遗传 ›› 2024, Vol. 46 ›› Issue (6): 478-489.doi: 10.16288/j.yczz.24-083
收稿日期:2024-03-25
修回日期:2024-05-02
出版日期:2024-06-20
发布日期:2024-05-21
通讯作者:
徐进,博士,教授,研究方向:传统和非传统的机体清理系统的构成、发育,以及它们在神经再生、机体稳态等方面的功能。E-mail: xujin@scut.edu.cn
作者简介:刘吉祥,硕士研究生,专业方向:细胞生物学。E-mail: 201920153297@mail.scut.edu.cn
基金资助:
Liu Jixiang( ), Lai Siting, Bai Jing, Xu Jin(
), Lai Siting, Bai Jing, Xu Jin( )
)
Received:2024-03-25
Revised:2024-05-02
Published:2024-06-20
Online:2024-05-21
Supported by:摘要:
甲硝唑(metronidazole,MTZ)是临床常用的抗感染药物,同时在科学研究中被用作细胞靶向消融系统的前体药物,具有极高的应用价值。但MTZ会引起一定程度的神经毒性症状,目前临床及科研使用过程中也缺乏规避其毒性的有效手段,这在一定程度上限制了其应用。因此,探究MTZ引起神经症状的具体机制并探讨应对措施将更大程度地发挥MTZ的实用价值。本研究利用斑马鱼(Danio rerio)脊髓损伤再生模型确认了MTZ的神经毒性导致斑马鱼中枢神经系统轴突再生障碍,通过在斑马鱼中枢神经系统中过表达il34消除了MTZ对轴突再生的抑制,并证明了这种抗MTZ神经毒性的促再生作用不是由白细胞介素34 (interleukin 34,Il34)趋化的过量巨噬细胞/小胶质细胞所介导。通过转录组测序分析组间差异表达基因的GO富集分析发现,Il34通过促进细胞间的黏附和细胞定位等生物学过程抗MTZ神经毒性从而促进脊髓损伤修复。综上所述,本研究揭示了MTZ神经毒性的可能原因,为消除MTZ毒性提供了一个新的视角。
刘吉祥, 赖思婷, 白晶, 徐进. Il34拯救甲硝唑导致的斑马鱼中枢神经系统轴突再生障碍[J]. 遗传, 2024, 46(6): 478-489.
Liu Jixiang, Lai Siting, Bai Jing, Xu Jin. Il34 rescues metronidazole-induced impairment of spinal cord regeneration in zebrafish central nervous system[J]. Hereditas(Beijing), 2024, 46(6): 478-489.
图1
il34过表达斑马鱼展现出良好的抗MTZ毒性 A:验证MTZ神经毒性的实验流程。首先对4 dpf大小Tg(neurod1:EGFP)转基因斑马鱼幼鱼背侧标红处进行脊髓横断处理,同时对照组使用含2%DMSO的亚甲基蓝养殖水继续培养,实验组用含2%DMSO (DMSO对MTZ起助溶作用)浓度为10 mmol/L MTZ的亚甲基蓝养殖水浸泡处理2天,于损伤后2天(2 day post-injury,2 dpi)即6 dpf时观察其脊髓轴突再生情况。B:Tg(neurod1:EGFP)转基因斑马鱼不同轴突再生情况展示。Tg(neurod1:EGFP)转基因斑马鱼可特异性标记斑马鱼脊髓神经细胞及其轴突从而呈现荧光信号,图中分别为脊髓横断2天(2 dpi)时轴突正常再生形成桥连(左侧)和未能再生形成桥连(右侧),白色箭头指示形成轴突桥连,红色箭头指示未见形成轴突桥连。此时,以各组斑马鱼的轴突形成桥连比例作为其再生能力的评价标准。C:各组别脊髓轴突再生情况示意图。白色箭头指示形成轴突桥连,红色箭头指示未见形成轴突桥连,右上角标注分数的分母代表该组样本量,分子代表示意图对应表型的样本量。野生型;DMSO组有明显轴突桥连的比例为91/129,Tg(Xla.Tubb:il34);DMSO组有明显轴突桥连的比例为84/109,野生型;MTZ组未能发生轴突桥连的比例为70/120,Tg(Xla.Tubb:il34);MTZ组有明显轴突桥连的比例为90/124。D:各组别损伤后轴突再生情况统计。n为样本量(该组别斑马鱼条数);百分数表示轴突桥连率,用以评价神经再生能力;相应组间进行卡方检验,*P<0.05,**P<0.01,***P<0.001,****P<0.0001,ns表示组间轴突再生情况无统计学差异。"
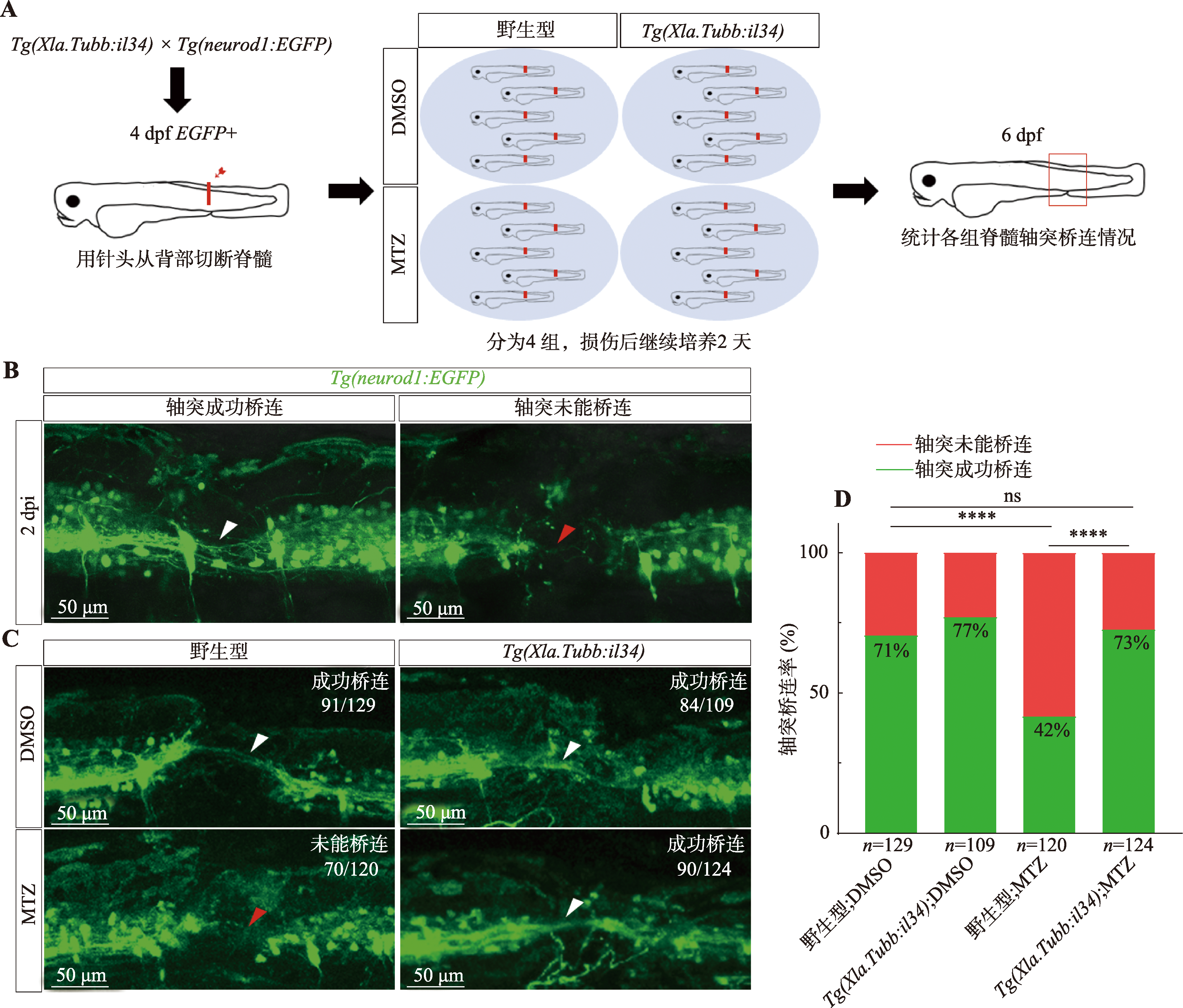
图2
巨噬细胞/小胶质细胞缺陷的Tg(Xla.Tubb:il34)斑马鱼仍表现出较强的轴突再生能力 A:激光共聚焦显微镜下4个组别斑马鱼损伤处的巨噬细胞/小胶质细胞荧光情况。图中红色荧光为Tg(mpeg1:DsRed)转基因斑马鱼的荧光信号,标记巨噬细胞/小胶质细胞;图中蓝色荧光信号为DAPI染料染色,标记细胞核。B:各组别斑马鱼损伤处巨噬细胞/小胶质细胞数量动态变化折线图。细胞数量统计包括该视野下所有具有完整细胞形态的红色荧光信号;*标记代表野生型;MTZ组和Tg(Xla.Tubb:il34);MTZ组间巨噬细胞/小胶质细胞数量的差异统计分析结果,***P<0.001,****P<0.0001。C:irf8突变体斑马鱼的巨噬细胞/小胶质细胞缺陷表型。斑马鱼整体原位杂交结果图中紫色信号为巨噬细胞/小胶质细胞特征基因mpeg1反义核酸探针染色,图左侧野生型损伤处信号较强,图右侧irf8突变斑马鱼基本未见信号。D:各组别损伤后2天(即6 dpf)时轴突再生情况。n为样本量(该组别斑马鱼条数);百分数表示轴突桥连率,用以评价神经再生能力;相应组间进行卡方检验,*P<0.05,**P<0.01,ns表示组间轴突再生情况无统计学差异。"
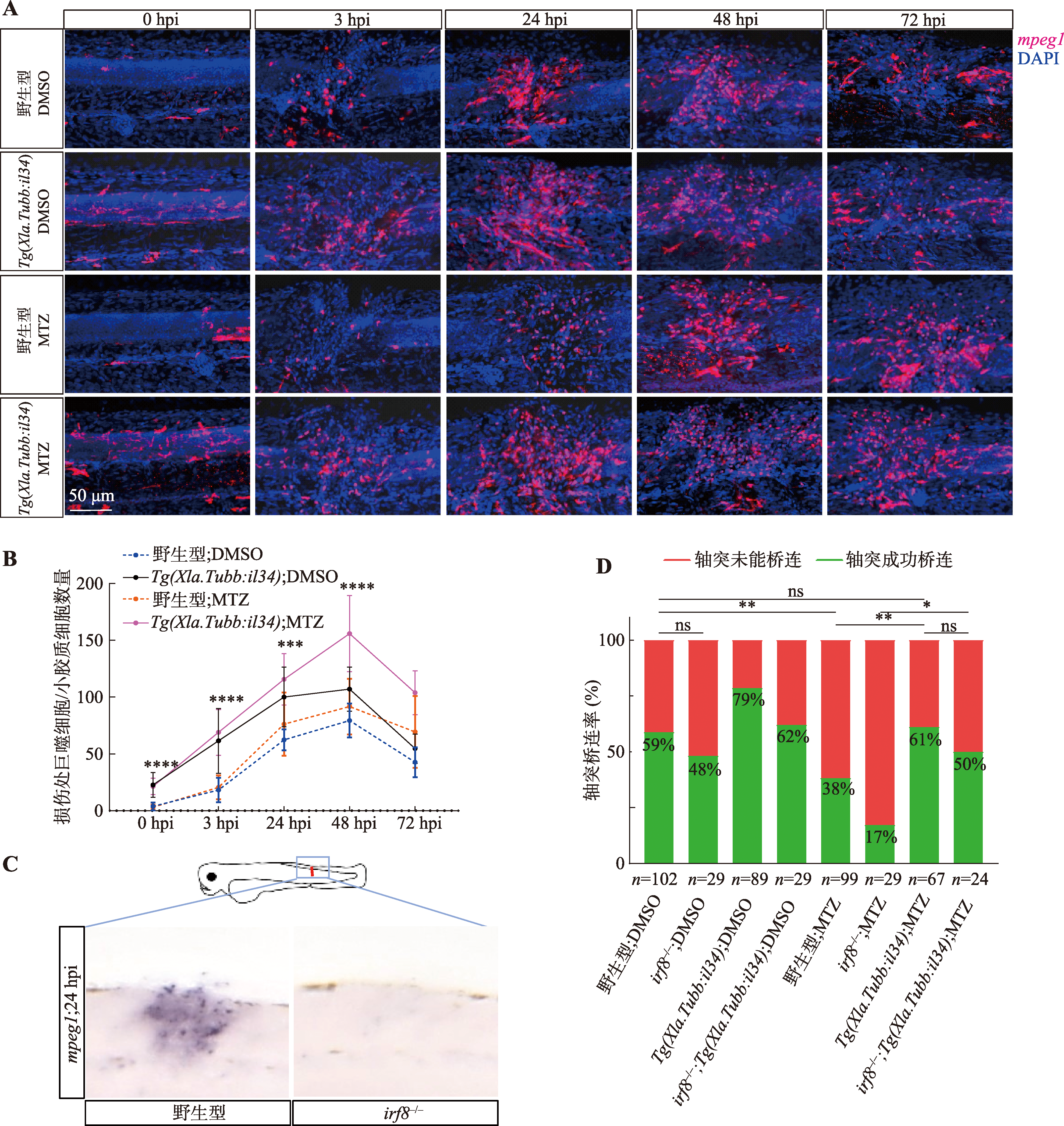
图4
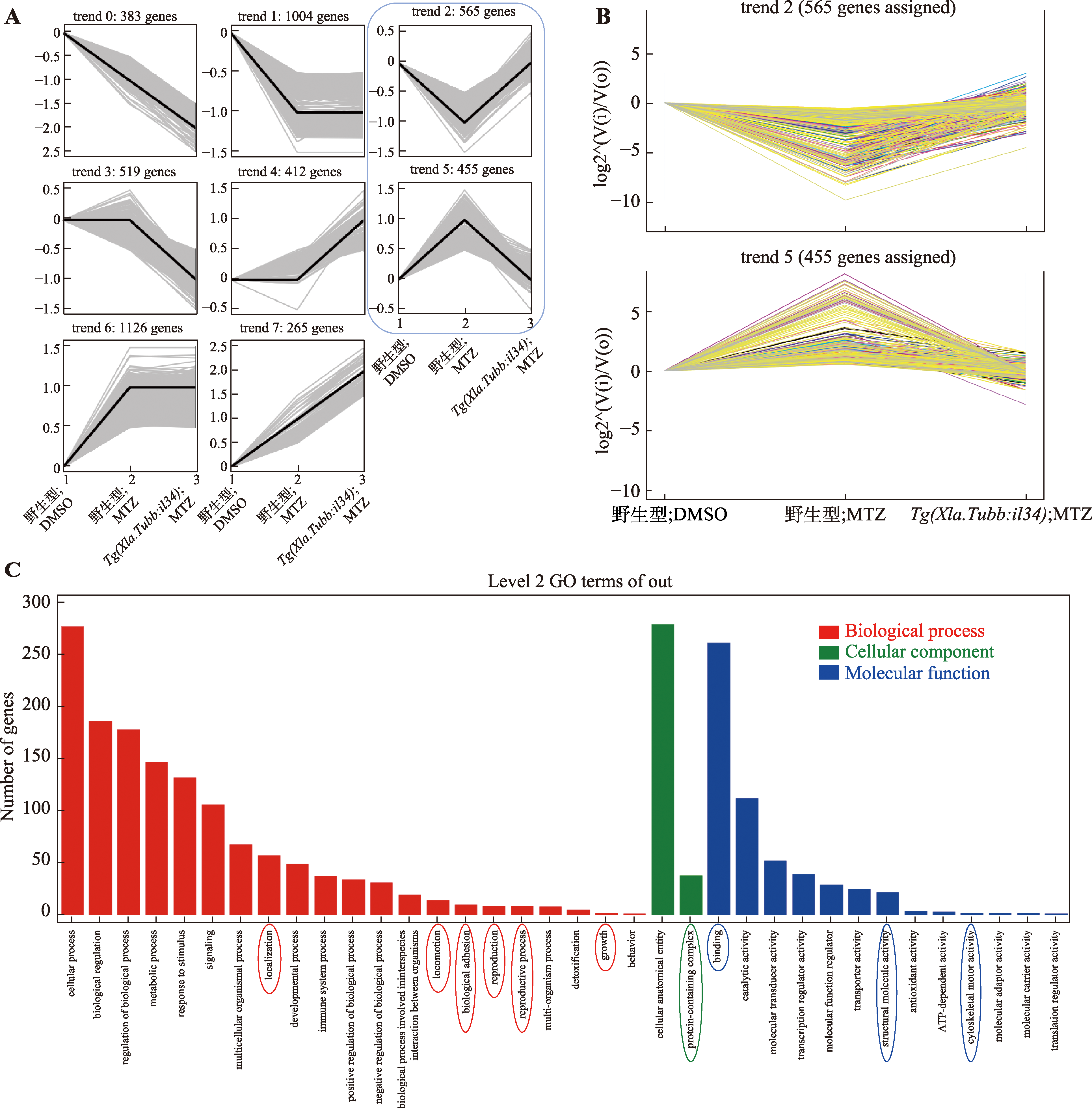
Il34通过恢复细胞黏附、细胞定位和骨架运动促进轴突再生 A:组间差异表达基因的趋势分析。图中从左至右3个节点分别代表野生型;DMSO组、野生型;MTZ组和Tg(Xla.Tubb:il34);MTZ组的基因表达情况,这些差异基因被分类为8个表达趋势,差异判定标准为该基因组间表达量的log2 fold change指数>1或<-1。B:趋势分析中trend 2和trend 5的基因表达情况折线图。纵坐标公式log2^(V(i)/V(o))为此处log2 fold change指数计算方法,V(o)表示野生型;DMSO组相关基因的表达量,V(i)表示与其作比较的组别中对应基因的表达量。C:trend 2中所包含基因的GO富集分析在level 2水平上的结果。图中红色、绿色、蓝色圆圈所圈出的条目分别为在生物学过程(biological process)、细胞学组分(cellular components)、分子生物学功能(molecular function)水平上富集的本研究重点关注的条目。"
| [1] | Cosar C, Julou L. The activity of 1-(2-hydroxyethyl)-2- methyl-5-nitroimidazole (R. P. 8823) against experimental Trichomonas vaginalis infections. Ann Inst Pasteur (Paris), 1959, 96(2): 238-241. |
| [2] |
Freeman CD, Klutman NE, Lamp KC. Metronidazole. A therapeutic review and update. Drugs, 1997, 54(5): 679-708.
doi: 10.2165/00003495-199754050-00003 pmid: 9360057 |
| [3] | Jokipii L, Jokipii AM. In vitro susceptibility of Giardia lamblia trophozoites to metronidazole and tinidazole. Infect Dis, 1980, 141(3): 317-325. |
| [4] | Farthing MJ, Inge PM. Antigiardial activity of the bile salt-like antibiotic sodium fusidate. J Antimicrob Chemother, 1986, 17(2): 165-171. |
| [5] |
Vinayak VK, Mahajan RC, Chitkara NL. In-vitro activity of methronidazole on the cysts of Entamoeba histolytica. Indian J Pathol Bacteriol, 1975, 18(1): 61-64.
pmid: 166039 |
| [6] |
Mahajan RC, Chitkara NL, Vinayak VK, Dutta DV. In vitro comparative evaluation of tinidazole and metronidazole on strains of Entamoeba histolytica. Indian J Pathol Bacteriol, 1974, 17(4): 226-228.
pmid: 4376127 |
| [7] |
Tally FP, Sutter VL, Finegold SM. Treatment of anaerobic infections with metronidazole. Antimicrob Agents Chemother, 1975, 7(5): 672-675.
doi: 10.1128/AAC.7.5.672 pmid: 1096810 |
| [8] |
Kusumi RK, Plouffe JF, Wyatt RH, Fass RJ. Central nervous system toxicity associated with metronidazole therapy. Ann Intern Med, 1980, 93(1): 59-60.
pmid: 7396319 |
| [9] |
Alvarez RS, Richardson DA, Bent AE, Ostergard DR. Central nervous system toxicity related to prolonged metronidazole therapy. Am J Obstet Gynecol, 1983, 145(5): 640-641.
pmid: 6829642 |
| [10] |
Guglielmo BJ. Metronidazole neurotoxicity: suspicions confirmed. Clin Infect Dis, 2021, 72(12): 2101-2102.
doi: 10.1093/cid/ciaa400 pmid: 32266372 |
| [11] | Quickfall D, Daneman N, Dmytriw AA, Juurlink DN. Metronidazole-induced neurotoxicity. CMAJ, 2021, 193(42): E1630. |
| [12] |
Kuriyama A, Jackson JL, Doi A, Kamiya T. Metronidazole- induced central nervous system toxicity: a systematic review. Clin Neuropharmacol, 2011, 34(6): 241-247.
doi: 10.1097/WNF.0b013e3182334b35 pmid: 21996645 |
| [13] | Patel K, Green-Hopkins I, Lu S, Tunkel AR. Cerebellar ataxia following prolonged use of metronidazole: case report and literature review. Int J Infect Dis, 2008, 12(6): e111-e114. |
| [14] |
White DT, Mumm JS. The nitroreductase system of inducible targeted ablation facilitates cell-specific regenerative studies in zebrafish. Methods, 2013, 62(3): 232-240.
doi: 10.1016/j.ymeth.2013.03.017 pmid: 23542552 |
| [15] | Lai ST, Kumari A, Liu JX, Zhang YY, Zhang WQ, Yen KY, Xu J. Chemical screening reveals Ronidazole is a superior prodrug to Metronidazole for nitroreductase-induced cell ablation system in zebrafish larvae. J Genet Genomics, 2021, 48(12): 1081-1090. |
| [16] |
Gürcü B, Koca YB, Özkut M, Tuğlu Mİ. Matrix changes due to the toxic effects of metronidazole in intestinal tissue of fish (Onchorhynchus mykiss). Chemosphere, 2016, 144: 1605-1610.
doi: 10.1016/j.chemosphere.2015.10.043 pmid: 26517388 |
| [17] |
Salter MW, Beggs S. Sublime microglia: expanding roles for the guardians of the CNS. Cell, 2014, 158(1): 15-24.
doi: 10.1016/j.cell.2014.06.008 pmid: 24995975 |
| [18] | Hu XM, Leak RK, Shi YJ, Suenaga J, Gao YQ, Zheng P, Chen J. Microglial and macrophage polarization-new prospects for brain repair. Nat Rev Neurol, 2015, 11(1): 56-64. |
| [19] |
Prinz M, Jung S, Priller J. Microglia biology: one century of evolving concepts. Cell, 2019, 179(2): 292-311.
doi: S0092-8674(19)31004-9 pmid: 31585077 |
| [20] |
Colonna M, Butovsky O. Microglia function in the central nervous system during health and neurodegeneration. Annu Rev Immunol, 2017, 35: 441-468.
doi: 10.1146/annurev-immunol-051116-052358 pmid: 28226226 |
| [21] |
Wright-Jin EC, Gutmann DH. Microglia as dynamic cellular mediators of brain function. Trends Mol Med, 2019, 25(11): 967-979.
doi: S1471-4914(19)30236-9 pmid: 31597593 |
| [22] |
Davies LC, Jenkins SJ, Allen JE, Taylor PR. Tissue- resident macrophages. Nat Immunol, 2013, 14(10): 986-995.
doi: 10.1038/ni.2705 pmid: 24048120 |
| [23] |
Zhou X, Wahane S, Friedl MS, Kluge M, Friedel CC, Avrampou K, Zachariou V, Guo L, Zhang B, He XJ, Friedel RH, Zou HY. Microglia and macrophages promote corralling, wound compaction and recovery after spinal cord injury via Plexin-B2. Nat Neurosci, 2020, 23(3): 337-350.
doi: 10.1038/s41593-020-0597-7 pmid: 32112058 |
| [24] | Li Y, He XL, Kawaguchi R, Zhang Y, Wang Q, Monavarfeshani A, Yang ZY, Chen B, Shi ZJ, Meng HY, Zhou SL, Zhu JJ, Jacobi A, Swarup V, Popovich PG, Geschwind DH, He ZG. Microglia-organized scar-free spinal cord repair in neonatal mice. Nature, 2020, 587(7835): 613-618. |
| [25] |
Wu ST, Xue RT, Hassan S, Nguyen TML, Wang TN, Pan HR, Xu J, Liu QF, Zhang WQ, Wen ZL. Il34-Csf1r pathway regulates the migration and colonization of microglial precursors. Dev Cell, 2018, 46(5): 552-563.e4.
doi: S1534-5807(18)30641-5 pmid: 30205037 |
| [26] | Baghdadi M, Umeyama Y, Hama N, Kobayashi T, Han N, Wada H, Seino KI. Interleukin-34, a comprehensive review. J Leukoc Biol, 2018, 104(5): 931-951. |
| [27] | Chitramuthu BP, Bennett HP. High resolution whole mount in situ hybridization within zebrafish embryos to study gene expression and function. J Vis Exp, 2013, (80): e50644. |
| [28] |
Zhou WB, Hildebrandt F. Inducible podocyte injury and proteinuria in transgenic zebrafish. J Am Soc Nephrol, 2012, 23(6): 1039-1047.
doi: 10.1681/ASN.2011080776 pmid: 22440901 |
| [29] |
Croese T, Castellani G, Schwartz M. Immune cell compartmentalization for brain surveillance and protection. Nat Immunol, 2021, 22(9): 1083-1092.
doi: 10.1038/s41590-021-00994-2 pmid: 34429552 |
| [30] |
Borst K, Dumas AA, Prinz M. Microglia: immune and non-immune functions. Immunity, 2021, 54(10): 2194-2208.
doi: 10.1016/j.immuni.2021.09.014 pmid: 34644556 |
| [31] |
Li QY, Barres BA. Microglia and macrophages in brain homeostasis and disease. Nat Rev Immunol, 2018, 18(4): 225-242.
doi: 10.1038/nri.2017.125 pmid: 29151590 |
| [32] |
Li L, Jin H, Xu J, Shi YY, Wen ZL. Irf8 regulates macrophage versus neutrophil fate during zebrafish primitive myelopoiesis. Blood, 2011, 117(4): 1359-1369.
doi: 10.1182/blood-2010-06-290700 pmid: 21079149 |
| [33] |
Wehner D, Tsarouchas TM, Michael A, Haase C, Weidinger G, Reimer MM, Becker T, Becker CG. Wnt signaling controls pro-regenerative Collagen XII in functional spinal cord regeneration in zebrafish. Nat Commun, 2017, 8(1): 126.
doi: 10.1038/s41467-017-00143-0 pmid: 28743881 |
| [34] | Tsata V, Möllmert S, Schweitzer C, Kolb J, Möckel C, Böhm B, Rosso G, Lange C, Lesche M, Hammer J, Kesavan G, Beis D, Guck J, Brand M, Wehner D. A switch in pdgfrb(+) cell-derived ECM composition prevents inhibitory scarring and promotes axon regeneration in the zebrafish spinal cord. Dev Cell, 2021, 56(4): 509-524.e9. |
| [35] |
O'Shea TM, Burda JE, Sofroniew MV. Cell biology of spinal cord injury and repair. J Clin Invest, 2017, 127(9): 3259-3270.
doi: 10.1172/JCI90608 pmid: 28737515 |
| [36] | Bloom O, Herman PE, Spungen AM. Systemic inflammation in traumatic spinal cord injury. Exp Neurol, 2020, 325: 113143. |
| [37] |
Morales RA, Allende ML. Peripheral macrophages promote tissue regeneration in zebrafish by fine-tuning the inflammatory response. Front Immunol, 2019, 10: 253.
doi: 10.3389/fimmu.2019.00253 pmid: 30891030 |
| [38] |
Benowitz LI, Popovich PG. Inflammation and axon regeneration. Curr Opin Neurol, 2011, 24(6): 577-583.
doi: 10.1097/WCO.0b013e32834c208d pmid: 21968547 |
| [39] | Jing X, Wang S, Tang H, Li DZ, Zhou FY, Xin LJ, He QQ, Hu SS, Zhang TW, Chen T, Song JL. Dynamically bioresponsive DNA hydrogel incorporated with dual-functional stem cells from apical papilla-derived exosomes promotes diabetic bone regeneration. ACS Appl Mater Interfaces, 2022, 14(14): 16082-16099. |
| [40] |
Tsarouchas TM, Wehner D, Cavone L, Munir T, Keatinge M, Lambertus M, Underhill A, Barrett T, Kassapis E, Ogryzko N, Feng Y, van Ham TJ, Becker T, Becker CG. Dynamic control of proinflammatory cytokines Il-1β and Tnf-α by macrophages in zebrafish spinal cord regeneration. Nat Commun, 2018, 9(1): 4670.
doi: 10.1038/s41467-018-07036-w pmid: 30405119 |
| [41] |
Eva R, Fawcett J. Integrin signalling and traffic during axon growth and regeneration. Curr Opin Neurobiol, 2014, 27: 179-185.
doi: 10.1016/j.conb.2014.03.018 pmid: 24793179 |
| [42] |
Nieuwenhuis B, Haenzi B, Andrews MR, Verhaagen J, Fawcett JW. Integrins promote axonal regeneration after injury of the nervous system. Biol Rev Camb Philos Soc, 2018, 93(3): 1339-1362.
doi: 10.1111/brv.12398 pmid: 29446228 |
| [43] |
Blanquie O, Bradke F. Cytoskeleton dynamics in axon regeneration. Curr Opin Neurobiol, 2018, 51: 60-69.
doi: S0959-4388(18)30008-4 pmid: 29544200 |
| [1] | 洪佳馨, 徐颂恩, 张文清, 刘伟. Pu.1和cMyb在斑马鱼中性粒细胞发育中的相互作用[J]. 遗传, 2024, 46(4): 319-332. |
| [2] | 孙飘, 李颖, 刘帆, 王璐. TPI缺乏症斑马鱼模型的构建及分析[J]. 遗传, 2024, 46(3): 232-241. |
| [3] | 李凯伦, 卢荆奥, 陈小辉, 张文清, 刘伟. 尿囊素促进破骨细胞缺陷斑马鱼骨折修复[J]. 遗传, 2023, 45(4): 341-353. |
| [4] | 胥腾, 黄海辉. 艰难梭菌抗菌药物耐药机制研究进展[J]. 遗传, 2023, 45(11): 1028-1038. |
| [5] | 卢荆澳, 黄春燕, 林芷茵, 唐政, 马宁, 黄志斌. cd99l2基因调控斑马鱼白细胞组织间的迁移机制[J]. 遗传, 2022, 44(9): 798-809. |
| [6] | 郑鹏飞, 谢海波, 朱盼盼, 赵呈天. 斑马鱼神经底板处神经元的分布及特征[J]. 遗传, 2022, 44(6): 510-520. |
| [7] | 张婷婷, 刘峰. 斑马鱼蛋白酪氨酸硫酸化修饰的检测方法研究[J]. 遗传, 2022, 44(2): 178-186. |
| [8] | 贾婷婷, 雷蕾, 吴歆媛, 蔡顺有, 陈艺璇, 薛钰. 二甲双胍对斑马鱼骨骼发育及损伤修复的机制研究[J]. 遗传, 2022, 44(1): 68-79. |
| [9] | 郭佳妮, 刘帆, 王璐. 斑马鱼血液疾病模型及应用[J]. 遗传, 2020, 42(8): 725-738. |
| [10] | 熊凤,谢训卫,潘鲁媛,李阔宇,柳力月,张昀,李玲璐,孙永华. 国家斑马鱼资源中心的资源、技术和服务建设[J]. 遗传, 2018, 40(8): 683-692. |
| [11] | 许璟瑾, 张文娟, 王静怡, 姚丽云, 潘裕添, 欧一新, 薛钰, . 金线莲抑制斑马鱼黑色素形成的活性组分筛选及机理研究[J]. 遗传, 2017, 39(12): 1178-1187. |
| [12] | 刘姗姗, 张翠珍, 彭刚. 饥饿对幼年斑马鱼下丘脑摄食相关性神经肽表达的影响[J]. 遗传, 2016, 38(9): 821-830. |
| [13] | 张峰华,王厚鹏,黄思雨,熊凤,朱作言,孙永华. 两种密码子优化的Cas9编码基因在斑马鱼胚胎中基因敲除效率的比较[J]. 遗传, 2016, 38(2): 144-154. |
| [14] | 顾爱华 严丽锋. 斑马鱼在再生医学研究中的应用及进展[J]. 遗传, 2013, 35(7): 856-866. |
| [15] | 李礼,罗凌飞. 以斑马鱼为模式动物研究器官的发育与再生[J]. 遗传, 2013, 35(4): 421-432. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||

www.chinagene.cn
备案号:京ICP备09063187号-4
总访问:,今日访问:,当前在线:
