遗传 ›› 2020, Vol. 42 ›› Issue (10): 979-992.doi: 10.16288/j.yczz.20-066
赵净颖1, 段小花1,2, 王秋婷1, 黄英1, 贾俊静1, 豆腾飞1( )
)
收稿日期:2020-03-11
修回日期:2020-06-14
出版日期:2020-10-20
发布日期:2020-07-29
通讯作者:
豆腾飞
E-mail:tengfeidou@sina.com
作者简介:赵净颖,在读硕士研究生,专业方向:动物营养与饲料科学。E-mail: 基金资助:
Jingying Zhao1, Xiaohua Duan1,2, Qiuting Wang1, Ying Huang1, Junjing Jia1, Tengfei Dou1( )
)
Received:2020-03-11
Revised:2020-06-14
Online:2020-10-20
Published:2020-07-29
Contact:
Dou Tengfei
E-mail:tengfeidou@sina.com
Supported by:摘要:
骨骼是组成脊椎动物内骨骼的坚硬器官,对机体起着运动、支撑和保护的作用。骨骼处于骨形成和骨吸收两种活动所组成的骨代谢的动态平衡状态,这种平衡对于维持骨量和矿物质稳态至关重要。在动物骨代谢过程中,存在着众多调节骨形成和骨吸收的信号通路,如BMP (bone morphogenetic protein)/SMADs、TGF-β (transforming growth factor β)、Wnt/β-catenin、OPG (osteoprotegerin)/RANKL (receptor activator of NF-κB ligand)/ RANK (receptor activator of NF-κB)、FGF (fibroblast growth factor)和Notch信号通路等。这些信号通路具有复杂的调控机制,参与骨代谢过程的调节。本文综述了在动物骨代谢过程中起关键调节作用的相关信号通路的作用机制及研究进展,以期为动物骨代谢研究奠定基础。
赵净颖, 段小花, 王秋婷, 黄英, 贾俊静, 豆腾飞. 动物骨代谢相关信号通路研究进展[J]. 遗传, 2020, 42(10): 979-992.
Jingying Zhao, Xiaohua Duan, Qiuting Wang, Ying Huang, Junjing Jia, Tengfei Dou. Progress on signal pathways related to bone metabolism in animals[J]. Hereditas(Beijing), 2020, 42(10): 979-992.
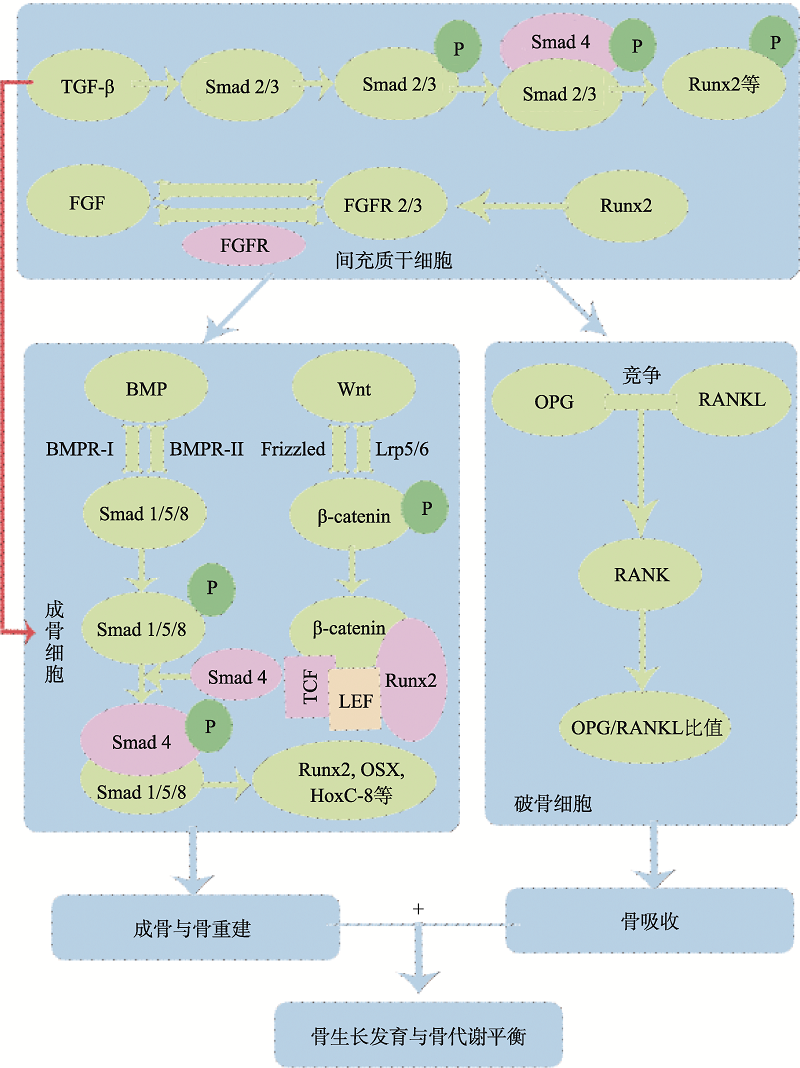
图1
骨代谢调控的关键信号通路图 TGF-β信号通路通过Smad2/3路径调节BMSCs的增殖、分化及其成骨细胞分化;FGF信号通路中FGF和FGFR可调节BMSCs的增殖和成骨分化;BMP/Smads信号通路通过激活Smads1/5/8,结合Smad4,再与Runx2和OSX等相互作用,调节成骨细胞分化与骨重建;Wnt/β-catenin信号通路通过Wnt蛋白与Frizzled 和Lrp5/6结合,激活β-catenin并将其转移到细胞核与TCF/LEF等相互作用,激活Wnt靶基因的转录,从而调控成骨细胞的增殖、分化及骨形成;OPG/RANKL/RANK信号通路中OPG和RANKL竞争性结合,阻止RANKL和RANK之间的结合,通过调节OPG/RANKL比值来调控骨吸收过程。该5条关键信号通路共同参与调节动物骨骼的生长发育与骨代谢平衡。"
| [1] |
Lu L, Huang JS, Xu FY, Xiao ZS, Wang J, Zhang B, David NV, Arends D, Gu WK, Ackert-Bicknell C, Sabik OL, Farber CR, Quarles LD, Williams RW . Genetic dissection of femoral and tibial microarchitecture. JBMR Plus, 2019,3(12):e10241.
doi: 10.1002/jbm4.10241 pmid: 31844829 |
| [2] |
Duren DL, Seselj M, Froehle AW, Nahhas RW, Sherwood RJ . Skeletal growth and the changing genetic landscape during childhood and adulthood. Am J Phys Anthropol, 2013,150(1):48-57.
doi: 10.1002/ajpa.22183 |
| [3] |
Berendsen AD, Olsen BR . Bone development. Bone, 2015,80:14-18.
doi: 10.1016/j.bone.2015.04.035 pmid: 26453494 |
| [4] |
Zhong ZD, Ethen NJ, Williams BO . WNT signaling in bone development and homeostasis. Wiley Interdiscip Rev Dev Biol, 2014,3(6):489-500.
doi: 10.1002/wdev.159 pmid: 25270716 |
| [5] | Tang ZR, Wang Z, Qing FZ, Ni YL, Fan YJ, Tan YF, Zhang XD . Bone morphogenetic protein Smads signaling in mesenchymal stem cells affected by osteoinductive calcium phosphate ceramics. J Biomed Mater Res A, 2015,103(3):1001-1010. |
| [6] |
Ko CH, Chan RL, Siu WS, Shum WT, Leung PC, Zhang L, Cho CH . Deteriorating effect on bone metabolism and microstructure by passive cigarette smoking through dual actions on osteoblast and osteoclast. Calcif Tissue Int, 2015,96(5):389-400.
doi: 10.1007/s00223-015-9966-8 pmid: 25694359 |
| [7] |
Nguyen A, Scott MA, Dry SM, James AW . Roles of bone morphogenetic protein signaling in osteosarcoma. Int Orthop, 2014,38(11):2313-2322.
doi: 10.1007/s00264-014-2512-x |
| [8] |
Carreira AC, Lojudice FH, Halcsik E, Navarro RD, Sogayar MC, Granjeiro JM . Bone morphogenetic proteins: facts, challenges, and future perspectives. J Dent Res, 2014,93(4):335-345.
doi: 10.1177/0022034513518561 |
| [9] |
Dorman LJ, Tucci M, Benghuzzi H . In vitro effects of bmp-2, bmp-7, and bmp-13 on proliferation and differentation of mouse mesenchymal stem cells. Biomed Sci Instrum, 2012,48:81-87.
pmid: 22846268 |
| [10] |
Mi M, Jin HT, Wang BL, Yukata K, Sheu TJ, Ke QH, Tong PJ, Im HJ, Xiao GZ, Chen D . Chondrocyte BMP2 signaling plays an essential role in bone fracture healing. Gene, 2013,512(2):211-218.
doi: 10.1016/j.gene.2012.09.130 |
| [11] |
Lin GL, Hankenson KD . Integration of BMP, Wnt, and notch signaling pathways in osteoblast differentiation. J Cell Biochem, 2011,112(12):3491-3501.
doi: 10.1002/jcb.23287 |
| [12] |
Miyazono K, Kamiya Y, Morikawa M . Bone morphogenetic protein receptors and signal transduction. J Biochem, 2010,147(1):35-51.
doi: 10.1093/jb/mvp148 pmid: 19762341 |
| [13] |
Jang WG, Kim EJ, Lee KN, Son HJ, Koh JT . AMP- activated protein kinase (AMPK) positively regulates osteoblast differentiation via induction of Dlx5-dependent Runx2 expression in MC3T3E1 cells. Biochem Biophys Res Commun, 2011,404(4):1004-1009.
doi: 10.1016/j.bbrc.2010.12.099 pmid: 21187071 |
| [14] |
Tang QQ, Lane MD . Adipogenesis: from stem cell to adipocyte. Annu Rev Biochem, 2012,81:715-736.
doi: 10.1146/annurev-biochem-052110-115718 pmid: 22463691 |
| [15] |
Liang WN, Lin MN, Li XH, Li CD, Gao BZ, Gan HJ, Yang ZY, Lin XJ, Liao LH, Yang M . Icariin promotes bone formation via the BMP-2/Smad4 signal transduction pathway in the hFOB 1.19 human osteoblastic cell line. Int J Mol Med, 2012,30(4):889-895.
doi: 10.3892/ijmm.2012.1079 |
| [16] |
Choi YH, Kim YJ, Jeong HM, Jin YH, Yeo CY, Lee KY . Akt enhances Runx2 protein stability by regulating Smurf2 function during osteoblast differentiation. FEBS J, 2014,281(16):3656-3666.
doi: 10.1111/febs.12887 |
| [17] |
Takimoto A, Kawatsu M, Yoshimoto Y, Kawamoto T, Seiryu M, Takano-Yamamoto T, Hiraki Y, Shukunami C . Scleraxis and osterix antagonistically regulate tensile force-responsive remodeling of the periodontal ligament and alveolar bone. Development, 2015,142(4):787-796.
doi: 10.1242/dev.116228 pmid: 25670797 |
| [18] |
Kruger C, Kappen C . Expression of cartilage developmental genes in Hoxc8- and Hoxd4-transgenic mice. PLoS One, 2010,5(2):e8978.
doi: 10.1371/journal.pone.0008978 pmid: 20126390 |
| [19] |
Li M, Liu XY, Liu XD, Ge BF . Calcium phosphate cement with bmp-2-loaded gelatin microspheres enhances bone healing in osteoporosis: A pilot study. Clin Orthop Relat Res, 2010,468(7):1978-1985.
doi: 10.1007/s11999-010-1321-9 pmid: 20306162 |
| [20] |
Dallari D, Savarino L, Greco M, Rani N, Del Piccolo N, Baldini N . Relevance of deep decortication and vascularization in a case of post-traumatic femoral non-union treated with grafts, platelet gel and bone marrow stromal cells. Knee Surg Sports Traumatol Arthrosc, 2012,20(9):1834-1838.
doi: 10.1007/s00167-011-1790-8 pmid: 22113222 |
| [21] |
Yang WC, Guo DY, Harris MA, Cui Y, Gluhak-Heinrich J, Wu JJ, Chen XD, Skinner C, Nyman JS, Edwards JR, Mundy GR, Lichtler A, Kream BE, Rowe DW, Kalajzic I, David V, Quarles DL, Villareal D, Scott G, Ray M, Liu S, Martin JF, Mishina Y, Harris SE . Bmp2 in osteoblasts of periosteum and trabecular bone links bone formation to vascularization and mesenchymal stem cells. J Cell Sci, 2013,126(18):4085-4098.
doi: 10.1242/jcs.118596 |
| [22] |
Zappitelli T, Chen F, Aubin JE . Up-regulation of BMP2/4 signaling increases both osteoblast-specific marker expression and bone marrow adipogenesis in Gja1Jrt/+ stromal cell cultures. Mol Biol Cell, 2015,26(5):832-842.
doi: 10.1091/mbc.E14-06-1136 pmid: 25568340 |
| [23] |
Zarrinkalam MR, Schultz CG, Ardern DW, Vernon- Roberts B, Moore RJ . Recombinant human bone morphogenetic protein-type 2 (rhBMP-2) enhances local bone formation in the lumbar spine of osteoporotic sheep. J Orthop Res, 2013,31(9):1390-1397.
doi: 10.1002/jor.22387 pmid: 23737220 |
| [24] |
Cipitria A, Reichert JC, Epari DR, Saifzadeh S, Berner A, Schell H, Mehta M, Schuetz MA, Duda GN, Hutmacher DW . Polycaprolactone scaffold and reduced rhBMP-7 dose for the regeneration of critical-sized defects in sheep tibiae. Biomaterials, 2013,34(38):9960-9968.
doi: 10.1016/j.biomaterials.2013.09.011 |
| [25] |
Mizrahi O, Sheyn D, Tawackoli W, Kallai I, Oh A, Su S, Da X, Zarrini P, Cook-Wiens G, Gazit D, Gazit Z . BMP-6 is more efficient in bone formation than BMP-2 when overexpressed in mesenchymal stem cells. Gene Ther, 2012,20(4):370-377.
doi: 10.1038/gt.2012.45 pmid: 22717741 |
| [26] |
Chen GQ, Deng CX, Li YP . TGF-β and BMP signaling in osteoblast differentiation and bone formation. Int J Biol Sci, 2012,8(2):272-288.
doi: 10.7150/ijbs.2929 pmid: 22298955 |
| [27] |
Xing LP, Zhang M, Chen D . Smurf control in bone cells. J Cell Biochem, 2010,110(3):554-563.
doi: 10.1002/jcb.22586 pmid: 20512916 |
| [28] |
Takeshita S, Fumoto T, Matsuoka K, Park KA, Aburatani H, Kato S, Ito M, Ikeda K . Osteoclast-secreted CTHRC1 in the coupling of bone resorption to formation. J Clin Invest, 2013,123(9):3914-3924.
doi: 10.1172/JCI69493 |
| [29] |
Chen JR, Lazarenko OP, Blackburn ML, Badeaux JV, Badger TM, Ronis MJJ . Infant formula promotes bone growth in neonatal piglets by enhancing osteoblastogenesis through bone morphogenic protein signaling. J Nutr, 2009,139(10):1839-1847.
doi: 10.3945/jn.109.109041 pmid: 19710159 |
| [30] |
Lienau J, Schmidt-Bleek K, Peters A, Weber H, Bail HJ, Duda GN, Perka C, Schell H . Insight into the molecular pathophysiology of delayed bone healing in a sheep model. Tissue Eng Part A, 2010,16(1):191-199.
pmid: 19678759 |
| [31] |
Feng C, Xiao L, Yu JC, Li DY, Tang TY, Liao W, Wang ZR, Lu AQ . Simvastatin promotes osteogenic differentiation of mesenchymal stem cells in rat model of osteoporosis through BMP-2/Smads signaling pathway. Eur Rev Med Pharmacol Sci, 2020,24(1):434-443.
doi: 10.26355/eurrev_202001_19943 pmid: 31957858 |
| [32] |
Chai S, Wan L, Wang JL, Huang JC, Huang HX . Gushukang inhibits osteocyte apoptosis and enhances BMP-2/Smads signaling pathway in ovariectomized rats. Phytomedicine, 2019,64:153063.
doi: 10.1016/j.phymed.2019.153063 pmid: 31419728 |
| [33] |
Yu B, Tang DZ, Li SY, Wu Y, Chen M . Daidzein promotes proliferation and differentiation in osteoblastic OCT1 cells via activation of the BMP-2/Smads pathway. Pharmazie, 2017,72(1):35-40.
doi: 10.1691/ph.2017.6502 pmid: 29441895 |
| [34] |
Feng XH, Derynck R . Specificity and versatility in TGF-β signaling through Smads. Annu Rev Cell Dev Biol, 2005,21:659-693.
doi: 10.1146/annurev.cellbio.21.022404.142018 pmid: 16212511 |
| [35] |
Chaudhry SS, Cain SA, Morgan A, Dallas SL, Shuttleworth CA, Kielty CM . Fibrillin-1 regulates the bioavailability of TGFβ1. J Cell Biol, 2007,176(3):355-367.
doi: 10.1083/jcb.200608167 pmid: 17242066 |
| [36] |
Dünker N, Krieglstein K . Tgfβ2 -/- Tgfβ3 -/- double knockout mice display severe midline fusion defects and early embryonic lethality. Anat Embryol (Berl), 2002,206(1-2):73-83.
doi: 10.1007/s00429-002-0273-6 |
| [37] |
Yasui T, Kadono Y, Nakamura M, Oshima Y, Matsumoto T, Masuda H, Hirose J, Omata Y, Yasuda H, Imamura T, Nakamura K, Tanaka S . Regulation of RANKL-induced osteoclastogenesis by TGF-β through molecular interaction between Smad3 and Traf6. J Bone Miner Res, 2011,26(7):1447-1456.
doi: 10.1002/jbmr.357 pmid: 21305609 |
| [38] |
Mohammad KS, Chen CG, Balooch G, Stebbins E, McKenna CR, Davis H, Niewolna M, Peng XH, Nguyen DH, Ionova-Martin SS, Bracey JW, Hogue WR, Wong DH, Ritchie RO, Suva LJ, Derynck R, Guise TA, Alliston T. Pharmacologic inhibition of the TGF-β type I receptor kinase has anabolic and anti-catabolic effects on bone. PLoS One, 2009,4(4):e5275.
doi: 10.1371/journal.pone.0005275 pmid: 19357790 |
| [39] |
Karst M, Gorny G, Galvin RJ, Oursler MJ . Roles of stromal cell RANKL, OPG, and M-CSF expression in biphasic TGF-β regulation of osteoclast differentiation. J Cell Physiol, 2004,200(1):99-106.
doi: 10.1002/jcp.20036 pmid: 15137062 |
| [40] |
Subramaniam M, Hawse JR, Bruinsma ES, Grygo SB, Cicek M, Oursler MJ, Spelsberg TC. TGFβ inducible early gene-1 directly binds to, represses, the OPG promoter in osteoblasts. Biochem Biophys Res Commun, 2010(1), 392(1):72-76.
doi: 10.1016/j.bbrc.2009.12.171 pmid: 20059964 |
| [41] |
Shi AY, Heinayati A, Bao DY, Liu HF, Ding XC, Tong X, Wang LD, Wang B, Qin HY . Small molecule inhibitor of TGF-β signaling enables robust osteogenesis of autologous GMSCs to successfully repair minipig severe maxillofacial bone defects. Stem Cell Res Ther, 2019,10(1):172.
doi: 10.1186/s13287-019-1281-2 pmid: 31196174 |
| [42] |
Zeng HC, Bae Y, Dawson BC, Chen YQ, Bertin T, Munivez E, Campeau PM, Tao JN, Chen R, Lee BH . MicroRNA miR-23a cluster promotes osteocyte differentiation by regulating TGF-β signalling in osteoblasts. Nat Commun, 2017,8:15000.
doi: 10.1038/ncomms15000 pmid: 28397831 |
| [43] |
Xu X, Zheng LW, Bian Q, Xie L, Liu WL, Zhen GH, Crane JL, Zhou XD, Cao X . Aberrant activation of TGF-β in subchondral bone at the onset of rheumatoid arthritis joint destruction. J Bone Miner Res, 2015,30(11):2033-2043.
doi: 10.1002/jbmr.2550 |
| [44] |
Tu XL, Delgado-Calle J, Condon KW, Maycas M, Zhang H, Carlesso N, Taketo MM, Burr DB, Plotkin LI, Bellido T . Osteocytes mediate the anabolic actions of canonical Wnt/β-catenin signaling in bone. Proc Natl Acad Sci USA, 2015,112(5):E478-486.
doi: 10.1073/pnas.1409857112 pmid: 25605937 |
| [45] |
Boyden LM, Mao JH, Belsky J, Mitzner L, Farhi A, Mitnick MA, Wu DQ, Insogna K, Lifton RP . High bone density due to a mutation in LDL-receptor-related protein 5. N Engl J Med, 2002,346(20):1513-1521.
doi: 10.1056/NEJMoa013444 pmid: 12015390 |
| [46] |
Little RD, Carulli JP, Del Mastro RG, Dupuis J, Osborne M, Folz C, Manning SP, Swain PM, Zhao SC, Eustace B, Lappe MM, Spitzer L, Zweier S, Braunschweiger K, Benchekroun Y, Hu XT, Adair R, Chee L, FitzGerald MG, Tulig C, Caruso A, Tzellas N, Bawa A, Franklin B, McGuire S, Nogues X, Gong G, Allen KM, Anisowicz A, Morales AJ, Lomedico PT, Recker SM, Van Eerdewegh P, Recker RR, Johnson ML. A mutation in the LDL receptor-related protein 5 gene results in the autosomal dominant high-bone-mass trait. Am J Hum Genet, 2002,70(1):11-19.
doi: 10.1086/338450 pmid: 11741193 |
| [47] |
Hu HL, Hilton MJ, Tu XL, Yu K, Ornitz DM, Long FX . Sequential roles of Hedgehog and Wnt signaling in osteoblast development. Development, 2005,132(1):49-60.
doi: 10.1242/dev.01564 pmid: 15576404 |
| [48] |
Day TF, Guo XZ, Garrett-Beal L, Yang YZ . Wnt/β-catenin signaling in mesenchymal progenitors controls osteoblast and chondrocyte differentiation during vertebrate skeletogenesis. Dev Cell, 2005,8(5):739-750.
doi: 10.1016/j.devcel.2005.03.016 pmid: 15866164 |
| [49] |
Hill TP, Später D, Taketo MM, Birchmeier W, Hartmann C . Canonical Wnt/β-catenin signaling prevents osteoblasts from differentiating into chondrocytes. Dev Cell, 2005,8(5):727-738.
pmid: 15866163 |
| [50] |
Rodda SJ, Mcmahon AP . Distinct roles for Hedgehog and canonical Wnt signaling in specification, differentiation and maintenance of osteoblast progenitors. Development, 2006,133(16):3231-3244.
doi: 10.1242/dev.02480 pmid: 16854976 |
| [51] |
Glass DA, Bialek P, Ahn JD, Starbuck M, Patel MS, Clevers H, Taketo MM, Long FX, McMahon AP, Lang RA, Karsenty G. Canonical Wnt signaling in differentiated osteoblasts controls osteoclast differentiation. Dev Cell, 2005,8(5):751-764.
doi: 10.1016/j.devcel.2005.02.017 pmid: 15866165 |
| [52] |
Holmen SL, Zylstra CR, Mukherjee A, Sigler RE, Faugere MC, Bouxsein ML, Deng LF, Clemens TL, Williams BO . Essential role of β-catenin in postnatal bone acquisition. J Biol Chem, 2005,280(22):21162-21168.
doi: 10.1074/jbc.M501900200 pmid: 15802266 |
| [53] |
Kramer I, Halleux C, Keller H, Pegurri M, Gooi JH, Weber PB, Feng JQ, Bonewald LF, Kneissel M . Osteocyte Wnt/β-catenin signaling is required for normal bone homeostasis. Mol Cell Biol, 2010,30(12):3071-3085.
doi: 10.1128/MCB.01428-09 pmid: 20404086 |
| [54] |
Bellido T, Ali AA, Gubrij I, Plotkin LI, Fu Q, O'Brien CA, Manolagas SC, Jilka RL. Chronic elevation of parathyroid hormone in mice reduces expression of sclerostin by osteocytes: A novel mechanism for hormonal control of osteoblastogenesis. Endocrinology, 2005,146(11):4577-4583.
doi: 10.1210/en.2005-0239 pmid: 16081646 |
| [55] |
O'Brien CA, Plotkin LI, Galli C, Goellner JJ, Gortazar AR, Allen MR, Robling AG, Bouxsein M, Schipani E, Turner CH, Jilka RL, Weinstein RS, Manolagas SC, Bellido T. Control of bone mass and remodeling by PTH receptor signaling in osteocytes. PLoS One, 2008,3(8):e2942.
doi: 10.1371/journal.pone.0002942 pmid: 18698360 |
| [56] |
Tu XL, Rhee Y, Condon KW, Bivi N, Allen MR, Dwyer D, Stolina M, Turner CH, Robling AG, Plotkin LI, Bellido T . Sost downregulation and local Wnt signaling are required for the osteogenic response to mechanical loading. Bone, 2012,50(1):209-217.
doi: 10.1016/j.bone.2011.10.025 |
| [57] |
Loots GG, Kneissel M, Keller H, Baptist M, Chang J, Collette NM, Ovcharenko D, Plajzer-Frick I, Rubin EM . Genomic deletion of a long-range bone enhancer misregulates sclerostin in Van Buchem disease. Genome Res, 2005,15(7):928-935.
doi: 10.1101/gr.3437105 pmid: 15965026 |
| [58] |
Balemans W, Ebeling M, Patel N, Van Hul E, Olson P, Dioszegi M, Lacza C, Wuyts W, Van Den Ende J, Willems P, Paes-Alves AF, Hill S, Bueno M, Ramos FJ, Tacconi P, Dikkers FG, Stratakis C, Lindpaintner K, Vickery B, Foernzler D, Van Hul W. Increased bone density in sclerosteosis is due to the deficiency of a novel secreted protein (SOST). Hum Mol Genet, 2001,10(5):537-543.
doi: 10.1093/hmg/10.5.537 pmid: 11181578 |
| [59] |
Wang YY, Zhang XH, Shao J, Liu HH, Liu X, Luo E . Adiponectin regulates BMSC osteogenic differentiation and osteogenesis through the Wnt/β-catenin pathway. Sci Rep, 2017,7(1):3652.
doi: 10.1038/s41598-017-03899-z pmid: 28623357 |
| [60] |
Zhu Y, Wang YM, Jia YC, Xu J, Chai YM . Catalpol promotes the osteogenic differentiation of bone marrow mesenchymal stem cells via the Wnt/β-catenin pathway. Stem Cell Res Ther, 2019,10(1):37.
pmid: 30670092 |
| [61] |
Molagoda IMN, Karunarathne WAHM, Choi YH, Park EK, Jeon YJ, Lee BJ, Kang CH, Kim GY . Fermented oyster extract promotes osteoblast differentiation by activating the Wnt/β-catenin signaling pathway, leading to bone formation. Biomolecules, 2019,9(11):711.
doi: 10.3390/biom9110711 |
| [62] |
Chen X, Wang ZQ, Duan N, Zhu GY, Schwarz EM, Xie C . Osteoblast-osteoclast interactions. Connect Tissue Res, 2018,59(2):99-107.
doi: 10.1080/03008207.2017.1290085 pmid: 28324674 |
| [63] |
Hamdy NAT . Targeting the RANK/RANKL/OPG signaling pathway: a novel approach in the management of osteoporosis. Curr Opin Investig Drugs, 2007,8(4):299-303.
pmid: 17458179 |
| [64] |
Hofbauer LC, Kühne CA, Viereck V . The OPG/RANKL/ RANK system in metabolic bone diseases. J Musculoskelet Neuronal Interact, 2004,4(3):268-275.
pmid: 15615494 |
| [65] | Sisay M, Abdela J, Molla Y . The molecular triad system involving RANK/RANKL/OPG as therapeutic targets for metabolic bone diseases. JDDT, 2016,6(6):31-39. |
| [66] |
Ono T, Hayashi M, Sasaki F, Nakashima T . RANKL biology: bone metabolism, the immune system, and beyond. Inflamm Regen, 2020,40:2.
doi: 10.1186/s41232-019-0111-3 pmid: 32047573 |
| [67] |
Wada T, Nakashima T, Hiroshi N, Penninger JM . RANKL-RANK signaling in osteoclastogenesis and bone disease. Trends Mol Med, 2006,12(1):17-25.
doi: 10.1016/j.molmed.2005.11.007 pmid: 16356770 |
| [68] |
Kawaida R, Ohtsuka T, Okutsu J, Takahashi T, Kadono Y, Oda H, Hikita A, Nakamura K, Tanaka S, Furukawa H . Jun dimerization protein 2 (JDP2), a member of the AP-1 family of transcription factor, mediates osteoclast differentiation induced by RANKL. J Exp Med, 2003,197(8):1029-1035.
doi: 10.1084/jem.20021321 pmid: 12707301 |
| [69] |
Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Lüthy R, Nguyen HQ, Wooden S, Bennett L, Boone T, Shimamoto G, DeRose M, Elliott R, Colombero A, Tan HL, Trail G, Sullivan J, Davy E, Bucay N, Renshaw-Gegg L, Hughes TM, Hill D, Pattison W, Campbell P, Sander S, Van G, Tarpley J, Derby P, Lee R, Boyle WJ. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell, 1997,89(2):309-319.
doi: 10.1016/s0092-8674(00)80209-3 pmid: 9108485 |
| [70] |
Melville KM, Kelly NH, Khan SA, Schimenti JC, Ross FP, Main RP, van der Meulen MCH,. Female mice lacking estrogen receptor-alpha in osteoblasts have compromised bone mass and strength. J Bone Miner Res, 2014,29(2):370-379.
doi: 10.1002/jbmr.2082 pmid: 24038209 |
| [71] |
Liu Y, Du HM, Wang YF, Liu MM, Deng SJ, Fan LL, Zhang LL, Sun Y, Zhang Q . Osteoprotegerin-knockout mice developed early onset root resorption. J Endod, 2016,42(10):1516-1522.
doi: 10.1016/j.joen.2016.07.008 |
| [72] |
Kostenuik PJ . Osteoprotegerin and RANKL regulate bone resorption, density, geometry and strength. Curr Opin Pharmacol, 2005,5(6):618-625.
doi: 10.1016/j.coph.2005.06.005 |
| [73] |
Titanji K, Vunnava A, Sheth AN, Delille C, Lennox JL, Sanford SE, Foster A, Knezevic A, Easley KA, Weitzmann MN, Ofotokun I . Dysregulated B cell expression of RANKL and OPG correlates with loss of bone mineral density in HIV infection. PLoS Pathog, 2014,10(10):e1004497.
doi: 10.1371/journal.ppat.1004497 pmid: 25393853 |
| [74] |
Huang SC, Zhang LH, Zhang JL, Rehman MU, Tong XL, Qiu G, Jiang X, Iqbal M, Shahzad M, Shen YQ, Li JK . Role and regulation of growth plate vascularization during coupling with osteogenesis in tibial dyschondroplasia of chickens. Sci Rep, 2018,8(1):3680.
doi: 10.1038/s41598-018-22109-y pmid: 29487404 |
| [75] |
Wu RX, Li Q, Pei XH, Hu KF . Effects of brucine on the OPG/RANKL/RANK signaling pathway in MDA-MB- 231 and MC3T3-E1 cell coculture system. Evid Based Complement Alternat Med, 2017,2017:1693643.
doi: 10.1155/2017/1693643 pmid: 29081815 |
| [76] |
Hou JM, Xue Y, Lin QM . Bovine lactoferrin improves bone mass and microstructure in ovariectomized rats via OPG/RANKL/RANK pathway. Acta Pharmacol Sin, 2012,33(10):1277-1284.
doi: 10.1038/aps.2012.83 |
| [77] |
Ma B, Zhang Q, Wu D, Wang YL, Hu YY, Cheng YP, Yang ZD, Zheng YY, Ying HJ. Strontium fructose 1, 6-diphosphate prevents bone loss in a rat model of postmenopausal osteoporosis via the OPG/RANKL/RANK pathway. Acta Pharmacol Sin, 2012, 33(4): 479‐489.
doi: 10.1038/aps.2011.177 |
| [78] |
Yun YR, Won JE, Jeon E, Lee S, Kang W, Jo H, Jang JH, Shin US, Kim HW . Fibroblast growth factors: Biology, function, and application for tissue regeneration. J Tissue Eng, 2010,2010:218142.
doi: 10.4061/2010/218142 pmid: 21350642 |
| [79] |
Ornitz DM, Marie PJ . FGF signaling pathways in endochondral and intramembranous bone development and human genetic disease. Genes Dev, 2002,16(12):1446-1465.
doi: 10.1101/gad.990702 pmid: 12080084 |
| [80] | Coutu DL, Galipeau J . Roles of FGF signaling in stem cell self-renewal, senescence and aging. Aging (Albany NY), 2011,3(10):920-933. |
| [81] |
Du XL, Xie YL, Xian CJ, Chen L . Role of FGFs/FGFRs in skeletal development and bone regeneration. J Cell Physiol, 2012,227(12):3731-3743.
doi: 10.1002/jcp.24083 |
| [82] |
Xiao GZ, Jiang D, Gopalakrishnan R, Franceschi RT . Fibroblast growth factor 2 induction of the osteocalcin gene requires MAPK activity and phosphorylation of the osteoblast transcription factor, Cbfa1/Runx2. J Biol Chem, 2002,277(39):36181-36187.
doi: 10.1074/jbc.M206057200 pmid: 12110689 |
| [83] |
Jacob AL, Smith C, Partanen J, Ornitz DM . Fibroblast growth factor receptor 1 signaling in the osteo- chondrogenic cell lineage regulates sequential steps of osteoblast maturation. Dev Biol, 2006,296(2):315-328.
doi: 10.1016/j.ydbio.2006.05.031 pmid: 16815385 |
| [84] |
Arvidson K, Abdallah BM, Applegate LA, Baldini N, Cenni E, Gomez-Barrena E, Granchi D, Kassem M, Konttinen YT, Mustafa K, Pioletti DP, Sillat T, Finne-Wistrand A . Bone regeneration and stem cells. J Cell Mol Med, 2011,15(4):718-746.
doi: 10.1111/j.1582-4934.2010.01224.x |
| [85] |
Huang W, Yang SY, Shao JZ, Li YP . Signaling and transcriptional regulation in osteoblast commitment and differentiation. Front Biosci, 2007,12:3068-3092.
doi: 10.2741/2296 pmid: 17485283 |
| [86] |
Kanda Y, Nishimura I, Sato T, Katayama A, Arano T, Ikada Y, Yoshinari M . Dynamic cultivation with radial flow bioreactor enhances proliferation or differentiation of rat bone marrow cells by fibroblast growth factor or osteogenic differentiation factor. Regen Ther, 2016,5:17-24.
doi: 10.1016/j.reth.2016.06.001 pmid: 31245496 |
| [87] |
Furuya H, Tabata Y, Kaneko K . Bone regeneration for murine femur fracture by gelatin hydrogels incorporating basic fibroblast growth factor with different release profiles. Tissue Eng Part A, 2014,20(9-10):1531-1541.
doi: 10.1089/ten.TEA.2012.0763 pmid: 24410201 |
| [88] |
D’Mello S, Elangovan S, Salem AK . FGF2 gene activated matrices promote proliferation of bone marrow stromal cells. Arch Oral Biol, 2015,60(12):1742-1749.
doi: 10.1016/j.archoralbio.2015.09.005 pmid: 26433191 |
| [89] |
Khorsand B, Nicholson N, Do AV, Femino JE, Martin JA, Petersen E, Guetschow B, Fredericks DC, Salem AK . Regeneration of bone using nanoplex delivery of FGF-2 and BMP-2 genes in diaphyseal long bone radial defects in a diabetic rabbit model. J Control Release, 2017,248:53-59.
doi: 10.1016/j.jconrel.2017.01.008 pmid: 28069556 |
| [90] |
Charles LF, Woodman JL, Ueno D, Gronowicz G, Hurley MM, Kuhn LT . Effects of low dose FGF-2 and BMP-2 on healing of calvarial defects in old mice. Exp Gerontol, 2015,64:62-69.
doi: 10.1016/j.exger.2015.02.006 pmid: 25681640 |
| [91] | Yuan SH, Pan Q, Fu CJ, Bi ZG . Effect of growth factors (BMP-4/7 & bFGF) on proliferation & osteogenic differentiation of bone marrow stromal cells. Indian J Med Res. 2013,138(1):104-110. |
| [92] |
Majidinia M, Alizadeh E, Yousefi B, Akbarzadeh M, Mihanfar A, Rahmati-Yamchi M, Zarghami N . Co-inhibition of notch and NF-κB signaling pathway decreases proliferation through downregulating IκB-α and hes-1 expression in human ovarian cancer OVCAR-3 cells. Drug Res (Stuttg), 2017,67(1):13-19.
doi: 10.1055/s-0042-115405 |
| [93] |
Hilton MJ, Tu XL, Wu XM, Bai ST, Zhao HB, Kobayashi T, Kronenberg HM, Teitelbaum SL, Ross FP, Kopan R, Long FX . Notch signaling maintains bone marrow mesenchymal progenitors by suppressing osteoblast differentiation. Nat Med, 2008,14(3):306-314.
doi: 10.1038/nm1716 pmid: 18297083 |
| [94] |
Tu XL, Chen JQ, Lim J, Karner CM, Lee SY, Heisig J, Wiese C, Surendran K, Kopan R, Gessler M, Long FX . Physiological notch signaling maintains bone homeostasis via RBPjk and Hey upstream of NFATc1. PLoS Genet, 2012,8(3):e1002577.
doi: 10.1371/journal.pgen.1002577 pmid: 22457635 |
| [95] |
Pan MX, Hong W, Yao Y, Gao XX, Zhou Y, Fu GX, Li YC, Guo Q, Rao XX, Tang PY, Chen SZ, Jin WF, Hua GQ, Gao JJ, Xu XY . Activated B lymphocyte inhibited the osteoblastogenesis of bone mesenchymal stem cells by Notch signaling. Stem Cells Int, 2019,2019:8150123.
doi: 10.1155/2019/8150123 pmid: 31281386 |
| [96] |
He Y, Zou LJ . Notch-1 inhibition reduces proliferation and promotes osteogenic differentiation of bone marrow mesenchymal stem cells. Exp Ther Med, 2019,18(3):1884-1890.
doi: 10.3892/etm.2019.7765 pmid: 31410150 |
| [97] |
Fukushima H, Nakao A, Okamoto F, Shin M, Kajiya H, Sakano S, Bigas A, Jimi E, Okabe K . The association of notch2 and nf-kappab accelerates rankl-induced osteoclastogenesis. Mol Cell Biol, 2008,28(20):6402-6412.
pmid: 18710934 |
| [98] |
Capurro M, Izumikawa T, Suarez P, Shi W, Cydzik M, Kaneiwa T, Gariepy J, Bonafe L, Filmus J . Glypican-6 promotes the growth of developing long bones by stimulating Hedgehog signaling. J Cell Biol, 2017,216(9):2911-2926.
doi: 10.1083/jcb.201605119 pmid: 28696225 |
| [99] |
St-Jacques B, Hammerschmidt M, McMahon AP. Indian hedgehog signaling regulates proliferation and differentiation of chondrocytes and is essential for bone formation. Genes Dev, 1999,13(16):2072-2086.
doi: 10.1101/gad.13.16.2072 pmid: 10465785 |
| [100] |
Vortkamp A, Lee K, Lanske B, Segre GV, Kronenberg HM, Tabin CJ . Regulation of rate of cartilage differentiation by Indian hedgehog and PTH-related protein. Science, 1996,273(5275):613-622.
doi: 10.1126/science.273.5275.613 |
| [101] |
Luo M, Huang HX, Huang H, Li ZT, Lai YY . Hedgehog signaling pathway and osteoporosis. Zhongguo Gu Shang, 2014,27(2):169-172.
pmid: 24826487 |
| [102] |
Zaman F, Zhao YH, Celvin B, Mehta HH, Wan JX, Chrysis D, Ohlsson C, Fadeel B, Cohen P, Sävendahl L . Humanin is a novel regulator of Hedgehog signaling and prevents glucocorticoid-induced bone growth impairment. FASEB J, 2019,33(4):4962-4974.
doi: 10.1096/fj.201801741R pmid: 30657335 |
| [103] |
Martini M, De Santis MC, Braccini L, Gulluni F, Hirsch E . PI3K/AKT signaling pathway and cancer: An updated review. Ann Med, 2014,46(6):372-383.
doi: 10.3109/07853890.2014.912836 |
| [104] |
Liu TJ, Gao YH, Sakamoto K, Minamizato T, Furukawa K, Tsukazaki T, Shibata Y, Bessho K, Komori T, Yamaguchi A . BMP-2 promotes differentiation of osteoblasts and chondroblasts in Runx2-deficient cell lines. J Cell Physiol, 2007,211(3):728-735.
doi: 10.1002/jcp.20988 pmid: 17226753 |
| [105] |
Ke K, Li Q, Yang XF, Xie ZJ, Wang Y, Shi J, Chi LF, Xu WJ, Hu LL, Shi HL . Asperosaponin VI promotes bone marrow stromal cell osteogenic differentiation through the PI3K/AKT signaling pathway in an osteoporosis model. Sci Rep, 2016,6:35233.
doi: 10.1038/srep35233 pmid: 27756897 |
| [106] |
Barradas AM, Fernandes HA, Groen N, Chai YC, Schrooten J, van de Peppel J, van Leeuwen JP, van Blitterswijk CA, de Boer J. A calcium-induced signaling cascade leading to osteogenic differentiation of human bone marrow-derived mesenchymal stromal cells. Biomaterials, 2012,33(11):3205-3215.
doi: 10.1016/j.biomaterials.2012.01.020 |
| [107] |
Liedert A, Kaspar D, Blakytny R, Claes L, Ignatius A . Signal transduction pathways involved in mechanotransduction in bone cells. Biochem Biophys Res Commun, 2006,349(1):1-5.
doi: 10.1016/j.bbrc.2006.07.214 pmid: 16930556 |
| [108] |
Liu JN, Lv FQ, Sun W, Tao CX, Ding GX, Karaplis A, Brown E, Goltzman D, Miao DS . The abnormal phenotypes of cartilage and bone in calcium-sensing receptor deficient mice are dependent on the actions of calcium, phosphorus, and PTH. PLoS Genet, 2011,7(9):e1002294.
doi: 10.1371/journal.pgen.1002294 pmid: 21966280 |
| [109] |
Chang WH, Tu CL, Chen TH, Bikle D, Shoback D. The extracellular calcium-sensing receptor(CaSR) is a critical modulator of skeletal development. Sci Signal, 2008, 1(35): ra1.
doi: 10.1126/scisignal.135pe40 pmid: 18765829 |
| [110] |
Wang B, Lin J, Zhang Q, Zhang XY, Yu H, Gong P, Xiang L . αCGRP Affects BMSCs' Migration and Osteogenesis via the Hippo-YAP Pathway. Cell Transplant, 2019,28(11):1420-1431.
doi: 10.1177/0963689719871000 pmid: 31426665 |
| [111] |
Chen Z, Luo Q, Lin CC, Kuang DD, Song GB . Simulated microgravity inhibits osteogenic differentiation of mesenchymal stem cells via depolymerizing F-actin to impede TAZ nuclear translocation. Sci Rep, 2016,6:30322.
doi: 10.1038/srep30322 pmid: 27444891 |
| [1] | 孙凤宇, 许强华. 血液发生相关microRNAs研究进展[J]. 遗传, 2022, 44(9): 756-771. |
| [2] | 唐湘薇, 楚丹, 颜赛娜, 尹艳飞, 卞桥, 翁波, 陈斌, 冉茂良. miR-191靶向BDNF基因通过激活PI3K/AKT信号通路促进猪未成熟支持细胞增殖[J]. 遗传, 2021, 43(7): 680-693. |
| [3] | 张春霞, 刘峰. 造血干细胞发育过程中的信号通路调控[J]. 遗传, 2021, 43(4): 295-307. |
| [4] | 杜倍倍, 刘磊, 朱洋洋. RNA结合蛋白Roquin负调控STING依赖的果蝇天然免疫反应[J]. 遗传, 2020, 42(12): 1201-1210. |
| [5] | 尹玲倩,冉金山,李菁菁,任鹏,张贤娴,刘益平. 禽类就巢性状的遗传调控[J]. 遗传, 2019, 41(5): 391-403. |
| [6] | 杨志, 姚俊, 曹新. FGF信号通路在内耳发育调控和毛细胞再生中的作用[J]. 遗传, 2018, 40(7): 515-524. |
| [7] | 孙书国, 吴世安, 张雷. Hippo信号通路在果蝇遗传学研究中的发现与扩展[J]. 遗传, 2017, 39(7): 537-545. |
| [8] | 吉新彦, 钟国轩, 赵斌. 哺乳动物Hippo信号通路分子机制研究进展[J]. 遗传, 2017, 39(7): 546-567. |
| [9] | 张平平,佟鑫,张天乐,黎子琛,龚清秋. 植物Hippo信号通路研究进展[J]. 遗传, 2017, 39(7): 568-575. |
| [10] | 顾远, 张雷, 余发星. Hippo信号通路在肠道稳态、再生及癌变过程中的作用及机制[J]. 遗传, 2017, 39(7): 588-596. |
| [11] | 付思玲,赵婉滢,张雯婧,宋海,季红斌,汤楠. Hippo信号通路在肺发育、再生和疾病中的功能[J]. 遗传, 2017, 39(7): 597-606. |
| [12] | 姚传波, 周鑫, 陈策实, 雷群英. Hippo信号通路在乳腺癌中的调控机制及作用[J]. 遗传, 2017, 39(7): 617-629. |
| [13] | 包笑妹, 何晴, 王莹, 黄智慧, 袁增强. Hippo/YAP信号通路在神经系统中的作用及机制研究进展[J]. 遗传, 2017, 39(7): 630-641. |
| [14] | 周欣,李伟芸,王红艳. MST1/2调控先天免疫的功能和机制[J]. 遗传, 2017, 39(7): 642-649. |
| [15] | 余淑娟,耿晶,陈兰芬. Hippo信号通路调控免疫细胞的功能[J]. 遗传, 2017, 39(7): 650-658. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||

www.chinagene.cn
备案号:京ICP备09063187号-4
总访问:,今日访问:,当前在线:
