遗传 ›› 2022, Vol. 44 ›› Issue (6): 531-542.doi: 10.16288/j.yczz.22-011
• 技术与方法 • 上一篇
宋绍征1( ), 何正义2(
), 何正义2( ), 成勇3(
), 成勇3( ), 于宝利3, 张婷3, 李丹1
), 于宝利3, 张婷3, 李丹1
收稿日期:2022-01-11
修回日期:2022-03-29
出版日期:2022-06-20
发布日期:2022-05-10
作者简介:宋绍征,博士,研究方向:转基因与胚胎工程。E-mail: 基金资助:
Shaozheng Song1( ), Zhengyi He2(
), Zhengyi He2( ), Yong Cheng3(
), Yong Cheng3( ), Baoli Yu3, Ting Zhang3, Dan Li1
), Baoli Yu3, Ting Zhang3, Dan Li1
Received:2022-01-11
Revised:2022-03-29
Online:2022-06-20
Published:2022-05-10
Supported by:摘要:
肌肉生长抑制素(myostatin, MSTN)是一种骨骼肌生长发育的负调控因子,可以抑制成肌细胞的增殖,是动物品种改良的重要候选基因,该基因突变能够引起骨骼肌的广泛性增生与肥大,产生“双肌”症状,导致动物脂肪分化减少、肌肉含量增加,从而满足市场动物肉品质消费的需求。为了获得“双肌”表型的MSTN基因突变克隆山羊,本研究在山羊MSTN基因第一外显子序列设计并构建TALENs表达载体,转染山羊胎儿成纤维细胞,经嘌呤霉素筛选获得抗性细胞株,通过PCR检测和基因测序筛选MSTN基因突变细胞株作为供核细胞进行山羊体细胞核移植,并鉴定MSTN基因突变类型;通过组织切片分析MSTN基因突变山羊肌肉组织形态结构,监测不同月龄克隆山羊体重并分析其不同生长发育阶段的体重增长趋势。结果表明,共获得109株MSTN基因突变细胞株,突变效率达到79.0% (109/138),其中46株为双等位基因突变,占细胞株总数的33.3% (46/138)。选取4株生长状态较好的MSTN基因突变细胞株(1株为双等位基因纯合突变,3株为非纯合突变)进行体细胞核移植至12只受体山羊,30 d时B超检查出受孕母羊4只,受孕率为33.3% (4/12),妊娠到期分娩2只克隆山羊,测序结果表明M-1克隆山羊MSTN双等位基因中的一个等位基因未产生突变,另一个等位基因缺失3 bp;M-2克隆山羊MSTN双等位基因中的一个等位基因产生1 bp碱基插入,另一个等位基因缺失13 bp,两种突变均造成MSTN在突变位点之后的蛋白序列丢失。此外,M-1克隆山羊肌纤维排列紧密且粗大,每月体重均高于普通野生型山羊,但与普通野生型山羊生长趋势一致,且能够健康发育至成年。本研究利用TALENs技术成功介导山羊MSTN基因定点修饰,并获得MSTN基因突变的山羊胎儿成纤维细胞,将其作为供核细胞成功生产MSTN基因突变克隆山羊,为培育“双肌”表型山羊新品系奠定了技术基础,也为将来其他转基因动物的制备提供了参考方法。
宋绍征, 何正义, 成勇, 于宝利, 张婷, 李丹. TALENs介导MSTN基因突变山羊的制备及性能分析[J]. 遗传, 2022, 44(6): 531-542.
Shaozheng Song, Zhengyi He, Yong Cheng, Baoli Yu, Ting Zhang, Dan Li. MSTN modification in goat mediated by TALENs and performance analysis[J]. Hereditas(Beijing), 2022, 44(6): 531-542.

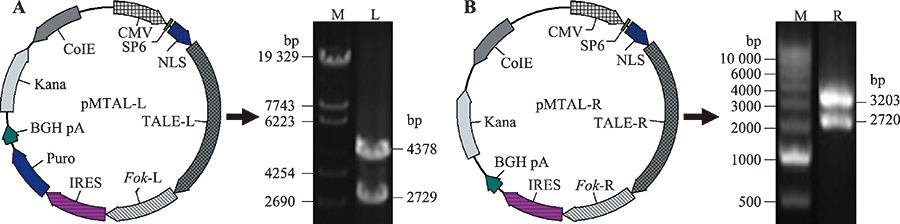
图1
TALENs载体示意图与质粒酶切鉴定 A:pMTAL-L质粒载体结构图及Cla I酶切电泳图谱。M:λ-EcoT14 digest DNA marker;L:pMTAL-L质粒。B:pMTAL-R质粒载体结构图及Cla I酶切电泳图谱。M:DL10000 DNA marker;L:pMTAL-R质粒。CMV:巨细胞病毒启动子;SP6:启动子/测序引物;NLS:核定位信号;TALE-L/R:转录激活因子样效应蛋白-左臂/右臂;Fok-L/R:限制性核酸内切酶-左臂/右臂;IRES:内部核糖体进入位点;BGH pA:嘌呤霉素筛选基因;Kana:卡那霉素筛选基因;CoIE:大肠杆菌复制子。"
| [1] |
Na RS, Ni WW, E GX,Zeng Y,Han YG,Huang YF. SNP screening of the MSTN gene and correlation analysis between genetic polymorphisms and growth traits in Dazu black goat. Anim Biotechnol, 2021, 32(5):558-565.
doi: 10.1080/10495398.2020.1727915 |
| [2] |
Jabbar A, Zulfiqar F, Mahnoor M, Mushtaq N, Zaman MH, Din ASU, Khan MA, Ahmad HI. Advances and perspectives in the application of CRISPR-Cas9 in livestock. Mol Biotechnol, 2021, 63(9):757-767.
doi: 10.1007/s12033-021-00347-2 pmid: 34041717 |
| [3] |
Tanihara F, Hirata M, Otoi T. Current status of the application of gene editing in pigs. J Reprod Dev, 2021, 67(3):177-187.
doi: 10.1262/jrd.2021-025 |
| [4] |
Lee JM, Kim U, Yang H, Ryu B, Kim J, Sakuma T, Yamamoto T, Park JH. TALEN-mediated generation of Nkx3.1 knockout rat model. Prostate, 2021, 81(3):182-193.
doi: 10.1002/pros.24095 |
| [5] |
Park JW, Lee JH, Han JS, Shin SP, Park TS. Muscle differentiation induced by p53 signaling pathway-related genes in myostatin-knockout quail myoblasts. Mol Biol Rep, 2020, 47(12):9531-9540.
doi: 10.1007/s11033-020-05935-0 |
| [6] | Yang CC, Tong HL, Ma XH, Du W, Liu D, Yang Y, Yan YQ. Myostatin knockout in bovine fetal fibroblasts by using TALEN. Hereditas(Beijing), 2014, 36(7):685-690. |
| 杨翠翠, 佟慧丽, 马兴红, 杜巍, 刘丹, 杨宇, 严云勤. 利用TALEN技术在牛胎儿成纤维细胞中敲除Myostatin基因. 遗传, 2014, 36(7):685-690. | |
| [7] |
Pino-Barrio MJ, Giménez Y, Villanueva M, Hildenbeutel M, Sánchez-Dominguez R, Rodríguez-Perales S, Pujol R, Surrallés J, Río P, Cathomen T, Mussolino C, Bueren JA, Navarro S. TALEN mediated gene editing in a mouse model of Fanconi anemia. Sci Rep, 2020, 10(1):6997.
doi: 10.1038/s41598-020-63971-z pmid: 32332829 |
| [8] | Zhu HM, Liu J, Cui CC, Song YJ, Ge HT, Hu LY, Li Q, Jin YP, Zhang Y. Targeting human α-lactalbumin gene insertion into the goat β-lactoglobulin locus by TALEN- mediated homologous recombination. PLoS One, 2016, 11(6): e0156636. |
| [9] |
Lee SJ, Lehar A, Meir JU, Koch C, Morgan A, Warren LE, Rydzik R, Youngstrom DW, Chandok H, George J, Gogain J, Michaud M, Stoklasek TA, Liu YW, Germain-Lee EL. Targeting myostatin/activin A protects against skeletal muscle and bone loss during spaceflight. Proc Natl Acad Sci USA, 2020, 117(38):23942-23951.
doi: 10.1073/pnas.2014716117 |
| [10] |
Zhang CY, Liu Y, Xu DQ, Wen QY, Li X, Zhang WM, Yang LG. Polymorphisms of myostatin gene (MSTN) in four goat breeds and their effects on Boer goat growth performance. Mol Biol Rep, 2012, 39(3):3081-3087.
doi: 10.1007/s11033-011-1071-0 |
| [11] |
Grobet L, Martin LJ, Poncelet D, Pirottin D, Brouwers B, Riquet J, Schoeberlein A, Dunner S, Ménissier F, Massabanda J, Fries R, Hanset R, Georges M. A deletion in the bovine myostatin gene causes the double-muscled phenotype in cattle. Nat Genet, 1997, 17(1):71-74.
pmid: 9288100 |
| [12] |
Grisolia AB, D'Angelo GT,Porto Neto LR,Siqueira F,Garcia JF,. Myostatin (GDF8) single nucleotide polymorphisms in Nellore cattle. Genet Mol Res, 2009, 8(3):822-830.
doi: 10.4238/vol8-3gmr548 pmid: 19731204 |
| [13] |
Boman IA, Klemetsdal G, Blichfeldt T, Nafstad O, Våge DI. A frameshift mutation in the coding region of the myostatin gene (MSTN) affects carcass conformation and fatness in Norwegian White Sheep(Ovis aries). Anim Genet, 2009, 40(4):418-422.
doi: 10.1111/j.1365-2052.2009.01855.x pmid: 19392824 |
| [14] | Crispo M, Mulet AP, Tesson L, Barrera N, Cuadro F,dos Santos-Neto PC,Nguyen TH,Crénéguy A,Brusselle L,Anegón I,Menchaca A. Efficient generation of myostatin knock-out sheep using CRISPR/Cas9 technology and microinjection into zygotes. PLoS One, 2015, 10(8): e0136690. |
| [15] |
Wang X, Niu Y, Zhou J, Zhu H, Ma B, Yu H, Yan H, Hua J, Huang X, Qu L, Chen Y. CRISPR/Cas9-mediated MSTN disruption and heritable mutagenesis in goats causes increased body mass. Anim Genet, 2018, 49(1):43-51.
doi: 10.1111/age.12626 pmid: 29446146 |
| [16] |
Cermak T, Doyle EL, Christian M, Wang L, Zhang Y, Schmidt C, Baller JA, Somia NV, Bogdanove AJ, Voytas DF. Efficient design and assembly of custom TALEN and other TAL effector-based constructs for DNA targeting. Nucleic Acids Res, 2011, 39(12):e82.
doi: 10.1093/nar/gkr218 |
| [17] |
Zhang HX, Zhang Y, Yin H. Genome editing with mRNA encoding ZFN, TALEN, and Cas9. Mol Ther, 2019, 27(4):735-746.
doi: 10.1016/j.ymthe.2019.01.014 |
| [18] |
Hryhorowicz M, Lipiński D, Zeyland J, Słomski R. CRISPR/Cas9 immune system as a tool for genome engineering. Arch Immunol Ther Exp (Warsz), 2017, 65(3):233-240.
doi: 10.1007/s00005-016-0427-5 |
| [19] |
Tesson L, Usal C, Ménoret S, Leung E, Niles BJ, Remy S, Santiago Y, Vincent AI, Meng XD, Zhang L, Gregory PD, Anegon I, Cost GJ. Knockout rats generated by embryo microinjection of TALENs. Nat Biotechnol, 2011, 29(8):695-696.
doi: 10.1038/nbt.1940 |
| [20] |
Sung YH, Baek IJ, Kim DH, Jeon J, Lee J, Lee K, Jeong D, Kim JS, Lee HW. Knockout mice created by TALEN- mediated gene targeting. Nat Biotechnol, 2013, 31(1):23-24.
doi: 10.1038/nbt.2477 |
| [21] |
Carlson DF, Tan WF, Lillico SG, Stverakova D, Proudfoot C, Christian M, Voytas DF, Long CR, Whitelaw CBA, Fahrenkrug SC. Efficient TALEN-mediated gene knockout in livestock. Proc Natl Acad Sci USA, 2012, 109(43):17382-17387.
doi: 10.1073/pnas.1211446109 |
| [22] |
Park KE, Telugu BPVL. Role of stem cells in large animal genetic engineering in the TALENs-CRISPR era. Reprod Fertil Dev, 2013, 26(1):65-73.
doi: 10.1071/RD13258 |
| [23] |
Christian M, Cermak T, Doyle EL, Schmidt C, Zhang F, Hummel A, Bogdanove AJ, Voytas DF. Targeting DNA double-strand breaks with TAL effector nucleases. Genetics, 2010, 186(2):757-761.
doi: 10.1534/genetics.110.120717 pmid: 20660643 |
| [24] |
Miller JC, Tan SY, Qiao GJ, Barlow KA, Wang JB, Xia DF, Meng XD, Paschon DE, Leung E, Hinkley SJ, Dulay GP, Hua KL, Ankoudinova I, Cost GJ, Urnov FD, Zhang HS, Holmes MC, Zhang L, Gregory PD, Rebar EJ. A TALE nuclease architecture for efficient genome editing. Nat Biotechnol, 2011, 29(2):143-148.
doi: 10.1038/nbt.1755 |
| [25] |
Reyon D, Tsai SQ, Khayter C, Foden JA, Sander JD, Joung JK. FLASH assembly of TALENs for high-throughput genome editing. Nat Biotechnol, 2012, 30(5):460-465.
doi: 10.1038/nbt.2170 |
| [26] |
Yu BL, Lu R, Yuan YG, Zhang T, Song SZ, Qi ZQ, Shao B, Zhu MM, Mi F, Cheng Y. Efficient TALEN-mediated myostatin gene editing in goats. BMC Dev Biol, 2016, 16(1):26.
doi: 10.1186/s12861-016-0126-9 |
| [27] | Song SZ, Zhu MM, Yuan YG, Rong Y, Xu S, Chen S, Mei JY, Cheng Y. BLG gene knockout and hLF gene knock-in at BLG locus in goat by TALENs. Chin J Biotechnol, 2016, 32(3):329-338. |
| 宋绍征, 朱孟敏, 袁玉国, 荣耀, 徐晟, 陈思, 梅珺琰, 成勇. 转录激活因子样效应物核酸酶介导的山羊β-乳球蛋白基因敲除和人乳铁蛋白基因定点整合. 生物工程学报, 2016, 32(3):329-338. | |
| [28] |
Clop A, Marcq F, Takeda H, Pirottin D, Tordoir X, Bibé B, Bouix J, Caiment F, Elsen JM, Eychenne F, Larzul C, Laville E, Meish F, Milenkovic D, Tobin J, Charlier C, Georges M. A mutation creating a potential illegitimate microRNA target site in the myostatin gene affects muscularity in sheep. Nat Genet, 2006, 38(7):813-818.
doi: 10.1038/ng1810 |
| [29] | Zhang T, Lu YY, Song SZ, Lu R, Zhou MY, He ZY, Yuan TT, Yan KN, Cheng Y. 'Double-muscling' and pelvic tilt phenomena in rabbits with the cystine-knot motif deficiency of myostatin on exon 3. Biosci Rep, 2019, 39(5): BSR20190207. |
| [30] |
Lee SJ, McPherron AC. Myostatin and the control of skeletal muscle mass. Curr Opin Genet Dev, 1999, 9(5):604-607.
pmid: 10508689 |
| [31] |
Zhang J, Cui ML, Nie YW, Dai B, Li FR, Liu DJ, Liang H, Cang M. CRISPR/Cas9-mediated specific integration of fat-1 at the goat MSTN locus. FEBS J, 2018, 285(15):2828-2839.
doi: 10.1111/febs.14520 pmid: 29802684 |
| [32] |
Moro LN, Viale DL, Bastón JI, Arnold V, Suvá M, Wiedenmann E, Olguín M, Miriuka S, Vichera G. Generation of myostatin edited horse embryos using CRISPR/Cas9 technology and somatic cell nuclear transfer. Sci Rep, 2020, 10(1):15587.
doi: 10.1038/s41598-020-72040-4 |
| [33] | Song SZ, Zhang T, Pan SQ, Lu R, Cheng Y, Zhou MM. hLF gene knock-in at the BLG locus of goat by CRISPR/ Cas9 system. J China Agric Univ, 2020, 25(7):111-119. |
| 宋绍征, 张婷, 潘生强, 陆睿, 成勇, 周鸣鸣. CRISPR/ Cas9系统介导的人乳铁蛋白基因在山羊β-乳球蛋白基因座定点敲入. 中国农业大学学报, 2020, 25(7):111-119. | |
| [34] |
An LY, Yuan YG, Yu BL, Yang TJ, Cheng Y. Generation of human lactoferrin transgenic cloned goats using donor cells with dual markers and a modified selection procedure. Theriogenology, 2012, 78(6):1303-1311.
doi: 10.1016/j.theriogenology.2012.05.027 |
| [35] |
Matoba S, Liu YT, Lu FL, Iwabuchi KA, Shen L, Inoue A, Zhang Y. Embryonic development following somatic cell nuclear transfer impeded by persisting histone methylation. Cell, 2014, 159(4):884-895.
doi: 10.1016/j.cell.2014.09.055 pmid: 25417163 |
| [1] | 贾觉睿智, 肖诚, 刘艺文, 李冉, 张化冰, 于淼. 二例GCK基因突变致先天性高胰岛素性低血糖症的诊疗和基因检测分析[J]. 遗传, 2022, 44(11): 1056-1062. |
| [2] | 肖诚, 刘洁颖, 杨春如, 于淼. LMNA基因突变相关脂肪萎缩综合征的研究进展[J]. 遗传, 2022, 44(10): 913-925. |
| [3] | 蒋琬姿, 张丽雯, 贺彩红, 阮梅花, 季勇, 于建荣, 周红文. 家族性高胆固醇血症研究进展[J]. 遗传, 2021, 43(11): 1011-1022. |
| [4] | 周俊, 赵成成, 吴霄, 石俊松, 周荣, 吴珍芳, 李紫聪. 猪耳成纤维细胞转录组异质性及对核移植胚胎发育的潜在影响[J]. 遗传, 2020, 42(9): 898-915. |
| [5] | 宋绍征, 于康英, 张婷, 陆睿, 潘生强, 周鸣鸣, 成勇. tPA/gGH双基因转染山羊乳腺上皮细胞表达分析[J]. 遗传, 2020, 42(4): 380-387. |
| [6] | 朱海美, 黄鹏程, 赵田田, 周长慧, 李若婉, 于春荣, 陈志勇, 顾林峰, 常艳. 纳米银颗粒及纳米二氧化钛颗粒的体外遗传毒性研究[J]. 遗传, 2020, 42(12): 1192-1200. |
| [7] | 王冰源, 牟玉莲, 李奎, 刘志国. 农业动物干细胞研究进展[J]. 遗传, 2020, 42(11): 1073-1080. |
| [8] | 敖政, 陈祥, 吴珍芳, 李紫聪. 体细胞克隆猪发育异常研究进展[J]. 遗传, 2020, 42(10): 993-1003. |
| [9] | 孙兆庆, 闫波. 转录因子GATA6在心血管疾病中的作用及其调控机制[J]. 遗传, 2019, 41(5): 375-383. |
| [10] | 李楠, 李亚娟, 郭海滨, 张向前. Ds插入突变体的遗传学综合性实验设计与探讨[J]. 遗传, 2019, 41(12): 1148-1155. |
| [11] | 杨旭琼, 吴珍芳, 李紫聪. 哺乳动物体细胞核移植表观遗传重编程研究进展[J]. 遗传, 2019, 41(12): 1099-1109. |
| [12] | 徐福如, 蒋文君, 张涛, 姜倩, 张瑞雪, 毕宏生. FBN2基因突变与遗传性结缔组织病的发生[J]. 遗传, 2019, 41(10): 919-927. |
| [13] | 王凤红,张磊,李晓凯,范一星,乔贤,龚高,严晓春,张令天,王志英,王瑞军,刘志红,王志新,何利兵,张燕军,李金泉,赵艳红,苏蕊. 山羊基因组研究进展[J]. 遗传, 2019, 41(10): 928-938. |
| [14] | 严婷婷, 张蕾, 李余动, 梁新乐. 基于微信的“微生物遗传育种实验”混合式教学模式探究[J]. 遗传, 2018, 40(7): 601-606. |
| [15] | 康岚, 陈嘉瑜, 高绍荣. 中国细胞重编程和多能干细胞研究进展[J]. 遗传, 2018, 40(10): 825-840. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||

www.chinagene.cn
备案号:京ICP备09063187号-4
总访问:,今日访问:,当前在线:
