遗传 ›› 2025, Vol. 47 ›› Issue (11): 1197-1213.doi: 10.16288/j.yczz.24-372
收稿日期:2025-04-12
修回日期:2025-06-20
出版日期:2025-07-07
发布日期:2025-07-07
通讯作者:
丁梅,博士,研究员,研究方向:神经系统发育的分子机制。E-mail: mding@genetics.ac.cn作者简介:陈佳强,博士研究生,专业方向:神经生物学。E-mail: jiaqiang.chen@genetics.ac.cn
基金资助:
Jiaqiang Chen1,2( ), Mei Ding1,2(
), Mei Ding1,2( )
)
Received:2025-04-12
Revised:2025-06-20
Published:2025-07-07
Online:2025-07-07
Supported by:摘要:
细胞外囊泡(extracellular vesicles, EVs)是由细胞释放到细胞外微环境的膜包被的颗粒。在神经系统中,EVs是介导生物分子运输和细胞间通讯的关键载体,它们深度参与调控生理稳态与病理级联反应,同时在疾病诊断与治疗领域展现出重要应用价值。本文系统综述了神经元与胶质细胞源性EVs的功能异质性及研究进展,以期为阐明EVs在神经系统的多样化角色提供理论依据。
陈佳强, 丁梅. 神经系统细胞外囊泡研究进展[J]. 遗传, 2025, 47(11): 1197-1213.
Jiaqiang Chen, Mei Ding. Progress on extracellular vesicles in the nervous system[J]. Hereditas(Beijing), 2025, 47(11): 1197-1213.

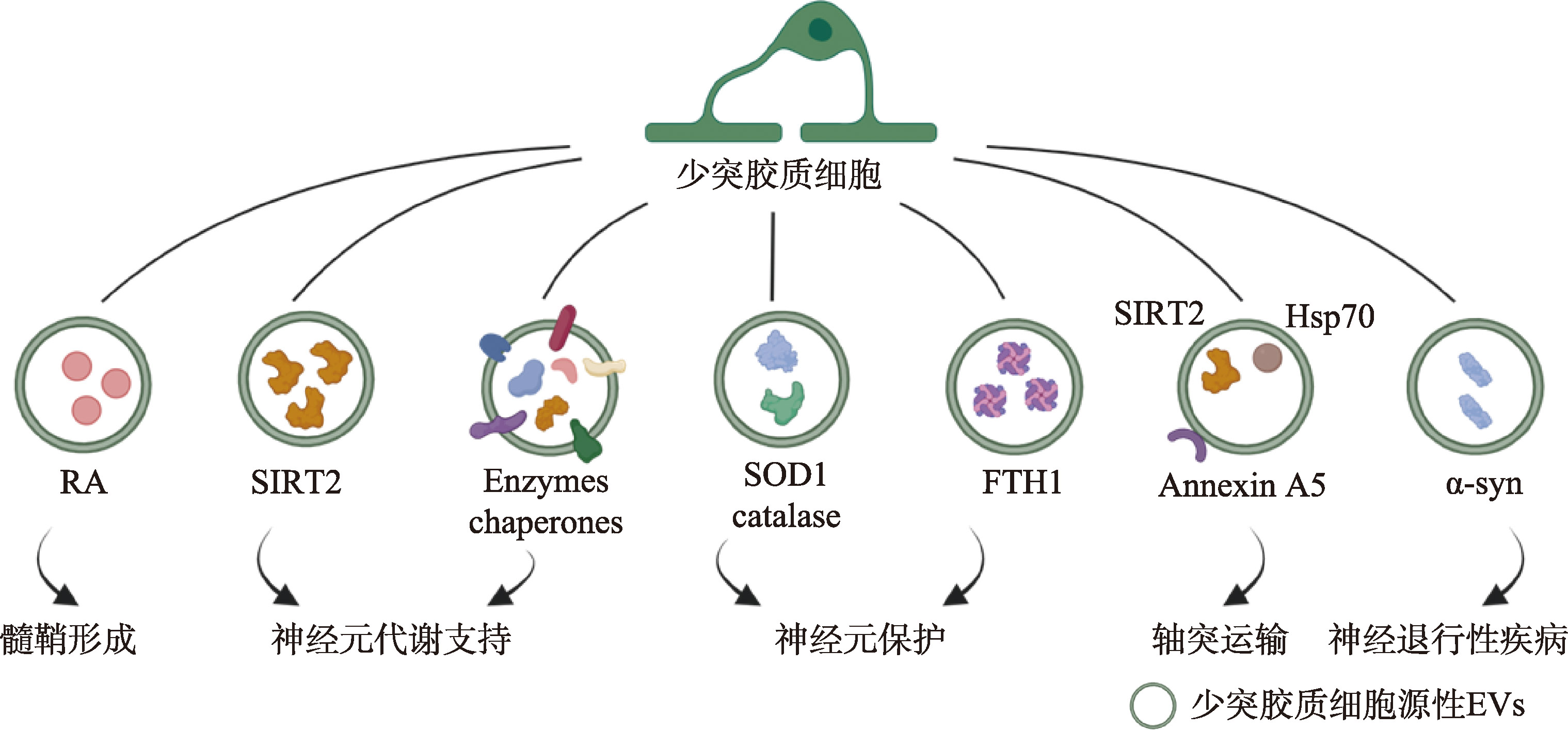
图4
少突胶质细胞源性EVs的功能示意图 少突胶质细胞源性EVs可通过多种途径参与调节神经元功能。例如,EVs携带代谢酶(如SIRT2)参与维持神经元代谢活动;携带抗氧化酶(如SOD1)增强神经元的抗氧化能力;通过减少特定组分(如维甲酸RA)的转运,提高该组分在少突胶质细胞祖细胞(OPC)内的含量,进而参与髓鞘形成调控。此外,少突胶质细胞源性EVs还可影响轴突运输和神经退行性疾病等过程。线条表示EVs是从标注的细胞释放的;EVs附近的分子表示其携带的组分;箭头指向的文字描述了EVs在靶细胞中发挥的主要生物学功能。示意图利用BioRender软件绘制, https://BioRender.com/fmlkh27。"
| [1] |
Agnati LF, Guidolin D, Guescini M, Genedani S, Fuxe K. Understanding wiring and volume transmission. Brain Res Rev, 2010, 64(1): 137-159.
pmid: 20347870 |
| [2] |
Budnik V, Ruiz-Cañada C, Wendler F. Extracellular vesicles round off communication in the nervous system. Nat Rev Neurosci, 2016, 17(3): 160-172.
pmid: 26891626 |
| [3] |
Ahmad S, Srivastava RK, Singh P, Naik UP, Srivastava AK. Role of extracellular vesicles in glia-neuron intercellular communication. Front Mol Neurosci, 2022, 15: 844194.
pmid: 35493327 |
| [4] |
Filannino FM, Panaro MA, Benameur T, Pizzolorusso I, Porro C. Extracellular vesicles in the central nervous system: a novel mechanism of neuronal cell communication. Int J Mol Sci, 2024, 25(3): 1629.
pmid: 38338906 |
| [5] |
Holm MM, Kaiser J, Schwab ME. Extracellular vesicles: multimodal envoys in neural maintenance and repair. Trends Neurosci, 2018, 41(6): 360-372.
pmid: 29605090 |
| [6] |
Gage FH. Adult neurogenesis in mammals. Science, 2019, 364(6443): 827-828.
pmid: 31147506 |
| [7] |
Sangani NB, Gomes AR, Curfs LMG, Reutelingsperger CP. The role of extracellular vesicles during CNS development. Prog Neurobiol, 2021, 205: 102124.
pmid: 34314775 |
| [8] |
Ma YZ, Li CH, Huang YL, Wang Y, Xia XH, Zheng JC. Exosomes released from neural progenitor cells and induced neural progenitor cells regulate neurogenesis through miR-21a. Cell Commun Signal, 2019, 17(1): 96.
pmid: 31419975 |
| [9] |
Sharma P, Mesci P, Carromeu C, McClatchy DR, Schiapparelli L, Yates JR, Muotri AR, Cline HT. Exosomes regulate neurogenesis and circuit assembly. Proc Natl Acad Sci USA, 2019, 116(32): 16086-16094.
pmid: 31320591 |
| [10] |
Upadhya R, Madhu LN, Attaluri S, Gitaí DLG, Pinson MR, Kodali M, Shetty G, Zanirati G, Kumar S, Shuai B, Weintraub ST, Shetty AK. Extracellular vesicles from human iPSC-derived neural stem cells: miRNA and protein signatures, and anti-inflammatory and neurogenic properties. J Extracell vesicles, 2020, 9(1): 1809064.
pmid: 32944193 |
| [11] |
Bassi MS, Iezzi E, Gilio L, Centonze D, Buttari F. Synaptic plasticity shapes brain connectivity: implications for network topology. Int J Mol Sci, 2019, 20(24): 6193.
pmid: 31817968 |
| [12] |
Vilcaes AA, Chanaday NL, Kavalali ET. Interneuronal exchange and functional integration of synaptobrevin via extracellular vesicles. Neuron, 2021, 109(6): 971-983.e5.
pmid: 33513363 |
| [13] |
Lee SH, Shin SM, Zhong P, Kim HT, Kim DI, Kim JM, Do Heo W, Kim DW, Yeo CY, Kim CH, Liu QS. Reciprocal control of excitatory synapse numbers by Wnt and Wnt inhibitor PRR7 secreted on exosomes. Nat Commun, 2018, 9(1): 3434.
pmid: 30143647 |
| [14] |
Korkut C, Ataman B, Ramachandran P, Ashley J, Barria R, Gherbesi N, Budnik V. Trans-synaptic transmission of vesicular Wnt signals through Evi/Wntless. Cell, 2009, 139(2): 393-404.
pmid: 19837038 |
| [15] |
Korkut C, Li YH, Koles K, Brewer C, Ashley J, Yoshihara M, Budnik V. Regulation of postsynaptic retrograde signaling by presynaptic exosome release. Neuron, 2013, 77(6): 1039-1046.
pmid: 23522040 |
| [16] |
Goldie BJ, Dun MD, Lin MJ, Smith ND, Verrills NM, Dayas CV, Cairns MJ. Activity-associated miRNA are packaged in Map1b-enriched exosomes released from depolarized neurons. Nucleic Acids Res, 2014, 42(14): 9195-9208.
pmid: 25053844 |
| [17] |
Pastuzyn ED, Day CE, Kearns RB, Kyrke-Smith M, Taibi AV, McCormick J, Yoder N, Belnap DM, Erlendsson S, Morado DR, Briggs JAG, Feschotte C, Shepherd JD. The neuronal gene Arc encodes a repurposed retrotransposon Gag protein that mediates intercellular RNA transfer. Cell, 2018, 172(1-2): 275-288.e18.
pmid: 29328916 |
| [18] |
Shepherd JD, Bear MF. New views of Arc, a master regulator of synaptic plasticity. Nat Neurosci, 2011, 14(3): 279-284.
pmid: 21278731 |
| [19] |
Ashley J, Cordy B, Lucia D, Fradkin LG, Budnik V, Thomson T. Retrovirus-like Gag protein Arc1 binds RNA and traffics across synaptic boutons. Cell, 2018, 172(1-2): 262-274.e11.
pmid: 29328915 |
| [20] |
Morel L, Regan M, Higashimori H, Ng SK, Esau C, Vidensky S, Rothstein J, Yang YJ. Neuronal exosomal miRNA-dependent translational regulation of astroglial glutamate transporter GLT1. J Biol Chem, 2013, 288(10): 7105-7116.
pmid: 23364798 |
| [21] |
Bahrini I, Song JH, Diez D, Hanayama R. Neuronal exosomes facilitate synaptic pruning by up-regulating complement factors in microglia. Sci Rep, 2015, 5(1): 7989.
pmid: 25612542 |
| [22] |
Peng H, Harvey BT, Richards CI, Nixon K. Neuron- derived extracellular vesicles modulate microglia activation and function. Biology (Basel), 2021, 10(10): 948.
pmid: 34681047 |
| [23] | Kaya Z, Belder N, Sever-Bahcekapili M, Donmez-Demir B, Erdener ŞE, Bozbeyoglu N, Bagci C, Eren-Kocak E, Yemisci M, Karatas H, Erdemli E, Gursel I, Dalkara T. Vesicular HMGB1 release from neurons stressed with spreading depolarization enables confined inflammatory signaling to astrocytes. J Neuroinflammation, 2023, 20(1): 295. [DOI] |
| [24] |
Xin DQ, Li TT, Zhao YJ, Guo XF, Gai CC, Jiang ZG, Yu SW, Cheng J, Song Y, Cheng YH, Luo Q, Gu B, Liu DX, Wang Z. MiR-100-5p-rich small extracellular vesicles from activated neuron to aggravate microglial activation and neuronal activity after stroke. J Nanobiotechnology, 2024, 22(1): 534.
pmid: 39227960 |
| [25] |
Cossetti C, Iraci N, Mercer TR, Leonardi T, Alpi E, Drago D, Alfaro-Cervello C, Saini HK, Davis MP, Schaeffer J, Vega B, Stefanini M, Zhao CJ, Muller W, Garcia-Verdugo JM, Mathivanan S, Bachi A, Enright AJ, Mattick JS, Pluchino S. Extracellular vesicles from neural stem cells transfer IFN-γ via Ifngr1 to activate Stat1 signaling in target cells. Mol Cell, 2014, 56(2): 193-204.
pmid: 25242146 |
| [26] |
Simeoli R, Montague K, Jones HR, Castaldi L, Chambers D, Kelleher JH, Vacca V, Pitcher T, Grist J, Al-Ahdal H, Wong LF, Perretti M, Lai J, Mouritzen P, Heppenstall P, Malcangio M. Exosomal cargo including microRNA regulates sensory neuron to macrophage communication after nerve trauma. Nat Commun, 2017, 8(1): 1778.
pmid: 29176651 |
| [27] |
Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron, 2008, 57(2): 178-201.
pmid: 18215617 |
| [28] |
Obermeier B, Daneman R, Ransohoff RM. Development, maintenance and disruption of the blood-brain barrier. Nat Med, 2013, 19(12): 1584-1596.
pmid: 24309662 |
| [29] |
Tam SJ, Watts RJ. Connecting vascular and nervous system development: angiogenesis and the blood-brain barrier. Annu Rev Neurosci, 2010, 33(1): 379-408.
pmid: 20367445 |
| [30] |
Xu B, Zhang Y, Du XF, Li J, Zi HX, Bu JW, Yan Y, Han H, Du JL. Neurons secrete miR-132-containing exosomes to regulate brain vascular integrity. Cell Res, 2017, 27(7): 882-897.
pmid: 28429770 |
| [31] |
Li Y, Jiang JW, Li JM, Liu SL, Wang C, Yu ZT, Xia Y. Exosome-derived CDC42 from hypoxia-pretreated neural stem cells inhibits ACSL4-related ferroptosis to alleviate vascular injury in Parkinson’s disease mice models. J neurochem, 2025, 169(3): e70027.
pmid: 40035385 |
| [32] |
Dong XH, Jiang DY, Wang L, Zhao J, Yu LL, Huang Y, Wu XH, Zhu YQ, Zhao YM, Zhao QS, Zhang GM, Li XY. VPS28 regulates brain vasculature by controlling neuronal VEGF trafficking through extracellular vesicle secretion. iScience, 2022, 25(4): 104042.
pmid: 35330682 |
| [33] |
Wang JW, Xie XF, Wu YG, Zhou Y, Li QF, Li Y, Xu X, Wang M, Murdiyarso L, Houck K, Hilton T, Chung D, Dong JF, Li M, Zhang JN. Brain-derived extracellular vesicles induce vasoconstriction and reduce cerebral blood flow in mice. J Neurotrauma, 2022, 39(11-12): 879-890.
pmid: 35316073 |
| [34] |
Yuyama K, Sun H, Sakai S, Mitsutake S, Okada M, Tahara H, Furukawa JI, Fujitani N, Shinohara Y, Igarashi Y. Decreased amyloid-β pathologies by intracerebral loading of glycosphingolipid-enriched exosomes in Alzheimer model mice. J Biol Chem, 2014, 289(35): 24488-24498.
pmid: 25037226 |
| [35] |
Yuyama K, Sun H, Usuki S, Sakai S, Hanamatsu H, Mioka T, Kimura N, Okada M, Tahara H, Furukawa JI, Fujitani N, Shinohara Y, Igarashi Y. A potential function for neuronal exosomes: sequestering intracerebral amyloid-β peptide. FEBS Lett, 2015, 589(1): 84-88.
pmid: 25436414 |
| [36] |
Melentijevic I, Toth ML, Arnold ML, Guasp RJ, Harinath G, Nguyen KC, Taub D, Parker JA, Neri C, Gabel CV, Hall DH, Driscoll M. C. elegans neurons jettison protein aggregates and mitochondria under neurotoxic stress. Nature, 2017, 542(7641): 367-371.
pmid: 28178240 |
| [37] |
Takeuchi T, Suzuki M, Fujikake N, Popiel HA, Kikuchi H, Futaki S, Wada K, Nagai Y. Intercellular chaperone transmission via exosomes contributes to maintenance of protein homeostasis at the organismal level. Proc Natl Acad Sci USA, 2015, 112(19): E2497-E2506.
pmid: 25918398 |
| [38] | Shang XK, Zhang SM, Ni JJ. Research progress of cathepsin B in brain aging and Alzheimer’s diseases. Hereditas(Beijing), 2023, 45(3): 212-220. |
| 商晓康, 张思萌, 倪军军. 组织蛋白酶B参与脑衰老及阿尔兹海默症发生发展研究进展. 遗传, 2023, 45(3): 212-220. | |
| [39] |
Ruan Z, Pathak D, Venkatesan Kalavai S, Yoshii- Kitahara A, Muraoka S, Bhatt N, Takamatsu-Yukawa K, Hu JQ, Wang YZ, Hersh S, Ericsson M, Gorantla S, Gendelman HE, Kayed R, Ikezu S, Luebke JI, Ikezu T. Alzheimer’s disease brain-derived extracellular vesicles spread tau pathology in interneurons. Brain, 2021, 144(1): 288-309.
pmid: 33246331 |
| [40] |
Sinha MS, Ansell-Schultz A, Civitelli L, Hildesjö C, Larsson M, Lannfelt L, Ingelsson M, Hallbeck M. Alzheimer’s disease pathology propagation by exosomes containing toxic amyloid-beta oligomers. Acta Neuropathol, 2018, 136(1): 41-56.
pmid: 29934873 |
| [41] |
Ding L, Yang XY, Xia XH, Li YX, Wang Y, Li CH, Sun YY, Gao G, Zhao S, Sheng SY, Liu JH, Zheng JC. Exosomes mediate APP dysregulation via APP-miR-185-5p axis. Front Cell Dev Biol, 2022, 10: 793388.
pmid: 35223832 |
| [42] |
Lizarraga-Valderrama LR, Sheridan GK. Extracellular vesicles and intercellular communication in the central nervous system. FEBS Lett, 2021, 595(10): 1391-1410.
pmid: 33728650 |
| [43] |
Freeman MR, Rowitch DH. Evolving concepts of gliogenesis: a look way back and ahead to the next 25 years. Neuron, 2013, 80(3): 613-623.
pmid: 24183014 |
| [44] |
Luarte A, Henzi R, Fernández A, Gaete D, Cisternas P, Pizarro M, Batiz LF, Villalobos I, Masalleras M, Vergara R, Varas-Godoy M, Abarzua-Catalan L, Herrera-Molina R, Lafourcade C, Wyneken U. Astrocyte-derived small extracellular vesicles regulate dendritic complexity through miR-26a-5p activity. Cells, 2020, 9(4): 930.
pmid: 32290095 |
| [45] |
You Y, Borgmann K, Edara VV, Stacy S, Ghorpade A, Ikezu T. Activated human astrocyte-derived extracellular vesicles modulate neuronal uptake, differentiation and firing. J Extracell Vesicles, 2019, 9(1): 1706801.
pmid: 32002171 |
| [46] |
Pascua-Maestro R, González E, Lillo C, Ganfornina MD, Falcón-Pérez JM, Sanchez D. Extracellular vesicles secreted by astroglial cells transport Apolipoprotein D to neurons and mediate neuronal survival upon oxidative stress. Front Cell Neurosci, 2019, 12: 526.
pmid: 30687015 |
| [47] |
Guitart K, Loers G, Buck F, Bork U, Schachner M, Kleene R. Improvement of neuronal cell survival by astrocyte-derived exosomes under hypoxic and ischemic conditions depends on prion protein. Glia, 2016, 64(6): 896-910.
pmid: 26992135 |
| [48] |
Bianco F, Perrotta C, Novellino L, Francolini M, Riganti L, Menna E, Saglietti L, Schuchman EH, Furlan R, Clementi E, Matteoli M, Verderio C. Acid sphingomyelinase activity triggers microparticle release from glial cells. EMBO J, 2009, 28(8): 1043-1054.
pmid: 19300439 |
| [49] |
Dickens AM, Tovar-Y-Romo LB, Yoo SW, Trout AL, Bae M, Kanmogne M, Megra B, Williams DW, Witwer KW, Gacias M, Tabatadze N, Cole RN, Casaccia P, Berman JW, Anthony DC, Haughey NJ. Astrocyte-shed extracellular vesicles regulate the peripheral leukocyte response to inflammatory brain lesions. Sci Signal, 2017, 10(473): eaai7696.
pmid: 28377412 |
| [50] |
Ibáñez F, Montesinos J, Ureña-Peralta JR, Guerri C, Pascual M. TLR4 participates in the transmission of ethanol-induced neuroinflammation via astrocyte- derived extracellular vesicles. J Neuroinflammation, 2019, 16(1): 136.
pmid: 31272469 |
| [51] |
Willis CM, Nicaise AM, Bongarzone ER, Givogri M, Reiter CR, Heintz O, Jellison ER, Sutter PA, TeHennepe G, Ananda G, Vella AT, Crocker SJ. Astrocyte support for oligodendrocyte differentiation can be conveyed via extracellular vesicles but diminishes with age. Sci Rep, 2020, 10(1): 828.
pmid: 31964978 |
| [52] |
Varcianna A, Myszczynska MA, Castelli LM, O'Neill B, Kim Y, Talbot J, Nyberg S, Nyamali I, Heath PR, Stopford MJ, Hautbergue GM, Ferraiuolo L. Micro- RNAs secreted through astrocyte-derived extracellular vesicles cause neuronal network degeneration in C9orf72 ALS. EBioMedicine, 2019, 40: 626-635.
pmid: 30711519 |
| [53] |
Basso M, Pozzi S, Tortarolo M, Fiordaliso F, Bisighini C, Pasetto L, Spaltro G, Lidonnici D, Gensano F, Battaglia E, Bendotti C, Bonetto V. Mutant copper-zinc superoxide dismutase (SOD1) induces protein secretion pathway alterations and exosome release in astrocytes: implications for disease spreading and motor neuron pathology in amyotrophic lateral sclerosis. J Biol Chem, 2013, 288(22): 15699-15711.
pmid: 23592792 |
| [54] |
Hickman S, Izzy S, Sen P, Morsett L, El Khoury J. Microglia in neurodegeneration. Nat Neurosci, 2018, 21(10): 1359-1369.
pmid: 30258234 |
| [55] |
Paolicelli RC, Bergamini G, Rajendran L. Cell-to-cell communication by extracellular vesicles: focus on microglia. Neuroscience, 2019, 405: 148-157.
pmid: 29660443 |
| [56] |
Antonucci F, Turola E, Riganti L, Caleo M, Gabrielli M, Perrotta C, Novellino L, Clementi E, Giussani P, Viani P, Matteoli M, Verderio C. Microvesicles released from microglia stimulate synaptic activity via enhanced sphingolipid metabolism. EMBO J, 2012, 31(5): 1231-1240.
pmid: 22246184 |
| [57] |
Gabrielli M, Battista N, Riganti L, Prada I, Antonucci F, Cantone L, Matteoli M, Maccarrone M, Verderio C. Active endocannabinoids are secreted on extracellular membrane vesicles. EMBO Rep, 2015, 16(2): 213-220.
pmid: 25568329 |
| [58] |
Prada I, Gabrielli M, Turola E, Iorio A, D’Arrigo G, Parolisi R, De Luca M, Pacifici M, Bastoni M, Lombardi M, Legname G, Cojoc D, Buffo A, Furlan R, Peruzzi F, Verderio C. Glia-to-neuron transfer of miRNAs via extracellular vesicles: a new mechanism underlying inflammation-induced synaptic alterations. Acta Neuropathol, 2018, 135(4): 529-550.
pmid: 29302779 |
| [59] |
Bianco F, Pravettoni E, Colombo A, Schenk U, Möller T, Matteoli M, Verderio C. Astrocyte-derived ATP induces vesicle shedding and IL-1β release from microglia. J Immunol, 2005, 174(11): 7268-7277.
pmid: 15905573 |
| [60] |
Yang YY, Boza-Serrano A, Dunning CJR, Clausen BH, Lambertsen KL, Deierborg T. Inflammation leads to distinct populations of extracellular vesicles from microglia. J Neuroinflammation, 2018, 15(1): 168.
pmid: 29807527 |
| [61] |
Kumar A, Stoica BA, Loane DJ, Yang M, Abulwerdi G, Khan N, Kumar A, Thom SR, Faden AI. Microglial- derived microparticles mediate neuroinflammation after traumatic brain injury. J Neuroinflammation, 2017, 14(1): 47.
pmid: 28292310 |
| [62] |
Huang S, Ge XT, Yu JW, Han ZL, Yin ZY, Li Y, Chen FL, Wang HC, Zhang JN, Lei P. Increased miR-124-3p in microglial exosomes following traumatic brain injury inhibits neuronal inflammation and contributes to neurite outgrowth via their transfer into neurons. FASEB J, 2018, 32(1): 512-528.
pmid: 28935818 |
| [63] |
Lombardi M, Parolisi R, Scaroni F, Bonfanti E, Gualerzi A, Gabrielli M, de Rosbo NK, Uccelli A, Giussani P, Viani P, Garlanda C, Abbracchio MP, Chaabane L, Buffo A, Fumagalli M, Verderio C. Detrimental and protective action of microglial extracellular vesicles on myelin lesions: astrocyte involvement in remyelination failure. Acta Neuropathol, 2019, 138(6): 987-1012.
pmid: 31363836 |
| [64] |
Aires ID, Ribeiro-Rodrigues T, Boia R, Ferreira- Rodrigues M, Girão H, Ambrósio AF, Santiago AR. Microglial extracellular vesicles as vehicles for neurodegeneration spreading. Biomolecules, 2021, 11(6): 770.
pmid: 34063832 |
| [65] |
Li J, Li XN, Jiang X, Yang M, Yang R, Burnstock G, Xiang ZH, Yuan HB. Microvesicles shed from microglia activated by the P2X7-p38 pathway are involved in neuropathic pain induced by spinal nerve ligation in rats. Purinergic Signal, 2017, 13(1): 13-26.
pmid: 27683228 |
| [66] |
Guo M, Wang J, Zhao YX, Feng YW, Han SD, Dong Q, Cui M, Tieu K. Microglial exosomes facilitate α-synuclein transmission in Parkinson’s disease. Brain, 2020, 143(5): 1476-1497.
pmid: 32355963 |
| [67] |
Joshi P, Turola E, Ruiz A, Bergami A, Libera DD, Benussi L, Giussani P, Magnani G, Comi G, Legname G, Ghidoni R, Furlan R, Matteoli M, Verderio C. Microglia convert aggregated amyloid-β into neurotoxic forms through the shedding of microvesicles. Cell Death Differ, 2014, 21(4): 582-593.
pmid: 24336048 |
| [68] |
Chen JF, Wang F, Huang NX, Xiao L, Mei F. Oligodendrocytes and myelin: active players in neurodegeneartive brains? Dev Neurobiol, 2022, 82(2): 160-174.
pmid: 35081276 |
| [69] |
Kuhn S, Gritti L, Crooks D, Dombrowski Y. Oligodendrocytes in development, myelin generation and beyond. Cells, 2019, 8(11): 1424.
pmid: 31726662 |
| [70] |
Yu Q, Guan T, Guo Y, Kong JM. The initial myelination in the central nervous system. ASN Neuro, 2023, 15: 17590914231163039.
pmid: 36974372 |
| [71] |
Bakhti M, Winter C, Simons M. Inhibition of myelin membrane sheath formation by oligodendrocyte-derived exosome-like vesicles. J Biol Chem, 2011, 286(1): 787-796.
pmid: 20978131 |
| [72] |
Goncalves MB, Wu Y, Clarke E, Grist J, Hobbs C, Trigo D, Jack J, Corcoran JPT. Regulation of myelination by exosome associated retinoic acid release from NG2-positive cells. J Neurosci, 2019, 39(16): 3013-3027.
pmid: 30760627 |
| [73] |
Chamberlain KA, Huang N, Xie YX, LiCausi F, Li SN, Li Y, Sheng ZH. Oligodendrocytes enhance axonal energy metabolism by deacetylation of mitochondrial proteins through transcellular delivery of SIRT2. Neuron, 2021, 109(21): 3456-3472.e8.
pmid: 34506725 |
| [74] |
Frühbeis C, Fröhlich D, Kuo WP, Amphornrat J, Thilemann S, Saab AS, Kirchhoff F, Möbius W, Goebbels S, Nave KA, Schneider A, Simons M, Klugmann M, Trotter J, Krämer-Albers EM. Neurotransmitter-triggered transfer of exosomes mediates oligodendrocyte-neuron communication. PLoS Biol, 2013, 11(7): e1001604.
pmid: 23874151 |
| [75] |
Fröhlich D, Kuo WP, Frühbeis C, Sun JJ, Zehendner CM, Luhmann HJ, Pinto S, Toedling J, Trotter J, Krämer- Albers EM. Multifaceted effects of oligodendroglial exosomes on neurons: impact on neuronal firing rate, signal transduction and gene regulation. Philos Trans R Soc Lond B Biol Sci, 2014, 369(1652): 20130510.
pmid: 25135971 |
| [76] |
Mukherjee C, Kling T, Russo B, Miebach K, Kess E, Schifferer M, Pedro LD, Weikert U, Fard MK, Kannaiyan N, Rossner M, Aicher ML, Goebbels S, Nave KA, Krämer-Albers EM, Schneider A, Simons M. Oligodendrocytes provide antioxidant defense function for neurons by secreting ferritin heavy chain. Cell Metab, 2020, 32(2): 259-272.e10.
pmid: 32531201 |
| [77] |
Frühbeis C, Kuo-Elsner WP, Müller C, Barth K, Peris L, Tenzer S, Möbius W, Werner HB, Nave KA, Fröhlich D, Krämer-Albers EM. Oligodendrocytes support axonal transport and maintenance via exosome secretion. PLoS Biol, 2020, 18(12): e3000621.
pmid: 33351792 |
| [78] |
Dutta S, Hornung S, Kruayatidee A, Maina KN, del Rosario I, Paul KC, Wong DY, Duarte Folle A, Markovic D, Palma JA, Serrano GE, Adler CH, Perlman SL, Poon WW, Kang UJ, Alcalay RN, Sklerov M, Gylys KH, Kaufmann H, Fogel BL, Bronstein JM, Ritz B, Bitan G. α-synuclein in blood exosomes immunoprecipitated using neuronal and oligodendroglial markers distinguishes Parkinson’s disease from multiple system atrophy. Acta Neuropathol, 2021, 142(3): 495-511.
pmid: 33991233 |
| [79] |
Kratzer I, Ek J, Stolp H. The molecular anatomy and functions of the choroid plexus in healthy and diseased brain. Biochim Biophys Acta Biomembr, 2020, 1862(11): 183430.
pmid: 32750317 |
| [80] |
Balusu S, Van Wonterghem E, De Rycke R, Raemdonck K, Stremersch S, Gevaert K, Brkic M, Demeestere D, Vanhooren V, Hendrix A, Libert C, Vandenbroucke RE. Identification of a novel mechanism of blood-brain communication during peripheral inflammation via choroid plexus-derived extracellular vesicles. EMBO Mol Med, 2016, 8(10): 1162-1183.
pmid: 27596437 |
| [81] |
Yang Z, Shi XF, Gao ZD, Chu L. miR-155-5p in extracellular vesicles derived from choroid plexus epithelial cells promotes autophagy and inflammation to aggravate ischemic brain injury in mice. Oxid Med Cell Longev, 2022, 2022: 8603427.
pmid: 35222806 |
| [82] |
Vandendriessche C, Balusu S, Van Cauwenberghe C, Brkic M, Pauwels M, Plehiers N, Bruggeman A, Dujardin P, Van Imschoot G, Van Wonterghem E, Hendrix A, Baeke F, De Rycke R, Gevaert K, Vandenbroucke RE. Importance of extracellular vesicle secretion at the blood-cerebrospinal fluid interface in the pathogenesis of Alzheimer’s disease. Acta Neuropathol Commun, 2021, 9(1): 143.
pmid: 34425919 |
| [83] |
O’Hara BA, Morris-Love J, Gee GV, Haley SA, Atwood WJ. JC virus infected choroid plexus epithelial cells produce extracellular vesicles that infect glial cells independently of the virus attachment receptor. PLoS Pathog, 2020, 16(3): e1008371.
pmid: 32130281 |
| [84] |
Grapp M, Wrede A, Schweizer M, Hüwel S, Galla HJ, Snaidero N, Simons M, Bückers J, Low PS, Urlaub H, Gärtner J, Steinfeld R. Choroid plexus transcytosis and exosome shuttling deliver folate into brain parenchyma. Nat Commun, 2013, 4(1): 2123.
pmid: 23828504 |
| [85] |
Ditte Z, Silbern I, Ditte P, Urlaub H, Eichele G. Extracellular vesicles derived from the choroid plexus trigger the differentiation of neural stem cells. J Extracell Vesicles, 2022, 11(11): e12276.
pmid: 36325603 |
| [86] |
Liu LL, Shannahan J, Zheng W. Choroid plexus modulates subventricular zone adult neurogenesis and olfaction through secretion of small extracellular vesicles. bioRxiv, 2023, doi: 10.1101/2023.03.16.532966.
pmid: 36993578 |
| [87] |
Kastriti ME, Adameyko I. Specification, plasticity and evolutionary origin of peripheral glial cells. Curr Opin Neurobiol, 2017, 47: 196-202.
pmid: 29161639 |
| [88] |
Taveggia C, Feltri ML. Beyond wrapping: canonical and noncanonical functions of Schwann cells. Annu Rev Neurosci, 2022, 45(1): 561-580.
pmid: 35440141 |
| [89] |
Wong FC, Ye LH, Demir IE, Kahlert C. Schwann cell-derived exosomes: janus-faced mediators of regeneration and disease. Glia, 2022, 70(1): 20-34.
pmid: 34519370 |
| [90] |
Lopez-Verrilli MA, Picou F, Court FA. Schwann cell- derived exosomes enhance axonal regeneration in the peripheral nervous system. Glia, 2013, 61(11): 1795-1806.
pmid: 24038411 |
| [91] |
López-Leal R, Díaz-Viraqué F, Catalán RJ, Saquel C, Enright A, Iraola G, Court FA. Schwann cell reprogramming into repair cells increases miRNA-21 expression in exosomes promoting axonal growth. J Cell Sci, 2020, 133(12): jcs239004.
pmid: 32409566 |
| [92] |
Zhou M, Hu M, He SM, Li BS, Liu C, Min J, Hong L. Effects of RSC96 Schwann cell-derived exosomes on proliferation, senescence, and apoptosis of dorsal root ganglion cells in vitro. Med Sci Monit, 2018, 24: 7841-7849.
pmid: 30387453 |
| [93] | Hyung S, Kim JY, Yu CJ, Jung HS, Hong JW. Neuroprotective effect of glial cell-derived exosomes on neurons. Immunother (Los Angel), 2019, 5(1): 156. |
| [94] |
Wu ZG, Pu PJ, Su Z, Zhang XC, Nie LY, Chang YM. Schwann cell-derived exosomes promote bone regeneration and repair by enhancing the biological activity of porous Ti6Al4V scaffolds. Biochem Biophys Res Commun, 2020, 531(4): 559-565.
pmid: 32811642 |
| [95] |
Jia LF, Chopp M, Wang L, Lu XR, Szalad A, Zhang ZG. Exosomes derived from high-glucose-stimulated Schwann cells promote development of diabetic peripheral neuropathy. FASEB J, 2018, 32(12): fj201800597R.
pmid: 29932869 |
| [96] |
Wang L, Chopp M, Szalad A, Lu XR, Zhang Y, Wang XL, Cepparulo P, Lu M, Li C, Zhang ZG. Exosomes derived from Schwann cells ameliorate peripheral neuropathy in type 2 diabetic mice. Diabetes, 2020, 69(4): 749-759.
pmid: 31915154 |
| [97] |
Chignon-Sicard B, Hofman V, Chevallier D, Cucchi JM, Ilié M, Dadone-Montaudié B, Paul F, Carpentier X, Quintens H, Bence-Gauchiez C, Caselles D, Rossant J, Durand M, Bertolotti R. Age-related schwannomatosis with potential exosome-mediated contribution to prostate hyperplasia: a case report and mini-review. Ther Adv Urol, 2019, 11: 1756287219875578.
pmid: 31632463 |
| [98] |
Milosavljević A, Jančić J, Mirčić A, Dožić A, Boljanović J, Milisavljević M, Ćetković M. Morphological and functional characteristics of satellite glial cells in the peripheral nervous system. Folia Morphol (Warsz), 2021, 80(4): 745-755.
pmid: 33330971 |
| [99] |
Vinterhøj HSH, Stensballe A, Duroux M, Gazerani P. Characterization of rat primary trigeminal satellite glial cells and associated extracellular vesicles under normal and inflammatory conditions. J Proteomics, 2019, 190: 27-34.
pmid: 29581063 |
| [100] |
Zhao LP, Liu SJ, Zhang XB, Yang J, Mao M, Zhang SS, Xu SQ, Feng SW, Wang X. Satellite glial cell-secreted exosomes after in-vitro oxaliplatin treatment presents a pro-nociceptive effect for dorsal root ganglion neurons and induce mechanical hypersensitivity in naïve mice. Mol Cell Neurosci, 2023, 126: 103881.
pmid: 37467904 |
| [101] |
Xin HQ, Li Y, Buller B, Katakowski M, Zhang Y, Wang XL, Shang X, Zhang ZG, Chopp M. Exosome-mediated transfer of miR-133b from multipotent mesenchymal stromal cells to neural cells contributes to neurite outgrowth. Stem Cells, 2012, 30(7): 1556-1564.
pmid: 22605481 |
| [102] |
Ridder K, Keller S, Dams M, Rupp AK, Schlaudraff J, Del Turco D, Starmann J, Macas J, Karpova D, Devraj K, Depboylu C, Landfried B, Arnold B, Plate KH, Höglinger G, Sültmann H, Altevogt P, Momma S. Extracellular vesicle-mediated transfer of genetic information between the hematopoietic system and the brain in response to inflammation. PLoS Biol, 2014, 12(6): e1001874.
pmid: 24893313 |
| [103] |
Picco F, Zeboudj L, Oggero S, Prato V, Burgoyne T, Gamper N, Malcangio M. Macrophage to neuron communication via extracellular vesicles in neuropathic pain conditions. Heliyon, 2024, 11(1): e41268.
pmid: 39811367 |
| [104] |
Sugihara Y, Onoue S, Tashiro K, Sato M, Hasegawa T, Katakura Y. Carnosine induces intestinal cells to secrete exosomes that activate neuronal cells. PLoS One, 2019, 14(5): e0217394.
pmid: 31136600 |
| [105] |
Verdi V, Bécot A, van Niel G, Verweij FJ. In vivo imaging of EVs in zebrafish: new perspectives from “the waterside”. FASEB BioAdv, 2021, 3(11): 918-929.
pmid: 34761174 |
| [106] |
Bécot A, Corona ML, van Niel G. In vivo imaging: an essential tool to better understand the biology of extracellular vesicles. Med Sci (Paris), 2021, 37(12): 1108-1115.
pmid: 34928213 |
| [107] |
Zhang RL, Mao WB, Niu LM, Bao WD, Wang YQ, Wang Y, Zhu YS, Yang ZH, Chen JC, Dong JW, Cai M, Yuan ZL, Song HK, Li GQ, Zhang M, Xiong NX, Wei J, Dong ZQ. NSC-derived exosomes enhance therapeutic effects of NSC transplantation on cerebral ischemia in mice. eLife, 2023, 12: e84493.
pmid: 37104115 |
| [108] |
Lu WC, Yan JF, Wang CY, Qin WP, Han XX, Qin ZX, Wei Y, Xu HQ, Gao JL, Gao CH, Ye T, Tay FR, Niu LN, Jiao K. Interorgan communication in neurogenic heterotopic ossification: the role of brain-derived extracellular vesicles. Bone Res, 2024, 12(1): 11.
pmid: 38383487 |
| [109] |
Arifin DR, Witwer KW, Bulte JWM. Non-invasive imaging of extracellular vesicles: quo vaditis in vivo? J Extracell vesicles, 2022, 11(7): e12241.
pmid: 35844061 |
| [1] | 寇涵婧, 黄志斌, 张文清, 陈琪. V-ATPase a3亚基调控小胶质细胞吞噬体成熟的机制[J]. 遗传, 2025, 47(11): 1256-1268. |
| [2] | 陈佳强, 丁梅. 细胞外囊泡的研究进展[J]. 遗传, 2025, 47(10): 1078-1098. |
| [3] | 刘吉祥, 赖思婷, 白晶, 徐进. Il34拯救甲硝唑导致的斑马鱼中枢神经系统轴突再生障碍[J]. 遗传, 2024, 46(6): 478-489. |
| [4] | 王奔, 李斯, 吴青峰, 穆文辉. 生酮饮食激活脂肪酸氧化促进ME区的少突胶质细胞前体细胞增殖[J]. 遗传, 2023, 45(5): 425-434. |
| [5] | 商晓康, 张思萌, 倪军军. 组织蛋白酶B参与脑衰老及阿尔兹海默症发生发展研究进展[J]. 遗传, 2023, 45(3): 212-220. |
| [6] | 韩熙, 罗富成. 单细胞转录组测序在少突胶质谱系细胞异质性与神经系统疾病中的应用[J]. 遗传, 2023, 45(3): 198-211. |
| [7] | 郑鹏飞, 谢海波, 朱盼盼, 赵呈天. 斑马鱼神经底板处神经元的分布及特征[J]. 遗传, 2022, 44(6): 510-520. |
| [8] | 王孟晓, 何淑君. 神经胶质细胞调控黑腹果蝇生理行为研究进展[J]. 遗传, 2022, 44(4): 300-312. |
| [9] | 吴安平, 庆宏, 全贞贞. Rab蛋白家族在神经类疾病中的作用[J]. 遗传, 2021, 43(1): 16-29. |
| [10] | 李芳,黄青芸,刘斯佳,郭忠信,熊欣欣,桂林,束会娟,黄绍明,谭国鹤,刘媛媛. Bmal1对小鼠胚胎期皮层神经元放射状迁移和轴突投射的影响[J]. 遗传, 2019, 41(6): 524-533. |
| [11] | 沈秀莲, 逯宜超, 甲芝莲, 吴强. N-WASP通过polyPro和VCA结构域调控大脑皮层神经元迁移[J]. 遗传, 2018, 40(5): 390-401. |
| [12] | 张秀妹, 高洁, 陈春红, 涂海军. 秀丽隐杆线虫固有免疫功能神经调控机制研究进展[J]. 遗传, 2018, 40(12): 1066-1074. |
| [13] | 包笑妹, 何晴, 王莹, 黄智慧, 袁增强. Hippo/YAP信号通路在神经系统中的作用及机制研究进展[J]. 遗传, 2017, 39(7): 630-641. |
| [14] | 陈万金, 张奇杰, 何瑾, 林翔, 王柠. 脊髓性肌萎缩症患者尿液细胞模型的建立[J]. 遗传, 2014, 36(11): 1168-1172. |
| [15] | 赖平,王凭青,张宝云,储明星,刘重旭,谭颖,樊奇. 哺乳动物季节性繁殖的神经内分泌调节机制[J]. 遗传, 2012, 34(3): 281-288. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||

www.chinagene.cn
备案号:京ICP备09063187号-4
总访问:,今日访问:,当前在线:
