1):9.
doi: 10.1186/s40348-018-0086-1 pmid: 30430274 |
| [34] |
Fang YD, Cheng Y, Lu D, Gong XL, Yang GH, Gong ZJ, Zhu YW, Sang X, Fan SY, Zhang JZ, Zeng FY . Treatment of β 654 -thalassaemia by TALENs in a mouse model . Cell Prolif, 2018,51(6):e12491.
doi: 10.1111/cpr.12491 pmid: 30070404 |
| [35] |
Xu SQ, Luk K, Yao QM, Shen AH, Zeng J, Wu YX, Luo HY, Brendel C, Pinello L, Chui DHK, Wolfe SA, Bauer DE . Editing aberrant splice sites efficiently restores β-globin expression in β-thalassemia. Blood, 2019,133(21):2255-2262.
doi: 10.1182/blood-2019-01-895094 pmid: 30704988 |
| [36] |
Park CY, Kim DH, Son JS, Sung JJ, Lee J, Bae S, Kim JH, Kim DW, Kim JS . Functional correction of large factor VIII gene chromosomal inversions in hemophilia A patient-derived iPSCs using CRISPR-Cas9. Cell Stem Cell, 2015,17(2):213-220.
doi: 10.1016/j.stem.2015.07.001 pmid: 26212079 |
| [37] |
Peng Z, Zhou WC, Fu WQ, Du RQ, Jin L, Zhang F . Correlation between frequency of non-allelic homologous recombination and homology properties: evidence from homology-mediated CNV mutations in the human genome. Hum Mol Genet, 2015,24(5):1225-1233.
doi: 10.1093/hmg/ddu533 pmid: 25324539 |
| [38] |
Sasaki M, Lange J, Keeney S . Genome destabilization by homologous recombination in the germ line. Nat Rev Mol Cell Biol, 2010,11(3):182-195.
doi: 10.1038/nrm2849 pmid: 20164840 |
| [39] |
Park CY, Kim J, Kweon J, Son JS, Lee JS, Yoo JE, Cho SR, Kim JH, Kim JS, Kim DW . Targeted inversion and reversion of the blood coagulation factor 8 gene in human iPS cells using TALENs. Proc Natl Acad Sci USA, 2014,111(25):9253-9258.
doi: 10.1073/pnas.1323941111 |
| [40] |
George CM, Alani E . Multiple cellular mechanisms prevent chromosomal rearrangements involving repetitive DNA. Crit Rev Biochem Mol Biol, 2012,47(3):297-313.
doi: 10.3109/10409238.2012.675644 pmid: 22494239 |
| [41] |
Zeng J, Wu YX, Ren CY, Bonanno J, Shen AH, Shea D, Gehrke JM, Clement K, Luk K, Yao QM, Kim R, Wolfe SA, Manis JP, Pinello L, Joung JK, Bauer DE . Therapeutic base editing of human hematopoietic stem cells. Nat Med, 2020,26(4):535-541.
doi: 10.1038/s41591-020-0790-y pmid: 32284612 |
| [42] |
Zhou CY, Sun YD, Yan R, Liu YJ, Zuo EW, Gu C, Han LX, Wei Y, Hu XD, Zeng R, Li YX, Zhou HB, Guo F, Yang H . Off-target RNA mutation induced by DNA base editing and its elimination by mutagenesis. Nature, 2019,571(7764):275-278.
doi: 10.1038/s41586-019-1314-0 pmid: 31181567 |
| [43] |
Thein SL . Molecular basis of β thalassemia and potential therapeutic targets. Blood Cells Mol Dis, 2018,70:54-65.
doi: 10.1016/j.bcmd.2017.06.001 pmid: 28651846 |
| [44] |
Mettananda S, Fisher CA, Hay D, Badat M, Quek L, Clark K, Hublitz P, Downes D, Kerry J, Gosden M, Telenius J, Sloane-Stanley JA, Faustino P, Coelho A, Doondeea J, Usukhbayar B, Sopp P, Sharpe JA, Hughes JR, Vyas P, Gibbons RJ, Higgs DR . Editing an α-globin enhancer in primary human hematopoietic stem cells as a treatment for β-thalassemia. Nat Commun, 2017,8(1):424.
doi: 10.1038/s41467-017-00479-7 pmid: 28871148 |
| [45] |
Wu YX, Zeng J, Roscoe BP, Liu PP, Yao QM, Lazzarotto CR, Clement K, Cole MA, Luk K, Baricordi C, Shen AH, Ren CY, Esrick EB, Manis JP, Dorfman DM, Williams DA, Biffi A, Brugnara C, Biasco L, Brendel C, Pinello L, Tsai SQ, Wolfe SA, Bauer DE . Highly efficient therapeutic gene editing of human hematopoietic stem cells. Nat Med, 2019,25(5):776-783.
doi: 10.1038/s41591-019-0401-y pmid: 30911135 |
| [46] |
Martyn GE, Wienert B, Yang L, Shah M, Norton LJ, Burdach J, Kurita R, Nakamura Y, Pearson RCM, Funnell APW, Quinlan KGR, Crossley M . Natural regulatory mutations elevate the fetal globin gene via disruption of BCL11A or ZBTB7A binding. Nat Genet, 2018,50(4):498-503.
doi: 10.1038/s41588-018-0085-0 pmid: 29610478 |
| [47] |
Martyn GE, Wienert B, Kurita R, Nakamura Y, Quinlan KGR, Crossley M . A natural regulatory mutation in the proximal promoter elevates fetal globin expression by creating a de novo GATA1 site. Blood, 2019,133(8):852-856.
doi: 10.1182/blood-2018-07-863951 pmid: 30617196 |
| [48] |
Antoniani C, Meneghini V, Lattanzi A, Felix T, Romano O, Magrin E, Weber L, Pavani G, Hoss SE, Kurita R, Nakamura Y, Cradick TJ, Lundberg AS, Porteus M, Amendola M, Nemer WE, Cavazzana M, Mavilio F, Miccio A . Induction of fetal hemoglobin synthesis by CRISPR/Cas9-mediated editing of the human β-globin locus. Blood, 2018,131(17):1960-1973.
doi: 10.1182/blood-2017-10-811505 pmid: 29519807 |
| [49] |
Canver MC, Smith EC, Sher F, Pinello L, Sanjana NE, Shalem O, Chen DD, Schupp PG, Vinjamur DS, Garcia SP, Luc S, Kurita R, Nakamura Y, Fujiwara Y, Maeda T, Yuan GC, Zhang F, Orkin SH, Bauer DE . BCL11A enhancer dissection by Cas9-mediated in situ saturating mutagenesis. Nature, 2015,527(7577):192-197.
doi: 10.1038/nature15521 pmid: 26375006 |
| [50] |
Liu PT, Keller JR, Ortiz M, Tessarollo L, Rachel RA, Nakamura T, Jenkins NA, Copeland NG . Bcl11a is essential for normal lymphoid development. Nat Immunol, 2003,4(6):525-532.
doi: 10.1038/ni925 pmid: 12717432 |
| [51] |
Li J, Lai YR, Shi LL . BCL11A down-regulation induces γ-globin in human β-thalassemia major erythroid cells. Hemoglobin, 2018,42(4):225-230.
doi: 10.1080/03630269.2018.1515774 pmid: 30821197 |
| [52] |
Wang LR, Li LX, Ma YL, Hu HD, Li Q, Yang Y, Liu WB, Yin SM, Li W, Fu B, Kurita R, Nakamura Y, Liu M, Lai YR, Li DL . Reactivation of γ-globin expression through Cas9 or base editor to treat β-hemoglobinopathies. Cell Res, 2020,30(3):276-278.
doi: 10.1038/s41422-019-0267-z pmid: 31911671 |
| [53] |
Grevet JD, Lan XJ, Hamagami N, Edwards CR, Sankaranarayanan L, Ji XJ, Bhardwaj SK, Face CJ, Posocco DF, Abdulmalik O, Keller CA, Giardine B, Sidoli S, Garcia BA, Chou ST, Liebhaber SA, Hardison RC, Shi JW, Blobel GA . Domain-focused CRISPR screen identifies HRI as a fetal hemoglobin regulator in human erythroid cells. Science, 2018,361(6399):285-290.
doi: 10.1126/science.aao0932 pmid: 30026227 |
| [54] |
Thompson AA, Walters MC, Kwiatkowski J, Rasko JEJ, Ribeil JA, Hongeng S, Magrin E, Schiller GJ, Payen E, Semeraro M, Moshous D, Lefrere F, Puy H, Bourget P, Magnani A, Caccavelli L, Diana JS, Suarez F, Monpoux F, Brousse V, Poirot C, Brouzes C, Meritet JF, Pondarré C, Beuzard Y, Chrétien S, Lefebvre T, Teachey DT, Anurathapan U, Ho PJ, von Kalle C, Kletzel M, Vichinsky E, Soni S, Veres G, Negre O, Ross RW, Davidson D, Petrusich A, Sandler L, Asmal M, Hermine O, Montalembert MD, Hacein-Bey-Abina S, Blanche S, Leboulch P, Cavazzana M,. Gene therapy in patients with transfusion-dependent β-thalassemia. N Engl J Med, 2018,378(16):1479-1493.
doi: 10.1056/NEJMoa1705342 pmid: 29669226 |
| [55] |
Chen YH, Keiser MS, Davidson BL . Viral vectors for gene transfer. Curr Protoc Mouse Biol, 2018,8(4):e58.
doi: 10.1002/cpmo.58 pmid: 30485696 |
| [56] |
Cunningham SC, Dane AP, Spinoulas A, Alexander IE . Gene delivery to the juvenile mouse liver using AAV2/8 vectors. Mol Ther, 2008,16(6):1081-1088.
doi: 10.1038/mt.2008.72 pmid: 18414478 |
| [57] |
George LA, Sullivan SK, Giermasz A, Rasko JEJ, Samelson-Jones BJ, Ducore J, Cuker A, Sullivan LM, Majumdar S, Teitel J, McGuinn CE, Ragni MV, Luk AY, Hui D, Wright JF, Chen YF, Liu Y, Wachtel K, Winters A, Tiefenbacher S, Arruda VR, van der Loo JCM, Zelenaia O, Takefman D, Carr ME, Couto LB, Anguela XM, High KA,. Hemophilia B gene therapy with a high-specific-activity factor IX variant. N Engl J Med, 2017,377(23):2215-2227.
doi: 10.1056/NEJMoa1708538 pmid: 29211678 |
| [58] |
Wang LL, Yang Y, Breton CA, White J, Zhang J, Che Y, Saveliev A, McMenamin D, He ZN, Latshaw C, Li MY, Wilson JM, . CRISPR/Cas9-mediated in vivo gene targeting corrects hemostasis in newborn and adult factor IX-knockout mice. Blood, 2019,133(26):2745-2752.
doi: 10.1182/blood.2019000790 pmid: 30975639 |
| [59] |
Baliou S, Adamaki M, Kyriakopoulos AM, Spandidos DA, Panayiotidis M, Christodoulou I, Zoumpourlis V . CRISPR therapeutic tools for complex genetic disorders and cancer (Review). Int J Oncol, 2018,53(2):443-468.
doi: 10.3892/ijo.2018.4434 pmid: 29901119 |
| [60] |
Cyranoski D . CRISPR gene-editing tested in a person for the first time. Nature, 2016,539(7630):479.
pmid: 27882996 |
| [61] |
Lu Y, Xue JX, Deng T, Zhou XJ, Yu K, Deng L, Huang MJ, Yi X, Liang MZ, Wang Y, Shen HG, Tong RZ, Wang WB, Li L, Song J, Li J, Su XX, Ding ZY, Gong YL, Zhu J, Wang YS, Zou BW, Zhang Y, Li YY, Zhou L, Liu YM, Yu M, Wang YQ, Zhang XW, Yin LM, Xia XF, Zeng Y, Zhou Q, Ying BW, Chen C, Wei YQ, Li WM, Mok T . Safety and feasibility of CRISPR-edited T cells in patients with refractory non-small-cell lung cancer. Nat Med, 2020,26(5):732-740.
doi: 10.1038/s41591-020-0840-5 pmid: 32341578 |
| [62] |
Stadtmauer EA, Fraietta JA, Davis MM, Cohen AD, Weber KL, Lancaster E, Mangan PA, Kulikovskaya I, Gupta M, Chen F, Tian LF, Gonzalez VE, Xu J, Jung IY, Melenhorst JJ, Plesa G, Shea J, Matlawski T, Cervini A, Gaymon AL, Desjardins S, Lamontagne A, Salas-Mckee J, Fesnak A, Siegel DL, Levine BL, Jadlowsky JK, Young RM, Chew A, Hwang WT, Hexner EO, Carreno BM, Nobles CL, Bushman FD, Parker KR, Qi YY, Satpathy AT, Chang HY, Zhao YB, Lacey SF, June CH. CRISPR-engineered T cells in patients with refractory cancer. Science, 2020, 367(6481): eaba7365.
pmid: 32108095 |
| [63] |
Xu L, Wang J, Liu YL, Xie LF, Su B, Mou DL, Wang LT, Liu TT, Wang XB, Zhang B, Zhao L, Hu LD, Ning HM, Zhang YF, Deng K, Liu LF, Lu XF, Zhang T, Xu J, Li C, Wu H, Deng HK, Chen H . CRISPR-edited stem cells in a patient with HIV and acute lymphocytic leukemia. N Engl J Med, 2019,381(13):1240-1247.
doi: 10.1056/NEJMoa1817426 pmid: 31509667 |
| [64] |
Wang DW, Wang K, Cai YJ . An overview of development in gene therapeutics in China. Gene Ther, 2020,27(7-8):338-348.
pmid: 32528163 |
| [65] |
Park JH, Geyer MB, Brentjens RJ . CD19-targeted CAR T-cell therapeutics for hematologic malignancies: interpreting clinical outcomes to date. Blood, 2016,127(26):3312-3320.
doi: 10.1182/blood-2016-02-629063 pmid: 27207800 |
| [66] |
Fu YF, Foden JA, Khayter C, Maeder ML, Reyon D, Joung JK, Sander JD . High-frequency off-target mutagenesis induced by CRISPR-Cas nucleases in human cells. Nat Biotechnol, 2013,31(9):822-826.
doi: 10.1038/nbt.2623 pmid: 23792628 |
| [67] |
Cullot G, Boutin J, Toutain J, Prat F, Pennamen P, Rooryck C, Teichmann M, Rousseau E, Lamrissi-Garcia I, Guyonnet-Duperat V, Bibeyran A, Lalanne M, Prouzet- Mauléon V, Turcq B, Ged C, Blouin JM, Richard E, Dabernat S, Moreau-Gaudry F, Bedel A . CRISPR-Cas9 genome editing induces megabase-scale chromosomal truncations. Nat Commun, 2019,10(1):1136.
doi: 10.1038/s41467-019-09006-2 pmid: 30850590 |
| [68] |
Lattanzi A, Meneghini V, Pavani G, Amor F, Ramadier S, Felix T, Antoniani C, Masson C, Alibeu O, Lee C, Porteus MH, Bao G, Amendola M, Mavilio F, Miccio A . Optimization of CRISPR/Cas9 delivery to human hematopoietic stem and progenitor cells for therapeutic genomic rearrangements. Mol Ther, 2019,27(1):137-150.
pmid: 30424953 |
| [69] |
Dever DP, Bak RO, Reinisch A, Camarena J, Washington G, Nicolas CE, Pavel-Dinu M, Saxena N, Wilkens AB, Mantri S, Uchida N, Hendel A, Narla A, Majeti R, Weinberg KI, Porteus MH . CRISPR/Cas9 β-globin gene targeting in human haematopoietic stem cells. Nature, 2016,539(7629):384-389.
pmid: 27820943 |
| [70] |
Doench JG, Fusi N, Sullender M, Hegde M, Vaimberg EW, Donovan KF, Smith I, Tothova Z, Wilen C, Orchard R, Virgin HW, Listgarten J, Root DE . Optimized sgRNA design to maximize activity and minimize off-target effects of CRISPR-Cas9. Nat Biotechnol, 2016,34(2):184-191.
doi: 10.1038/nbt.3437 pmid: 26780180 |
| [71] |
Hendel A, Bak RO, Clark JT, Kennedy AB, Ryan DE, Roy S, Steinfeld I, Lunstad BD, Kaiser RJ, Wilkens AB, Bacchetta R, Tsalenko A, Dellinger D, Bruhn L, Porteus MH . Chemically modified guide RNAs enhance CRISPR- Cas genome editing in human primary cells. Nat Biotechnol, 2015,33(9):985-989.
doi: 10.1038/nbt.3290 pmid: 26121415 |
| [72] |
Vakulskas CA, Dever DP, Rettig GR, Turk R, Jacobi AM, Collingwood MA, Bode NM, McNeill MS, Yan SQ, Camarena J, Lee CM, Park SH, Wiebking V, Bak RO, Gomez-Ospina N, Pavel-Dinu M, Sun WC, Bao G, Porteus MH, Behlke MA,. A high-fidelity Cas9 mutant delivered as a ribonucleoprotein complex enables efficient gene editing in human hematopoietic stem and progenitor cells. Nat Med, 2018,24(8):1216-1224.
doi: 10.1038/s41591-018-0137-0 pmid: 30082871 |
| [73] |
Shen MW, Arbab M, Hsu JY, Worstell D, Culbertson SJ, Krabbe O, Cassa CA, Liu DR, Gifford DK, Sherwood RI . Predictable and precise template-free CRISPR editing of pathogenic variants. Nature, 2018,563(7733):646-651.
pmid: 30405244 |
| [74] |
Yang YY, Zhang XB, Yi L, Hou ZZ, Chen JY, Kou XC, Zhao YH, Wang H, Sun XF, Jiang CZ, Wang YX, Gao SR . Naïve induced pluripotent stem cells generated from β-thalassemia fibroblasts allow efficient gene correction with CRISPR/Cas9. Stem Cells Transl Med, 2016,5(2):267.
doi: 10.5966/sctm.2015-0157erratum |
| [75] |
Volarevic V, Markovic BS, Gazdic M, Volarevic A, Jovicic N, Arsenijevic N, Armstrong L, Djonov V, Lako M, Stojkovic M . Ethical and safety issues of stem cell-based therapy. Int J Med Sci, 2018,15(1):36-45.
doi: 10.7150/ijms.21666 pmid: 29333086 |
| [76] |
Paes BCMF, Moço PD, Pereira CG, Porto GS, de Sousa Russo EM, Reis LCJ, Covas DT, Picanço-Castro V,. Ten years of iPSC: clinical potential and advances in vitro hematopoietic differentiation. Cell Biol Toxicol, 2017,33(3):233-250.
doi: 10.1007/s10565-016-9377-2 pmid: 28039590 |
| [77] |
Tan YT, Ye L, Xie F, Beyer AI, Muench MO, Wang JM, Chen Z, Liu H, Chen SJ, Kan YW . Respecifying human iPSC-derived blood cells into highly engraftable hematopoietic stem and progenitor cells with a single factor. Proc Natl Acad Sci USA, 2018,115(9):2180-2185.
pmid: 29386396 |
| [78] |
Uchida N, Haro-Mora JJ, Fujita A, Lee DY, Winkler T, Hsieh MM, Tisdale JF . Efficient generation of β-globin- expressing erythroid cells using stromal cell-derived induced pluripotent stem cells from patients with sickle cell disease. Stem Cells, 2017,35(3):586-596.
doi: 10.1002/stem.2517 pmid: 27739611 |
| [1] | Bingzheng Wang, Chao Zhang, Jiali Zhang, Jin Sun. Conditional editing of the Drosophila melanogaster genome using single transcripts expressing Cas9 and sgRNA [J]. Hereditas(Beijing), 2023, 45(7): 593-601. |
| [2] | Meizhen Liu, Liren Wang, Yongmei Li, Xueyun Ma, Honghui Han, Dali Li. Generation of genetically modified rat models via the CRISPR/Cas9 technology [J]. Hereditas(Beijing), 2023, 45(1): 78-87. |
| [3] | Xiaojun Zhang, Kun Xu, Juncen Shen, Lu Mu, Hongrun Qian, Jieyu Cui, Baoxia Ma, Zhilong Chen, Zhiying Zhang, Zehui Wei. A CRISPR/Cas9-Gal4BD donor adapting system for enhancing homology-directed repair [J]. Hereditas(Beijing), 2022, 44(8): 708-719. |
| [4] | Chong Zhang, Zixuan Wei, Min Wang, Yaosheng Chen, Zuyong He. Editing MC1R in human melanoma cells by CRISPR/Cas9 and functional analysis [J]. Hereditas(Beijing), 2022, 44(7): 581-590. |
| [5] | Yao Liu, Xianhui Zhou, Shuhong Huang, Xiaolong Wang. Prime editing: a search and replace tool with versatile base changes [J]. Hereditas(Beijing), 2022, 44(11): 993-1008. |
| [6] | Yuting Han, Bowen Xu, Yutong Li, Xinyi Lu, Xizhi Dong, Yuhao Qiu, Qinyun Che, Ruibao Zhu, Li Zheng, Xiaochen Li, Xu Si, Jianquan Ni. The cutting edge of gene regulation approaches in model organism Drosophila [J]. Hereditas(Beijing), 2022, 44(1): 3-14. |
| [7] | Guangwu Yang, Yuan Tian. The F-box gene Ppa promotes lipid storage in Drosophila [J]. Hereditas(Beijing), 2021, 43(6): 615-622. |
| [8] | Dingwei Peng, Ruiqiang Li, Wu Zeng, Min Wang, Xuan Shi, Jianhua Zeng, Xiaohong Liu, Yaoshen Chen, Zuyong He. Editing the cystine knot motif of MSTN enhances muscle development of Liang Guang Small Spotted pigs [J]. Hereditas(Beijing), 2021, 43(3): 261-270. |
| [9] | Na Wang, Zhilian Jia, Qiang Wu. RFX5 regulates gene expression of the Pcdhα cluster [J]. Hereditas(Beijing), 2020, 42(8): 760-774. |
| [10] | Guoling Li, Shanxin Yang, Zhenfang Wu, Xianwei Zhang. Recent developments in enhancing the efficiency of CRISPR/Cas9- mediated knock-in in animals [J]. Hereditas(Beijing), 2020, 42(7): 641-656. |
| [11] | Yingnan Chen, Jing Lu. Application of CRISPR/Cas9 mediated gene editing in trees [J]. Hereditas(Beijing), 2020, 42(7): 657-668. |
| [12] | Siyuan Liu, Guoqiang Yi, Zhonglin Tang, Bin Chen. Progress on genome-wide CRISPR/Cas9 screening for functional genes and regulatory elements [J]. Hereditas(Beijing), 2020, 42(5): 435-443. |
| [13] | Minting Lin, Lulu Lai, Miao Zhao, Biwei Lin, Xiangping Yao. Construction of a striatum-specific Slc20a2 gene knockout mice model by CRISPR/Cas9 AAV system [J]. Hereditas(Beijing), 2020, 42(10): 1017-1027. |
| [14] | Peifeng Liu, Qiang Wu. Probing 3D genome by CRISPR/Cas9 [J]. Hereditas(Beijing), 2020, 42(1): 18-31. |
| [15] | Xuran Niu,Shuming Yin,Xi Chen,Tingting Shao,Dali Li. Gene editing technology and its recent progress in disease therapy [J]. Hereditas(Beijing), 2019, 41(7): 582-598. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||