遗传 ›› 2025, Vol. 47 ›› Issue (5): 573-588.doi: 10.16288/j.yczz.24-288
收稿日期:2024-10-08
修回日期:2024-12-11
出版日期:2025-05-20
发布日期:2025-02-18
通讯作者:
张文清,博士,教授,研究方向:人类疾病斑马鱼动物模型的建立、造血发育以及调控。E-mail: mczhangwq@scut.edu.cn;作者简介:林杰豪,硕士,专业方向:生物学。E-mail: 2213763056@qq.com
基金资助:
Jiehao Lin( ), Tongshu Yang, Wenqing Zhang(
), Tongshu Yang, Wenqing Zhang( ), Wei Liu(
), Wei Liu( )
)
Received:2024-10-08
Revised:2024-12-11
Published:2025-05-20
Online:2025-02-18
Supported by:摘要:
原始造血是生物体内至关重要的发育过程,其产生的血液细胞不仅在早期胚胎时期负责氧气和营养物质的运输,同时也为免疫系统发育奠定了基础。在原始造血过程中,造血相关转录因子及其辅因子相互作用形成复杂的调控网络,共同调控原始造血的发生与成熟。其中,bHLH转录因子家族中的SCL和LYL1在胚胎造血中起着核心作用。SCL参与原始造血的启动,而LYL1则被认为是SCL的旁系同源,能够在成年后SCL缺失时弥补其对造血的影响。然而,目前LYL1在原始造血中的具体作用尚不明确。本研究通过分析斑马鱼血液细胞单细胞RNA测序(scRNA-seq)数据,发现CABZ01066694.1在造血干/祖细胞中高表达。序列比对显示,CABZ01066694.1是lyl1基因的一种短型转录本。本研究通过 5′端快速扩增的cDNA末端测序(5′RACE)验证了斑马鱼和人类的lyl1基因均存在长型转录本(lyl1f)和短型转录本(lyl1s)。进一步通过分析公共数据库中的scRNA-seq和RNA-seq数据,发现在斑马鱼原始造血细胞中,lyl1主要转录lyl1s。最后,本研究通过Morpholino技术分别敲低lyl1f和lyl1s,发现lyl1s的敲低显著抑制了原始髓系祖细胞和原始粒系细胞的产生,而lyl1f的敲低则促进了原始巨噬细胞的生成。综上所述,本研究揭示了斑马鱼源lyl1和人源LYL1均存在长、短型转录本,并且这两种转录本在调控原始髓系的发生过程中具有不同作用,为理解原始造血的分子调控提供了新的线索。
林杰豪, 杨童舒, 张文清, 刘伟. Lyl1不同转录本在斑马鱼原始造血中的作用[J]. 遗传, 2025, 47(5): 573-588.
Jiehao Lin, Tongshu Yang, Wenqing Zhang, Wei Liu. Role of different Lyl1 transcripts in zebrafish primitive hematopoiesis[J]. Hereditas(Beijing), 2025, 47(5): 573-588.
表1
本研究所用的引物序列"
| 引物名称 | 引物序列(5′→3′) | 用途 |
|---|---|---|
| qPCR1 | F:CATCTCCATCTGTCCTCCAGC R:TGTGTCCTGAGCTCTGGGTT | 荧光定量PCR |
| qPCR2 | F:GCTGAGCAAGAACGAGATCCT R:CTGTCAGTGTCCCCATAGCAG | |
| mfap4 | F:GTTTACACCATCTATCCAGCC R:GTTCTCTAGTCCCAGCCA | |
| lyz | F:AAAGCAGGTTTAAGACCCAC R:CTGTCAGTGTCCCCATAGCAG | |
| gata1a | F:TCCAGTTCGCCAAGTTTACTCA R:GGAGGTGTGAGAGTGGAGAGGT | |
| βe1 | F:CTGGCAAGGTGTCTCATCG R:GCAGCACACTTAAATCAGCA | |
| alas2 | F:AGGAGAGCCCATCAGAGAAATG R:TTATCGCCTGCACGTAGATGTT | |
| z_GSP | GATTACGCCAAGCTTCCGGCGGGTGCGTCGGGATCAGT | 5′RACE |
| h_GSP | GATTACGCCAAGCTTCGTCGGCAGCAGCTTCCTCAGCT | |
| FP1 | F:AGAACCCAGAGCTCAGGACA | PCR |
| RP1 | R:AGCGTTACTGAAGATCCCGA | |
| FP2 | F:CCCAGCACACGCGTGTTCAA | |
| RP2 | R:GGCAGAGATGCGGAGCTGGA |
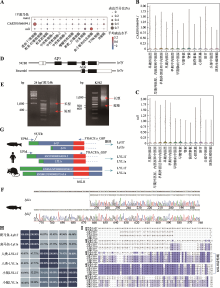
图1
CABZ01066694.1基因鉴定和解析 A:runx1、CABZ01066694.1和tal1基因在1Y AB野生型斑马鱼肾髓血单细胞测序各类血液细胞中表达的气泡图;B、C:CABZ01066694.1和tal1表达分布图。利用CellOracle application分析AB野生型斑马鱼CABZ01066694.1和tal1在内、中、外三胚层中的表达分布情况;D:lyl1在NCBI和Ensembl中转录本信息示意图。将NCBI中基因对应的转录本命名为lyl1f,Ensembl中的命名为lyl1s;E、F:24 hpf AB野生型斑马鱼和K562细胞系lyl1基因5′RACE扩增产物的琼脂糖凝胶电泳结果及5′RACE扩增产物中lyl1s和lyl1f测序部分结果;G:人LYL1和斑马鱼Lyl1的5′RACE结果示意图及与小鼠Lyl1不同转录本的比对。其中UPM(Universal Primer Mix)和GSP(Gene Specific Primer)为5′RACE 实验扩增引物,f:长型蛋白,s:短型蛋白;H:斑马鱼源Lyl1、小鼠源LYL1和人源LYL1长型蛋白和短型蛋白的同源性百分比;I:斑马鱼源Lyl1、小鼠源LYL1和人源LYL1长型蛋白和短型蛋白的氨基酸序列比对结果。"
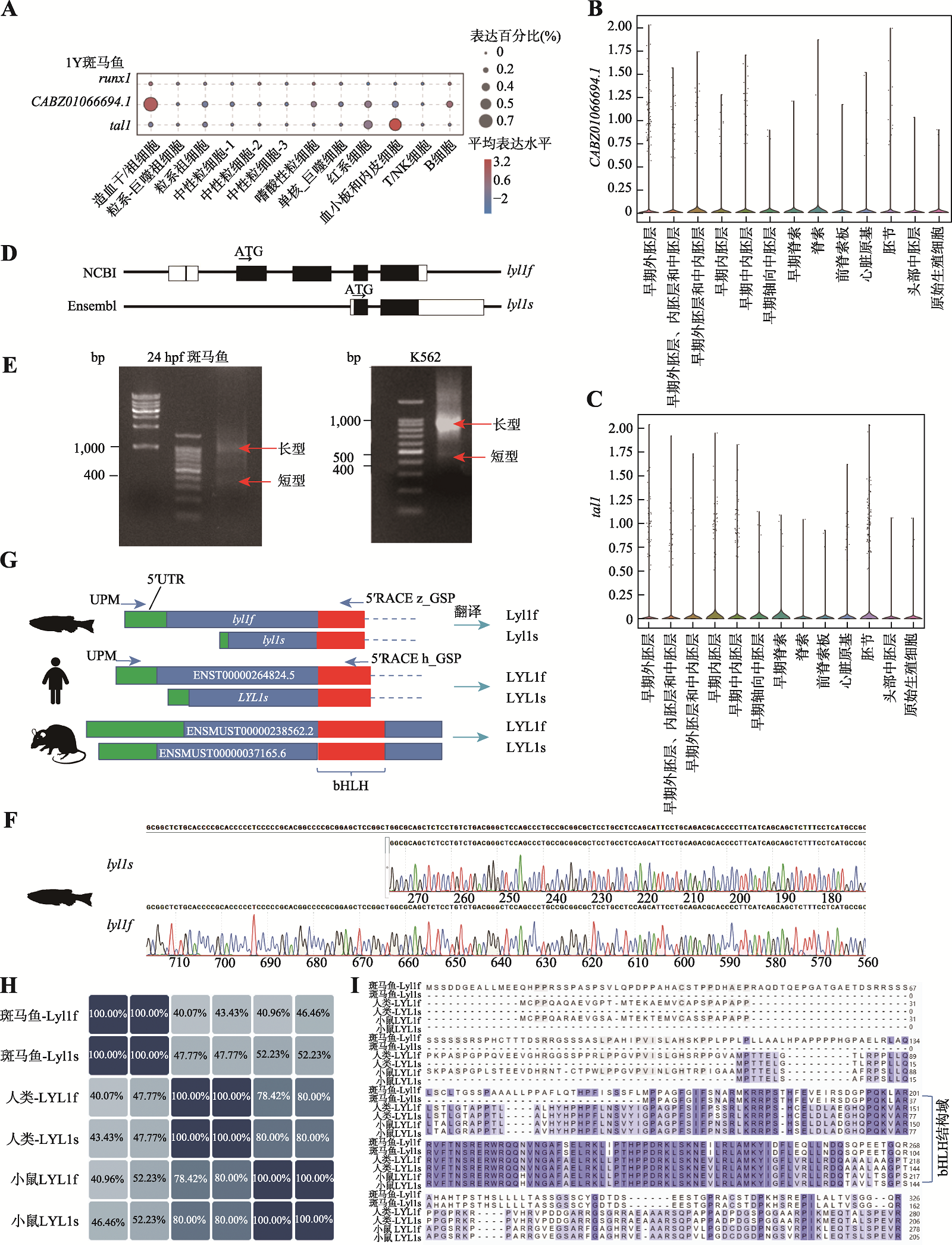
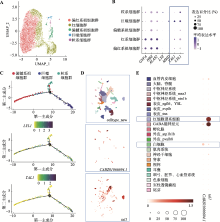
图2
lyl1在原始造血细胞中的表达情况 A、B:细胞分群图及相关基因表达气泡图。利用Seurat分析scRNA-seq数据(GSM7749027)并进行分群和观察相关标志性基因的表达情况。蓝色框为TAL1和LYL1在原始造血细胞群中的表达情况;C:髓系细胞命运轨迹图。利用R包Monocle进行拟时序分析,展示了人源iPSC诱导原始造血数据中的偏髓系祖细胞群向巨噬细胞群和粒系细胞群分化;D、E:细胞分群图及CABZ01066694.1和tal1在24 hpf AB野生型斑马鱼中的表达气泡图。在Liu等[35]所发布的在线平台中分析24 hpf AB野生型斑马鱼scRNA-seq数据,分群方式选择“celltype_new”,观察CABZ01066694.1和tal1在24 hpf AB野生型斑马鱼中其他组织中的表达情况。蓝色框为CABZ01066694.1和tal1在原始红系和原始白细胞群中的表达情况。"
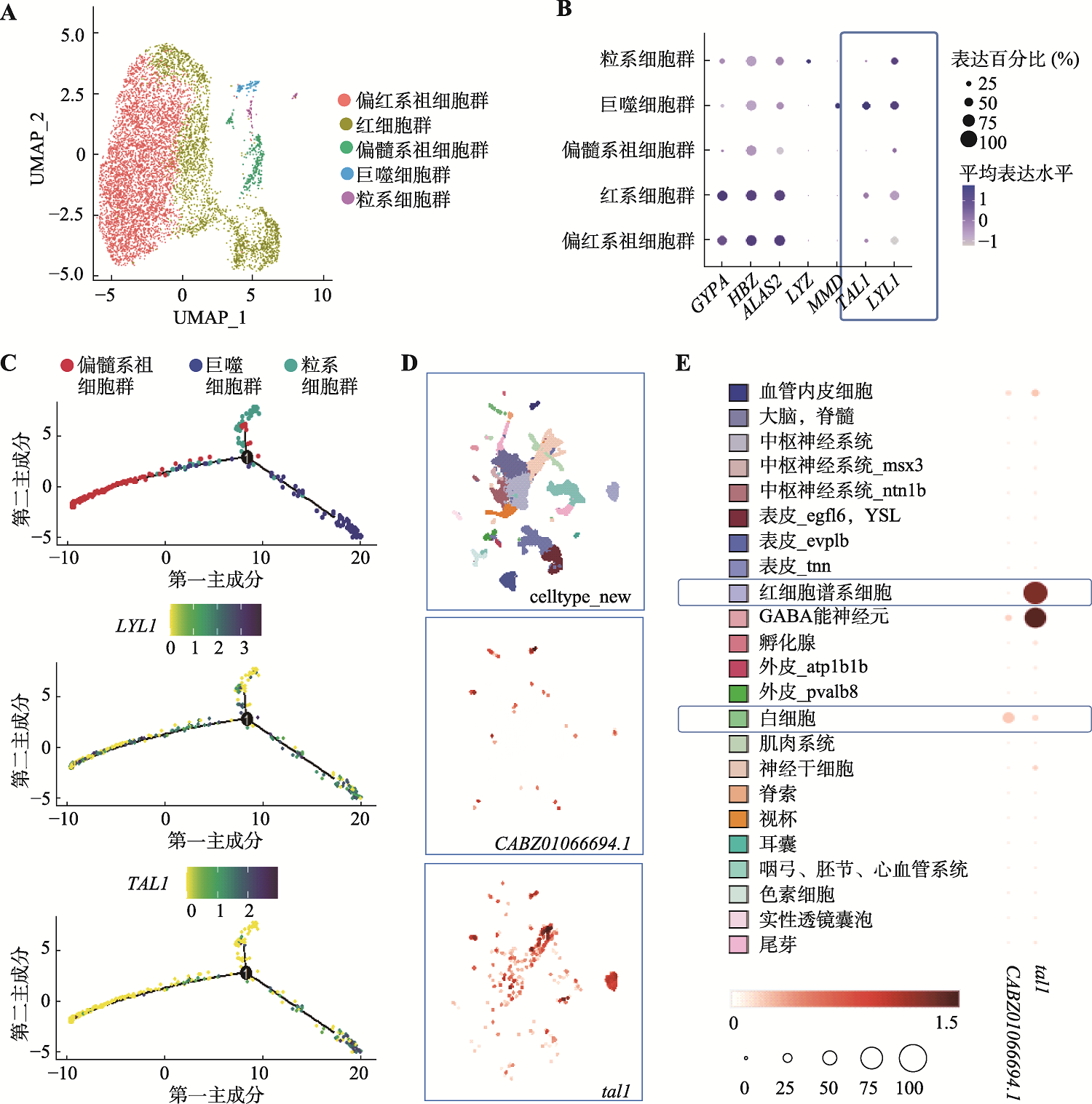
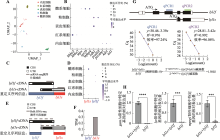
图3
lyl1在原始造血细胞中的表达情况 A、B:24 hpf斑马鱼整体组织scRNA-seq数据(SRR10095968)UMAP降维图及部分基因表达气泡图;C:重新定义scRNA-seq数据(SRR10095968)中lyl1信息示意图。在24 hpf野生型斑马鱼整体组织scRNA-seq数据(SRR10095968)所用的参考基因组中,对lyl1s和lyl1f序列信息的重新定义。黑色短横线为测序中的序列捕获片段,蓝色为lyl1f定义序列,红色为lyl1s定义序列;D:24 hpf野生型斑马鱼整体组织scRNA-seq数据(SRR10095968)中lyl1s和lyl1f在不同细胞群中的表达情况;E:重新定义Bulk RNA-seq数据(SRR25181149)中lyl1信息的示意图。在24 hpf gata1a阳性红细胞RNA-seq数据(SRR25181149)所用的参考基因组中对lyl1s和lyl1f序列信息的重新定义。黑色短横线为测序中的序列捕获片段,蓝色为lyl1f定义序列,红色为lyl1s定义序列;F:24 hpf gata1a阳性红细胞RNA-seq数据(SRR25181149)中lyl1s和lyl1f的表达情况;G:lyl1基因经不同位置的引物扩增后qPCR结果及扩增曲线图。其中qPCR1检测lyl1f转录水平,qPCR2检测lyl1的总体转录水平;H:lyl1s和lyl1f在红细胞、粒细胞和巨噬细胞中的表达柱形图。qPCR结果显示流式细胞术分选出的24 hpf Tg(gata1a:dsred)、Tg(lyz:dsred)和Tg(mpeg1:eGFP) 转基因斑马鱼中,gata1a-dsred阳性细胞、lyz-dsred阳性细胞和mpeg1-eGFP阳性细胞中lyl1总体表达量(引物qPCR2)和lyl1f (引物qPCR1)的相对表达量(student-t检验,***P<0.001,****P<0.0001)。"
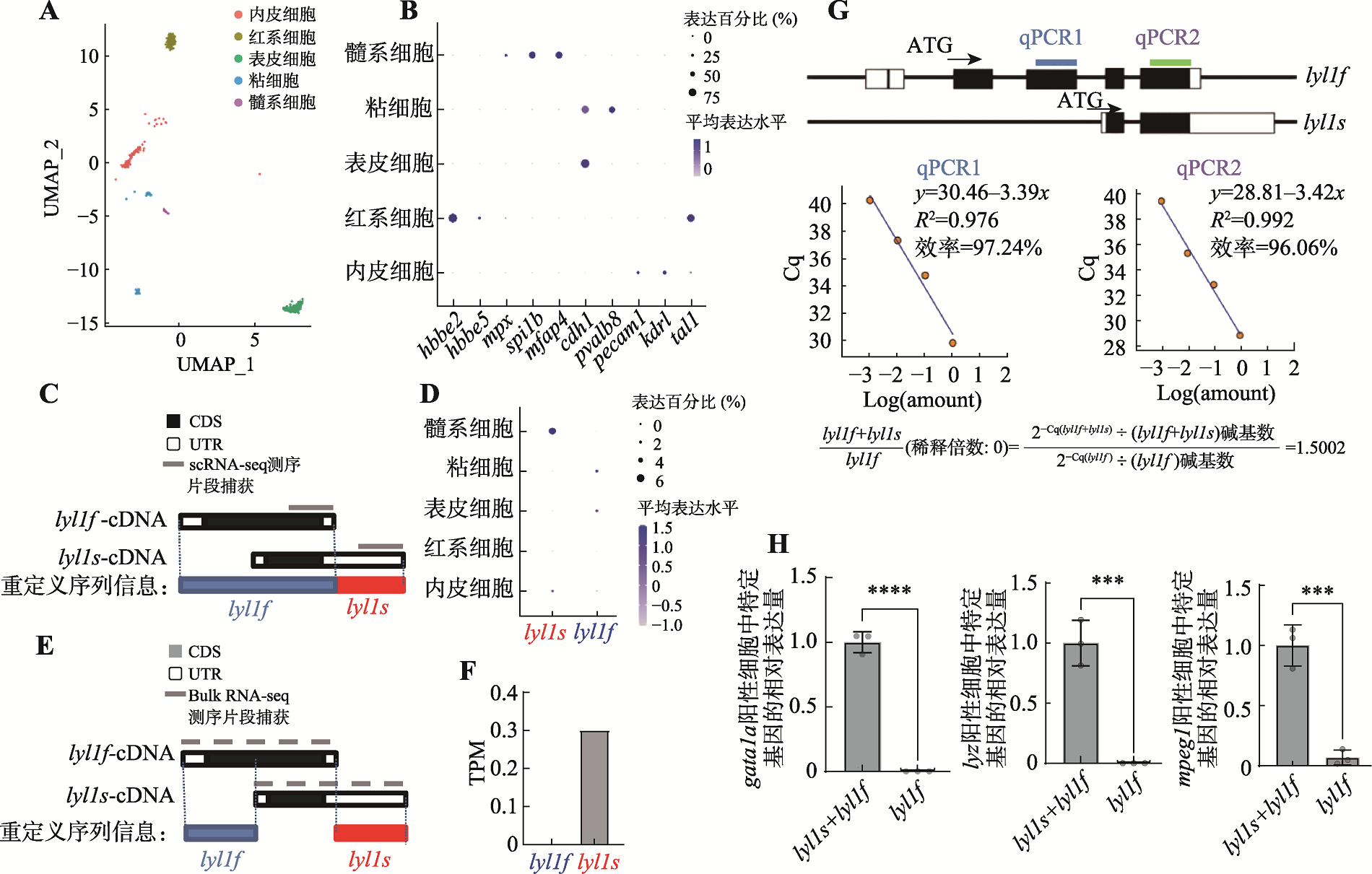
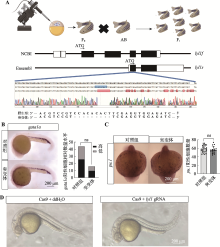
图4
24 hpf lyl1(-9,+0)突变体原始红系和髓系的表型图 A:CRISPR/Cas9实验示意图及测序结果。通过显微注射技术向单细胞阶段AB野生型斑马鱼胚胎注射gRNA和Cas9蛋白,通过筛选获取lyl1突变体(图片素材来源于Biorender官网),测序结果展示突变位置序列;B:24 hpf野生型(对照组)和24 hpf lyl1(-9,+0)纯合突变体gata1a mRNA整体原位杂交(50×倍镜,卡方检验,n>10;ns表示无显著差异);C:野生型(对照组)和lyl1(-9,+0)纯合突变体pu.1 mRNA整体原位杂交(63×倍镜,student-t检验,ns表示无显著差异);D:CRISPR/Cas9技术瞬时敲除lyl1后36 hpf 的F0代斑马鱼胚胎发育形态变化。"
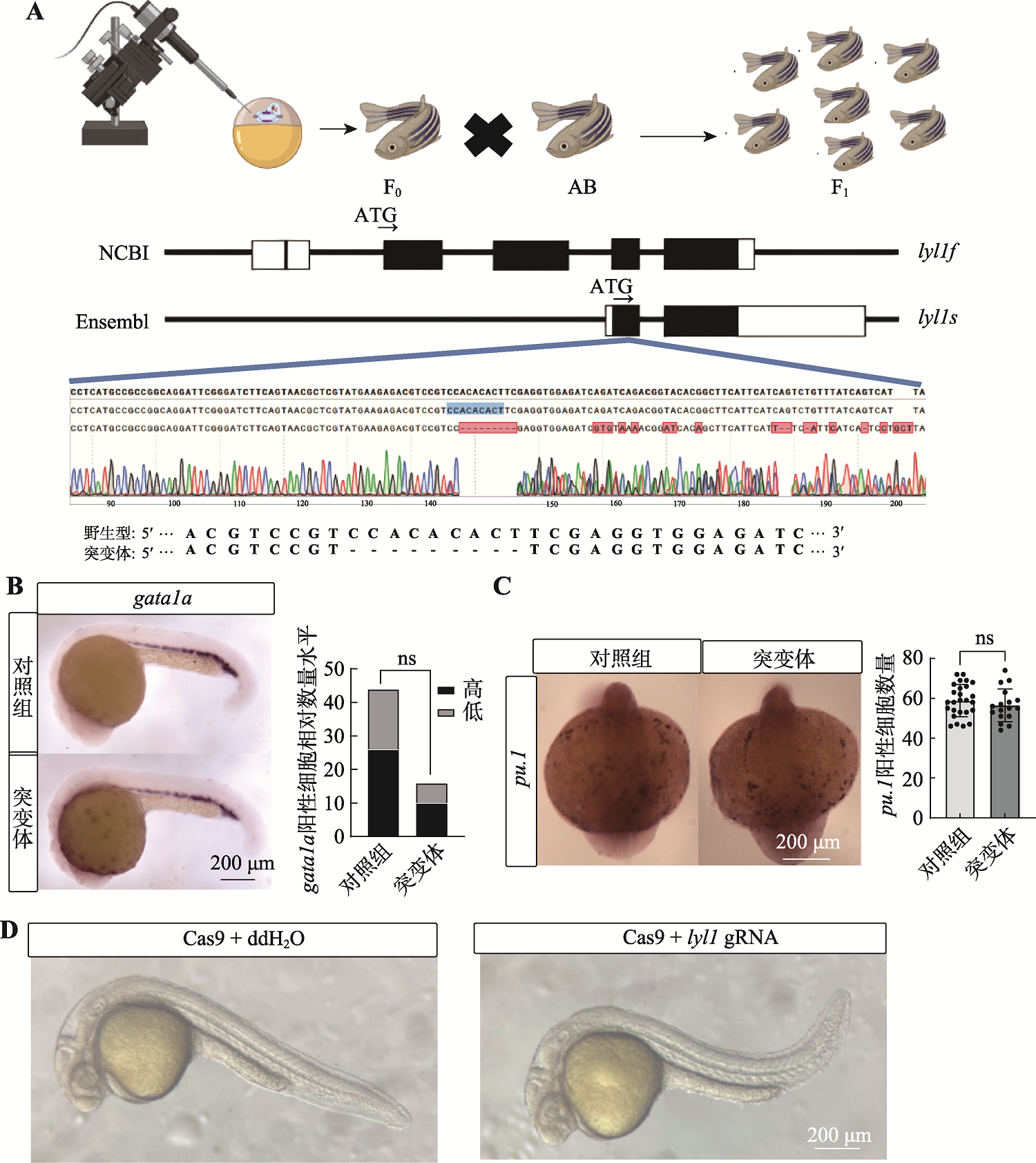
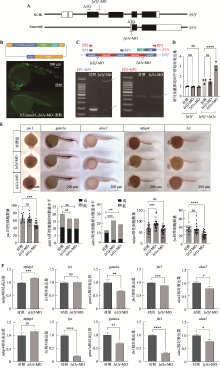
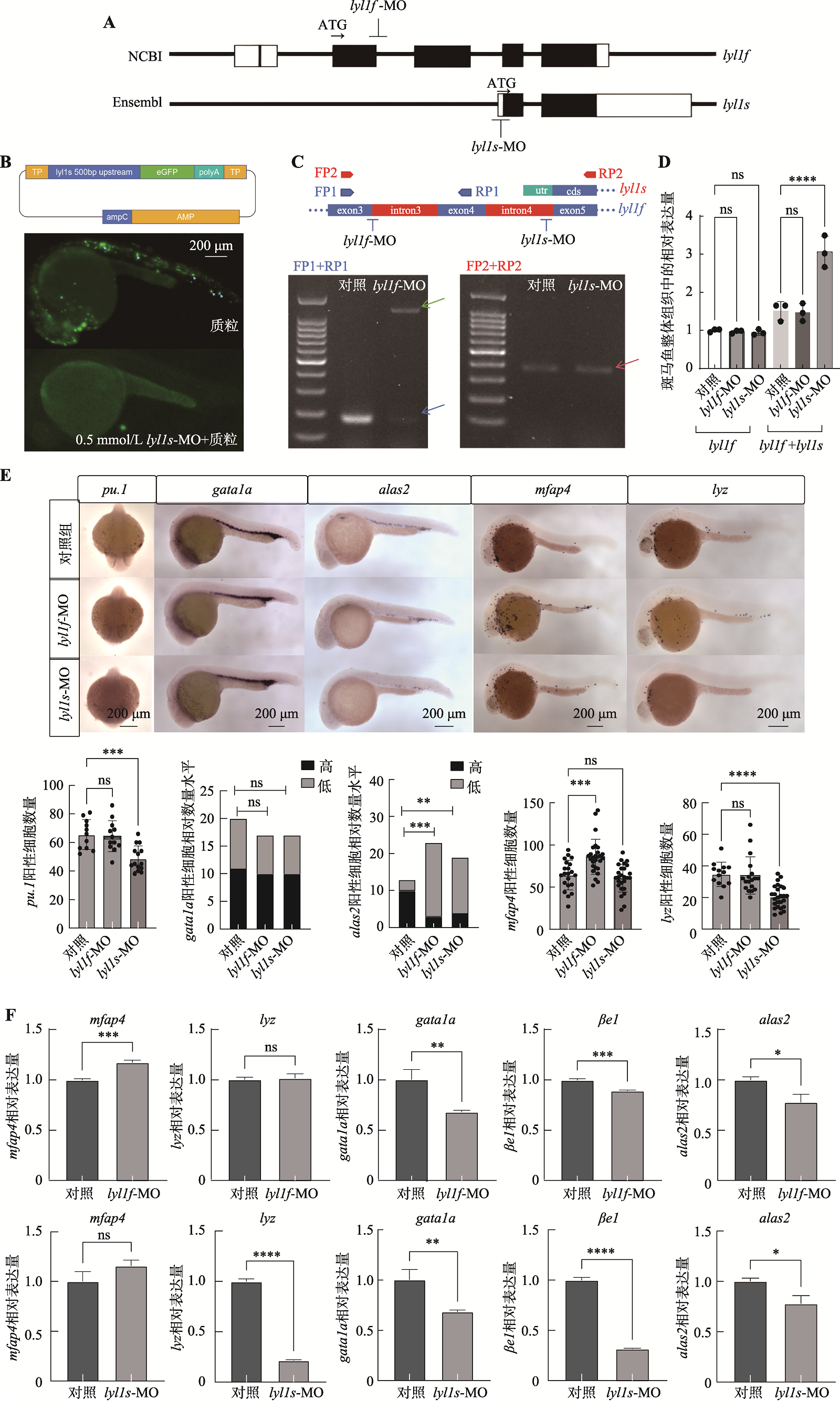
图5
探究lyl1不同转录本对原始造血的影响 A:针对不同转录本的Morpholino设计示意图。其中lyl1f-MO设计在外显子3-内含子4交界处,lyl1s-MO设计在起始密码子处;B:荧光报告质粒示意图和显微注射荧光报告质粒后24 hpf AB野生型斑马鱼的荧光表达情况。取lyl1s上游500 bp作为启动子,启动eGFP的CDS序列,构建荧光报告质粒,通过在AB野生型斑马鱼胚胎中注射lyl1s-MO单液和lyl1s-MO与荧光报告质粒混合液后,检测24 hpf 斑马鱼胚胎荧光信号;C:检测lyl1f和lyl1s存在的PCR引物设计示意图及琼脂糖凝胶电泳图。在lyl1f的外显子3设计正向引物(FP1、FP2)以及外显子4和外显子5处设计反向引物(RP1和RP2),并分别提取单细胞阶段被注射ddH2O和被注射lyl1f-MO/lyl1s-MO的24 hpf AB野生型斑马鱼 cDNA作为模板,进行PCR扩增并通过琼脂糖凝胶电泳进行检测扩增产物(其中引物FP1与RP1扩增结果展示lyl1f在外显子3与内含子3交界处的剪切情况,蓝色箭头表示lyl1f在该处正常剪切后条带,绿色箭头表示lyl1f在该处非正常剪切后条带;引物FP2和RP2扩增结果展示注射lyl1s-MO后,lyl1f在外显子5和内含子4交界处的剪切情况,红色箭头表示lyl1f在该处正常剪切后条带);D:在单细胞阶段,分别注射了lyl1f-MO和lyl1s-MO的24 hpf AB野生型斑马鱼中lyl1s和lyl1f的表达情况。分别取注射lyl1f-MO和lyl1s-MO的24 hpf AB野生型斑马鱼cDNA为模板,以引物qPCR1/qPCR2进行qPCR检测(qPCR1引物检测lyl1f的相对表达量,qPCR2引物检测lyl1f和lyl1s的总体相对表达量);E:原位杂交实验显示单细胞阶段分别注射ddH2O(对照组)、lyl1f-MO和lyl1s-MO后,24 hpf AB野生型斑马鱼中 pu.1、gata1a、alas2、mfap4和lyz的表达信号变化及统计图(pu.1、mfap4和lyz进行student-t检验,gata1a和alas2进行卡方检验;*P<0.05,**P<0.01,***P<0.001,****P<0.0001),原位杂交信号图片于50×倍镜下拍摄;F:qPCR实验检测单细胞阶段分别注射ddH2O(对照组)、lyl1f-MO和lyl1s-MO后,24 hpf AB野生型斑马鱼中mfap4、lyz、gata1a、βe1、和alas2的相对表达量(以上均进行student-t检验,*P<0.05,**P<0.01,***P<0.001,****P<0.0001)"
| [1] |
Davidson AJ, Zon LI. Turning mesoderm into blood: the formation of hematopoietic stem cells during embryogenesis. Curr Top Dev Biol, 2000, 50: 45-60.
pmid: 10948449 |
| [2] |
Zizioli D, Mione M, Varinelli M, Malagola M, Bernardi S, Alghisi E, Borsani G, Finazzi D, Monti E, Presta M, Russo D. Zebrafish disease models in hematology: highlights on biological and translational impact. Biochim Biophys Acta Mol Basis Dis, 2019, 1865(3): 620-633.
pmid: 30593895 |
| [3] |
Ciau-Uitz A, Monteiro R, Kirmizitas A, Patient R. Developmental hematopoiesis: ontogeny, genetic programming and conservation. Exp Hematol, 2014, 42(8): 669-683.
pmid: 24950425 |
| [4] |
Canu G, Ruhrberg C. First blood: the endothelial origins of hematopoietic progenitors. Angiogenesis, 2021, 24(2): 199-211.
pmid: 33783643 |
| [5] |
Palis J. Erythropoiesis in the mammalian embryo. Exp Hematol, 2024, 136: 104283.
pmid: 39048071 |
| [6] |
Bertrand JY, Kim AD, Violette EP, Stachura DL, Cisson JL, Traver D. Definitive hematopoiesis initiates through a committed erythromyeloid progenitor in the zebrafish embryo. Development, 2007, 134(23): 4147-4156.
pmid: 17959717 |
| [7] |
Moore MA, Metcalf D. Ontogeny of the haemopoietic system: yolk sac origin of in vivo and in vitro colony forming cells in the developing mouse embryo. Br J Haematol, 1970, 18(3): 279-296.
pmid: 5491581 |
| [8] |
Goldfarb AN, Lewandowska K. Inhibition of cellular differentiation by the SCL/tal oncoprotein: transcriptional repression by an Id-like mechanism. Blood, 1995, 85(2): 465-471.
pmid: 7812000 |
| [9] |
Hofmann TJ, Cole MD. The TAL1/Scl basic helix-loop- helix protein blocks myogenic differentiation and E-box dependent transactivation. Oncogene, 1996, 13(3): 617-624.
pmid: 8760303 |
| [10] |
Hsu HL, Wadman I, Tsan JT, Baer R. Positive and negative transcriptional control by the TAL1 helix-loop- helix protein. Proc Natl Acad Sci USA, 1994, 91(13): 5947-5951.
pmid: 8016094 |
| [11] |
Wadman IA, Osada H, Grütz GG, Agulnick AD, Westphal H, Forster A, Rabbitts TH. The LIM-only protein Lmo2 is a bridging molecule assembling an erythroid, DNA-binding complex which includes the TAL1, E47, GATA-1 and Ldb1/NLI proteins. EMBO J, 1997, 16(11): 3145-3157.
pmid: 9214632 |
| [12] |
Hoang T, Lambert JA, Martin R. SCL/TAL1 in hematopoiesis and cellular reprogramming. Curr Top Dev Biol, 2016, 118: 163-204.
pmid: 27137657 |
| [13] |
Real PJ, Ligero G, Ayllon V, Ramos-Mejia V, Bueno C, Gutierrez-Aranda I, Navarro-Montero O, Lako M, Menendez P. SCL/TAL1 regulates hematopoietic specification from human embryonic stem cells. Mol Ther, 2012, 20(7): 1443-1453.
pmid: 22491213 |
| [14] |
Lukov GL, Goodell MA. LYL1 degradation by the proteasome is directed by a N-terminal PEST rich site in a phosphorylation-independent manner. PLoS One, 2010, 5(9): e12692.
pmid: 20844761 |
| [15] |
Giroux S, Kaushik AL, Capron C, Jalil A, Kelaidi C, Sablitzky F, Dumenil D, Albagli O, Godin I. lyl-1 and tal-1/scl, two genes encoding closely related bHLH transcription factors, display highly overlapping expression patterns during cardiovascular and hematopoietic ontogeny. Gene Expr Patterns, 2007, 7(3): 215-226.
pmid: 17112790 |
| [16] |
Visvader J, Begley CG, Adams JM. Differential expression of the LYL, SCL and E2A helix-loop-helix genes within the hemopoietic system. Oncogene, 1991, 6(2): 187-194.
pmid: 2000219 |
| [17] |
Chiu SK, Orive SL, Moon MJ, Saw J, Ellis S, Kile BT, Huang YZ, Chacon D, Pimanda JE, Beck D, Hamilton JR, Tremblay CS, Curtis DJ. Shared roles for Scl and Lyl1 in murine platelet production and function. Blood, 2019, 134(10): 826-835.
pmid: 31300405 |
| [18] |
Kuo SS, Mellentin JD, Copeland NG, Gilbert DJ, Jenkins NA, Cleary ML. Structure, chromosome mapping, and expression of the mouse Lyl-1 gene. Oncogene, 1991, 6(6): 961-968.
pmid: 2067848 |
| [19] |
Capron C, Lécluse Y, Kaushik AL, Foudi A, Lacout C, Sekkai D, Godin I, Albagli O, Poullion I, Svinartchouk F, Schanze E, Vainchenker W, Sablitzky F, Bennaceur- Griscelli A, Duménil D. The SCL relative LYL-1 is required for fetal and adult hematopoietic stem cell function and B-cell differentiation. Blood, 2006, 107(12): 4678-4686.
pmid: 16514064 |
| [20] |
Capron C, Lacout C, Lécluse Y, Wagner-Ballon O, Kaushik AL, Cramer-Bordé E, Sablitzky F, Duménil D, Vainchenker W. LYL-1 deficiency induces a stress erythropoiesis. Exp Hematol, 2011, 39(6): 629-642.
pmid: 21420467 |
| [21] |
Chan WYI, Follows GA, Lacaud G, Pimanda JE, Landry JR, Kinston S, Knezevic K, Piltz S, Donaldson IJ, Gambardella L, Sablitzky F, Green AR, Kouskoff V, Göttgens B. The paralogous hematopoietic regulators Lyl1 and Scl are coregulated by Ets and GATA factors, but Lyl1 cannot rescue the early Scl-/- phenotype. Blood, 2007, 109(5): 1908-1916.
pmid: 17053063 |
| [22] |
Wang ST, Ren DS, Arkoun B, Kaushik AL, Matherat G, Lécluse Y, Filipp D, Vainchenker W, Raslova H, Plo I, Godin I. Lyl-1 regulates primitive macrophages and microglia development. Commun Biol, 2021, 4(1): 1382.
pmid: 34887504 |
| [23] |
Zhou ZY, Huang B, Li S, Huang XH, Tang JY, Kwan YW, Hoi PM, Lee SMY. Sodium tanshinone IIA sulfonate promotes endothelial integrity via regulating VE-cadherin dynamics and RhoA/ROCK-mediated cellular contractility and prevents atorvastatin-induced intracerebral hemorrhage in zebrafish. Toxicol Appl Pharmacol, 2018, 350: 32-42.
pmid: 29730311 |
| [24] |
Hall C, Flores MV, Storm T, Crosier K, Crosier P. The zebrafish lysozyme C promoter drives myeloid-specific expression in transgenic fish. BMC Dev Biol, 2007, 7: 42.
pmid: 17477879 |
| [25] |
Mazzolini J, Chia K, Sieger D. Isolation and RNA extraction of neurons, macrophages and microglia fromlarval zebrafish brains. J Vis Exp, 2018, (134): 57431.
pmid: 29757273 |
| [26] | Westerfield M. The zebrafish book: a guide for the laboratory use of zebrafish (Danio rerio). University of Oregon Press, Eugene, 1995. |
| [27] |
Thisse C, Thisse B. High-resolution in situ hybridization to whole-mount zebrafish embryos. Nat Protoc, 2008, 3(1): 59-69.
pmid: 18193022 |
| [28] |
Songhet P, Adzic D, Reibe S, Rohr KB. fgf1 is required for normal differentiation of erythrocytes in zebrafish primitive hematopoiesis. Dev Dyn, 2007, 236(3): 633-643.
pmid: 17219402 |
| [29] |
Kitaguchi T, Kawakami K, Kawahara A. Transcriptional regulation of a myeloid-lineage specific gene lysozyme C during zebrafish myelopoiesis. Mech Dev, 2009, 126(5-6): 314-323.
pmid: 19275935 |
| [30] |
Li XG, Xiong JW, Shelley CS, Park H, Arnaout MA. The transcription factor ZBP-89 controls generation of the hematopoietic lineage in zebrafish and mouse embryonic stem cells. Development, 2006, 133(18): 3641-3650.
pmid: 16914492 |
| [31] |
Li JY, Li K, Dong XH, Liang D, Zhao QS. Ncor1 and Ncor2 play essential but distinct roles in zebrafish primitive myelopoiesis. Dev Dyn, 2014, 243(12): 1544-1553.
pmid: 25156564 |
| [32] |
Chang NN, Sun CH, Gao L, Zhu D, Xu XF, Zhu XJ, Xiong JW, Xi JZJ. Genome editing with RNA-guided Cas9 nuclease in zebrafish embryos. Cell Res, 2013, 23(4): 465-472.
pmid: 23528705 |
| [33] | Hong JX, Xu SE, Zhang WQ, Liu W. The interaction of Pu.1 and cMyb in zebrafish neutrophil development. Hereditas(Beijing), 2024, 46(4): 319-332. |
| 洪佳馨, 徐颂恩, 张文清, 刘伟. Pu.1和cMyb在斑马鱼中性粒细胞发育中的相互作用. 遗传, 2024, 46(4): 319-332. | |
| [34] |
Pavani G, Klein JG, Nations CC, Sussman JH, Tan K, An HH, Abdulmalik O, Thom CS, Gearhart PA, Willett CM, Maguire JA, Chou ST, French DL, Gadue P. Modeling primitive and definitive erythropoiesis with induced pluripotent stem cells. Blood Adv, 2024, 8(6): 1449-1463.
pmid: 38290102 |
| [35] |
Liu C, Li R, Li Y, Lin XM, Zhao KC, Liu Q, Wang SW, Yang XQ, Shi XY, Ma YT, Pei CY, Wang H, Bao WD, Hui JH, Yang T, Xu ZC, Lai TT, Berberoglu MA, Sahu SK, Esteban MA, Ma KL, Fan GY, Li YX, Liu SP, Chen A, Xu X, Dong ZQ, Liu LQ. Spatiotemporal mapping of gene expression landscapes and developmental trajectories during zebrafish embryogenesis. Dev Cell, 2022, 57(10): 1284-1298.e5.
pmid: 35512701 |
| [36] |
Farnsworth DR, Saunders LM, Miller AC. A single-cell transcriptome atlas for zebrafish development. Dev Biol, 2020, 459(2): 100-108.
pmid: 31782996 |
| [37] |
Yang SY, Cao SH, Xu XB, Li Q, Li JT, Guo J, Wang F, Bao YH, Jiang ZA, Zhang T, Wang L, Sun SG. adducin 1 is essential for the survival of erythroid precursors via regulating p53 transcription in zebrafish. iScience, 2023, 26(9): 107516.
pmid: 37636049 |
| [38] |
Qian F, Zhen FH, Xu J, Huang M, Li WY, Wen ZL. Distinct functions for different scl isoforms in zebrafish primitive and definitive hematopoiesis. PLoS Biol, 2007, 5(5): e132.
pmid: 17472439 |
| [39] |
Ren X, Gomez GA, Zhang B, Lin S. Scl isoforms act downstream of etsrp to specify angioblasts and definitive hematopoietic stem cells. Blood, 2010, 115(26): 5338-5346.
pmid: 20185582 |
| [40] |
Zhen FH, Lan YH, Yan B, Zhang WQ, Wen ZL. Hemogenic endothelium specification and hematopoietic stem cell maintenance employ distinct Scl isoforms. Development, 2013, 140(19): 3977-3985.
pmid: 24046317 |
| [1] | 刘吉祥, 赖思婷, 白晶, 徐进. Il34拯救甲硝唑导致的斑马鱼中枢神经系统轴突再生障碍[J]. 遗传, 2024, 46(6): 478-489. |
| [2] | 洪佳馨, 徐颂恩, 张文清, 刘伟. Pu.1和cMyb在斑马鱼中性粒细胞发育中的相互作用[J]. 遗传, 2024, 46(4): 319-332. |
| [3] | 孙飘, 李颖, 刘帆, 王璐. TPI缺乏症斑马鱼模型的构建及分析[J]. 遗传, 2024, 46(3): 232-241. |
| [4] | 杨晓君, 黄振瀚, 刘伟, 张文清, 黄志斌. CD209同源基因在斑马鱼中的鉴定及功能表征[J]. 遗传, 2024, 46(11): 947-957. |
| [5] | 李凯伦, 卢荆奥, 陈小辉, 张文清, 刘伟. 尿囊素促进破骨细胞缺陷斑马鱼骨折修复[J]. 遗传, 2023, 45(4): 341-353. |
| [6] | 卢荆澳, 黄春燕, 林芷茵, 唐政, 马宁, 黄志斌. cd99l2基因调控斑马鱼白细胞组织间的迁移机制[J]. 遗传, 2022, 44(9): 798-809. |
| [7] | 孙凤宇, 许强华. 血液发生相关microRNAs研究进展[J]. 遗传, 2022, 44(9): 756-771. |
| [8] | 慕蓉蓉, 牛晴晴, 孙玉强, 梅俊, 苗蒙. 陆地棉MYB类转录因子基因GhTT2克隆及功能初步分析[J]. 遗传, 2022, 44(8): 720-728. |
| [9] | 郑鹏飞, 谢海波, 朱盼盼, 赵呈天. 斑马鱼神经底板处神经元的分布及特征[J]. 遗传, 2022, 44(6): 510-520. |
| [10] | 张婷婷, 刘峰. 斑马鱼蛋白酪氨酸硫酸化修饰的检测方法研究[J]. 遗传, 2022, 44(2): 178-186. |
| [11] | 贾婷婷, 雷蕾, 吴歆媛, 蔡顺有, 陈艺璇, 薛钰. 二甲双胍对斑马鱼骨骼发育及损伤修复的机制研究[J]. 遗传, 2022, 44(1): 68-79. |
| [12] | 吕孟冈, 刘艾嘉, 李庆伟, 苏鹏. RHR转录因子家族起源、功能以及进化机制的研究进展[J]. 遗传, 2021, 43(3): 215-225. |
| [13] | 邱晓芬, 汤冬娥, 虞海燕, 廖秋燕, 胡芷洋, 周俊, 赵鑫, 何慧燕, 梁灼健, 许承明, 杨明, 戴勇. 基于单细胞ATAC测序技术对18-三体综合征染色质开放性区域转录因子的分析[J]. 遗传, 2021, 43(1): 74-83. |
| [14] | 妥晓梅, 朱东丽, 陈晓峰, 荣誉, 郭燕, 杨铁林. 骨质疏松易感SNP rs4325274通过增强子远程调控SOX6基因的功能机制研究[J]. 遗传, 2020, 42(9): 889-897. |
| [15] | 郭佳妮, 刘帆, 王璐. 斑马鱼血液疾病模型及应用[J]. 遗传, 2020, 42(8): 725-738. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||

www.chinagene.cn
备案号:京ICP备09063187号-4
总访问:,今日访问:,当前在线:
